Abstract
Background
Intercropping (IC) has been widely adopted by farmers for enhancing crop productivity and economic returns; however, the underpinning mechanisms from the perspective of below-ground interspecific interactions are only partly understood especially when intercropping practices under saline soil conditions. By using permeable (100 μm) and impermeable (solid) root barriers in a multi-site field experiment, we aimed to study the impact of root-root interactions on nutrient accumulation, soil microbial communities, crop yield, and economic returns in a peanut/cotton IC system under non-saline, secondary-saline, and coastal saline soil conditions of China.
Results
The results indicate that IC decreased the peanut pods yield by 14.00, 10.01, and 16.52% while increased the seed cotton yield by 61.99, 66.00, and 58.51%, respectively in three experimental positions, and consequently enhanced the economic returns by compared with monoculture of peanut (MP) and cotton (MC). The higher accumulations of nutrients such as nitrogen (N), phosphorus (P), and potassium (K) were also observed in IC not only in the soil but also in vegetative tissues and reproductive organs of peanut. Bacterial community structure analysis under normal growth conditions reveals that IC dramatically altered the soil bacterial abundance composition in both peanut and cotton strips of the top soil whereas the bacterial diversity was barely affected compared with MP and MC. At blossom-needling stage, the metabolic functional features of the bacterial communities such as fatty acid biosynthesis, lipoic acid metabolism, peptidoglycan biosynthesis, and biosynthesis of ansamycins were significantly enriched in MP compared with other treatments. Conversely, these metabolic functional features were dramatically depleted in MP while significantly enriched in IC at podding stage. Permeable root barrier treatments (NC-P and NC-C) counteracted the benefits of IC and the side effects were more pronounced in impermeable treatments (SC-P and SC-C).
Conclusion
Peanut/cotton intercropping increases crop yield as well as economic returns under non-saline, secondary-saline, and coastal saline soil conditions probably by modulating the soil bacterial abundance composition and accelerating plant nutrients accumulation.
Similar content being viewed by others
Background
Intercropping has been defined as simultaneously cultivation of two or more crop species in close proximity [1,2,3]. Generally, intercropping system has been widely adopted by farmers for it shows a positive relationship between plant diversity and agricultural productivity worldwide [4,5,6]. Compared with monocropping systems, the advantages of intercropping in over-yielding have been explained by niche complementarity and interspecific facilitation [7,8,9]. In a cropping system, the interspecific facilitation might come from the above-ground and below-ground parts of the crops. Literatures indicated that planting arrangements e.g. plant density and strip width achieve a dominant position in controlling the strength of interspecific crop interactions and yields [10,11,12]. An earlier report indicated that cotton/peanut intercropping system could increase the crop productivity through regulating the photosystem and the maximum leaf area index of peanut [13, 14]. These studies documented the profound role of the above-ground organs in the interspecific facilitation. Alternatively, from the perspective of the below-ground part of the crops, the interspecific facilitation may occur via transfer of nutrients through co-cultivated crop species or stimulation of beneficial soil microbes as affected by root exudates [15,16,17,18]. Although intercropping has been proved to be an efficient land use and sustainable agricultural practice that is widely practiced worldwide, knowledge on this intercropping system is still lacking from the perspective of saline growth conditions [19, 20].
Salinity is one of the major abiotic stresses and has become an ever-increasing threat to agricultural production around the globe [21,22,23,24]. It is estimated that over 50% of the arable land on our planet will be salinized by the middle of this century [25, 26]. Crop species show great variability in their inherent saline tolerance. Plants have evolved sophisticated physiological mechanisms to cope with salinity [27,28,29]. Upon a salinity stress, the first and rapid phase is to accumulate osmolytes to maintain the turgor pressure [30, 31]. In the past decade, plant growth regulators have been extensively applied in researches to alleviate crop salinity stress including polyamines [32], epibrassinolide [33], jasmonate [34], melatonin [35], and silicon [36]; however, sustainable agricultural practices are still needed to achieve the goal of green ecological agriculture.
Being a leguminous crop, peanut (Arachis hypogaea L.) is a good source of protein and vegetable oil for humans [21, 37]. Peanut is relatively sensitive to saline stress [38,39,40]. Soil saline severely decreases seed germination, morphogenesis, and production of peanut [41,42,43]. In spite of this, peanut is often grown under poor soil conditions such as saline affected soil because nearly one-third of the global arable irrigated ploughland is already affected by salinity [44, 45]. As an important source of fiber, commercial cotton (Gossypium hirsutum L.) is moderately salt-tolerant [46, 47]. Recent years, peanut/cotton intercropping combined with rotation system has been increasingly adopted by farmers in North China Plain [13, 48] and elsewhere in the world [49]. The advantages of this cropping system could be to alleviate the constraints of continuous cropping and increase the productivity; however, whether this kind of intercropping system could be conducted under salinity conditions remains unknown. To address this concern, the current research was performed to elucidate the effects of peanut/cotton intercropping on crop productivity and economic returns under both normal and saline soil conditions. By using different root barriers, we were able to detect the interspecific facilitation between the below-ground parts of peanut strip and cotton strip. We therefore hypothesized that peanut/cotton intercropping could increase crop yield and economic returns through regulation of soil microbial communities and accumulation of nutrients in the crops. Our study may guide management decisions to enhance productivity in the era of soil salinization and develop sustainable agriculture.
Methods
Field experimental site
The multi-site field experiments were conducted at PingDu experimental station (120.12 °E, 36.55 °N), GaoTang experimental station (116.27 °E, 36.86 °N), and LiJin experimental station (118.27 °E, 37.50 °N), Shandong Province of China in 2018. All of the experimental sites are located in a warm semi-arid monsoon region with a continental climate during summer and autumn. No extreme weather conditions were observed during crop growth seasons in 2018. The temperature and rainfall were slightly different between these experimental sites from May to September in 2018. The soil was considered non-saline soil, secondary-saline soil, and coastal saline soil in PingDu, GaoTang, and LiJin, respectively which was classified according to the previous research [50]. The soil chemical properties of the top soil (0–20 cm and 20–40 cm) in each experimental site were listed in Table 1. Commercial cotton (var. Lumianyan 37) seeds obtained from Shandong Cotton Research Center and peanut (var. Huayu 25) seeds obtained from Shandong Peanut Research Institute were used in all of the experimental sites. Both peanut and cotton are major cultivated high-yield varieties in Shandong Province.
Experimental design
The managements were identical in all the 3 experimental sites. The fields were successively planted with peanuts, wheat, and maize for at least 10 consecutive years. Basal synthetic fertilizer (975 kg/hm2; N: P2O5: K2O = 1:1.5:1.5) was applied homogeneously into the soils before sowing. The peanut and cotton seeds with uniform sides were manually selected and sowed on 7 May, 23 April, and 3 May 2018 in PingDu, GaoTang, and LiJin, respectively.
The cropping system was modified as previously reported with minor modifications [13]. Peanut was sowed on a raised bed (Height 10 cm and width 80 cm). Two rows of peanut were arranged on a ridge (Row spacing 30 cm and plant distance 17 cm). Cotton was sowed in equal rows (Row spacing 60 cm and plant distance 25 cm). The spacing between cotton and peanut was 65 cm. There were 6 rows of peanut and 4 rows of cotton on each intercropping plot and the two crops occupied the same land area (270 cm in width) (Fig. 1). In order to eliminate the root interactions between peanut and cotton, two kinds of barriers were placed below-ground in the middle of the peanut strip and cotton strip. The permeable (100 μm nylon mesh) barrier was 15-m long and 1.5-m deep (No root contact whereas majority of root exudates and microbes can pass through) (NC) and the impermeable (solid plastic) barrier was 15-m long and 1.5-m deep (No below-ground contact) (SC). For no barrier intercropping treatment (IC), the ground between peanut strip and cotton strip has also been digged for 1.5-m deep and refilled as a negative control. The monocropping of peanut (MP) and cotton (MC) were identical with the intercropping system as shown in Fig. 1. Taken together, a total of five cropping systems were established, namely, monocropping of peanut (MP), monocropping of cotton (MC), intercropping of peanut/cotton without barriers (IC), intercropping of peanut/cotton with 100 μm nylon mesh barrier (NC), and intercropping of peanut/cotton with solid barrier (SC).
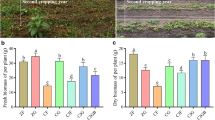
Schematic diagrams of experimental design used in different cropping systems and root barriers (a). MP: Monocropping of peanut, MC: Monocropping of cotton, IC: Intercropping of peanut/cotton without barriers, NC: Intercropping of peanut/cotton with 100 μm nylon mesh barrier, SC: Intercropping of peanut/cotton with solid barrier. Solid triangles represent soil sampling positions. Pictures showing MP (b), MC (c), both peanut and cotton in IC (d), peanut strips in IC (e), and cotton strips in IC (f)
The experiments were set up using a randomized complete block design with three biological replicates in each treatment and the area of each plot was 162 m2 (15 m × 10.8 m). The peanut was manually harvested on 9 September, 2 September, and 22 September 2018 in PingDu, GaoTang, and LiJin, respectively while the cotton was manually harvested on 29 October, 20 October, and 22 October 2018 in PingDu, GaoTang, and LiJin, respectively.
Measurements and data collection
Nutrient analyses from plant and soil samples
The plant and soil samples were taken simultaneously at peanut podding stage. Plant samples were firstly heated at 105 °C for 30 min to deactivate enzymes and dried at 75 °C to constant weight, and then the dry weights were immediately recorded. The samples were finely ground to powder and digested with H2SO4-H2O2 as separate plant parts. Nitrogen (N) content was assayed using the micro Kjeldahl analysis [51]. Phosphorus (P) content was determined by a flow analyzer according to the manufacturer’s instructions [52]. Potassium (K) content was measured using a flame photometer as described by K Chakraborty, D Bhaduri, HN Meena and K Kalariya [53].
Freshly collected soil samples of the top soil (0–40 cm) which were taken at 15 cm away from the plants in the peanut strip and cotton strip (as shown in Fig. 1) were sieved to 2 mm and the identical methodology was followed except the extraction to determine the content of soil N, P, and K.
Yield and yield components
Yield samples were taken at 1 day before harvest. After sun-drying for 15 days, the yield and yield components of peanut and cotton were measured, respectively. The sampling area for both sole peanut and sole cotton was 13.5 m2 (5 m in length and 2.7 m in width). For peanut, the pods yield, pod density, and 100-pod weight were measured for all plants in sampling area. For cotton, all plants were collected in sampling area to quantify the seed cotton yield, boll density, and boll weight.
Competition parameters
Land equivalent ratio (LER) was utilized to evaluate the land use advantage provided by intercropping [54]. LER was calculated as:
where LERp and LERc are partial LERs for peanut and cotton, respectively, Yp and Mp are the yields of peanut in intercropping and monocropping systems, respectively, and Yc and Mc are the yields of cotton in intercropping and monocropping systems, respectively. Ratios of 1.0 indicate the same land productivity for intercropping and monocropping systems, ratios greater than 1.0 indicate that intercropping is advantageous, whereas ratios smaller than 1.0 indicate a land use disadvantage for intercropping.
Benefit-cost measurement
Material inputs such as seeds, fertilizer, pesticide, irrigation system, and labor cost including fertilizer application, irrigation, insect and weed control, and harvesting were recorded in each experimental station. The input cost was calculated based on the local prices of the material and labor days, meanwhile, the output cost of the peanut pods and seed cotton was determined according to the average prices of the local market in 2018.
Soil sampling and determination of soil bacterial communities
Soil samples were taken from both 0–20 cm and 20–40 cm depth on 15 July (blossom-needling stage of peanut) and 25 August (podding stage of peanut) in PingDu. The sampling positions were 15 cm away from the plants in the peanut strip and cotton strip as marked in solid triangles (Fig. 1). Soil samples were collected from 3 random points within each replicate plot and mixed thoroughly, and each treatment composed of 3 replicates. In MP, MC, and IC, soil samples from the two strips were further mixed together to make the composite samples. In NC and SC, the peanut strip and cotton strip samples were collected separately as NC-P, NC-C, SC-P, and SC-C, respectively. The obtained soil samples were firstly frozen in liquid nitrogen and then immediately transported to Gene Denovo Biotechnology Co., Ltd., Guangzhou, China in drikold for DNA extraction and soil microbial determinations.
High-throughput sequencing analysis of the 16S rRNA gene was performed with Illumina Novaseq 6000 to determine soil bacterial diversities and communities as previously described [52]. Total genomic DNA was extracted from 0.5 g soil using the HiPure Soil DNA Kit (Magen, Guangzhou, China) following the manufacturer’s protocols [55]. The hypervariable regions of V3 and V4 of the 16S rRNA genes were amplified using the specific primers 341F (5′-CCT ACG GGN GGC WGC AG-3′) and 806R (5′-GGA CTA CHV GGG TAT CTA AT-3′) according to A Ali, M Imran Ghani, Y Li, H Ding, H Meng and Z Cheng [56]. The concentration and quality of the DNA samples were firstly determined before polymerase chain reaction (PCR) amplification was carried out. Amplicons extracted from 2% agarose gels were further purified with a AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA). The purified triplicate amplification products were pooled in equimolar amounts and quantified using NanoDrop (Thermo Scientific, USA) according to standard protocols [42]. Afterwards, the prepared libraries were sequenced on an Illumina HiSeq 2500 PE 250 platform. The raw sequences data generated in the current research were deposited into the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database under Submission ID of SUB10633613 and Accession of PRJNA779843.
Raw data containing adapters or low quality reads would affect the following assembly and analysis. To get high quality clean reads, raw reads were further filtered when they were containing more than 10% of unknown nucleotides or containing less than 80% of bases with quality (Q-value) > 20. Consequently, effective tags were used for the subsequent analysis. Then, paired end clean reads were merged as raw tags using FLSAH (V1.2.11) according to T Magoč and SL Salzberg [57] with a minimum overlap of 10 bp and mismatch error rates of 2%. Noisy sequences of raw tags were filtered by QIIME (V1.9.1) [58] pipeline under specific filtering conditions [59] to obtain the high quality clean tags. Then, clean tags were searched against the reference database (http://drive5.com/uchime/uchime_download.html) to perform Reference based chimera checking using UCHIME algorithm (http://www.drive5.com/usearch/manual/uchime_algo.html). All chimeric tags were removed and finally obtained effective tags were used for further analysis.
The effective tags were clustered into operational taxonomic units OTUs of ≥97% similarity using UPARSE [60] pipeline. The tag sequence with highest abundance was selected as reprehensive sequence within each cluster. The representative sequences were classified into organisms by a naive Bayesian model using RDP classifier (V2.2) [61] based on UNITE Database (https://unite.ut.ee/). The abundance statistics of each taxonomy and phylogenetic tree was construction in a Perl script and visualized using SVG [62]. Biomarker features in each group were screened by Metastats and LEfSe software. Additionally, Chao1, Simpson, and all other alpha diversity index were calculation in QIIME. OTU rarefaction curve and Rank abundance curves was plotted in QIIME. Alpha index comparing among groups was computed by a Tukey’s HSD test and a Kruskal-Wallis H test in R. The principal coordinates analysis (PCoA) in Hellinger distance was calculated and plotted in R. The metabolic functional features of the bacterial communities were predicted using Tax4Fun (version 1.0) [63] with Kyoto Encyclopedia of Genes and Genomes (KEGG) database [64].
Statistical analysis
The physiological data were firstly tested for homogeneity of variance with boxplot and subjected to the one-way analysis of variance (ANOVA). The difference was considered to be statistically significant when P < 0.05 using Tukey’s test.
Results
Yield and yield components of peanut and cotton
The highest peanut pods yield was observed in treatment MP (5192 kg/ha in PingDu, 4967 kg/ha in GaoTang, and 5146 kg/ha in LiJin), while the lowest peanut pods yield was obtained under treatment SC (3816 kg/ha in PingDu, 3775 kg/ha in GaoTang, and 3726 kg/ha in LiJin) (Table 2). In addition, the maximum seed cotton yield was found under IC treatment (7160 kg/ha in PingDu, 6846 kg/ha in GaoTang, and 6778 kg/ha in LiJin), whereas the lowest seed cotton yield (4420 kg/ha in PingDu, 4124 kg/ha in GaoTang, and 4276 kg/ha in LiJin) was recorded in MC treatment in 3 positions (Table 3). Intercropping with root barriers significantly reduced peanut pods yield by 9.34% (NC) and 14.54% (SC) in PingDu, 11.10% (NC) and 15.55% (SC) in GaoTang, and 8.43% (NC) and 13.27% (SC) in LiJin, compared with no root barrier treatment (IC) (Table 2). In seed cotton yield, the reduction was 17.26% (NC) and 20.67% (SC) in PingDu, 12.05% (NC) and 16.87% (SC) in GaoTang, and 10.70% (NC) and 14.46% (SC) in LiJin, compared with IC (Table 3).
We then measured the peanut pod density and cotton boll density where intercropping (IC) significantly decreased peanut pod density by 14.61 and 17.79% in PingDu and LiJin, respectively, while increased peanut pod density by 18.81% in GaoTang compared with MP. The boll density of cotton in IC was significantly increased by 91.66, 43.67, and 48.06% in PingDu, GaoTang, and LiJin, respectively compared with MC. Root barriers significantly reduced the pod density of peanut and the reduction was 6.63% (NC) and 11.10% (SC) in PingDu, 18.76% (NC) and 18.85% (SC) in GaoTang, and 6.06% (NC) and 8.91% (SC) in LiJin compared with IC. The changes of boll density of cotton by root barriers were − 15.43% (NC) and − 33.08% (SC) in PingDu, 1.12% (NC) and − 6.12% (SC) in GaoTang, and 5.10% (NC) and − 9.05% (SC) in LiJin, compared with IC. Intercropping did not change 100-pod weight of peanut except for that in GaoTang where 100-pod weight was significantly decreased by 24.05% compared with MP. Intercropping significantly reduced boll weight of cotton by 15.49% in PingDu, while increased boll weight of cotton by 15.66 and 7.03% in GaoTang and LiJin, respectively, compared with IC. Compared with IC, the 100-pod weight was reduced (2.92% of NC and 4.01% of SC) in PingDu and (2.63% of NC and 4.89% of SC) in LiJin, while induced (9.05% of NC and 4.02% of SC) in GaoTang. In PingDu, SC significantly increased boll weight of cotton by 18.54% while NC did not change this parameter, compared with IC. In GaoTang and LiJin, root barriers significantly reduced boll weight of cotton by (13.03% of NC and 11.51% of SC) and (15.06% of NC and 5.93% of SC), respectively, compared with IC (Tables 2 and 3).
Competition parameters
In general, the value of LER for all of the treatments were found higher than one suggesting yield advantage of peanut/cotton intercropping system. Among all the treatments, IC had the maximum LER: 1.24, 1.28, and 1.21 in PingDu, GaoTang, and LiJin, respectively, while lowest LER was recorded by 1.01, 10.7, and 1.04 in PingDu, GaoTang, and LiJin, respectively, under treatment SC. Relative to NC, treatment SC significantly reduced LER by 4.72, 5.31, and 4.59% in PingDu, GaoTang, and LiJin, respectively. Similar changes of LERp and LERc were further observed where root barriers significantly reduced LERp and LERc in all of the experimental positions except that LERp did not show significant differences between SC and NC in 3 positions (Table 4).
Economic analysis
Input value, output value, and net return were significantly affected by the cropping systems. Overall, the cost of inputs for IC was between MP and MC in all experimental positions. IC produced a significantly higher output value (24.21% of MP and 4.24% of MC) in PingDu, (22.49% of MP and 5.38% of MC) in GaoTang, and (22.92% of MP and 5.38% of MC) in LiJin. Averaged for 3 positions, IC produced a significantly higher output value by 23.76 and 4.98% in MP and MC, respectively. Compared with MP, the net returns in IC were significantly increased by 20.08, 10.90, and 15.22% in PingDu, GaoTang, and LiJin, respectively. The net returns in IC were significantly increased by 54.95, 67.31, and 65.78% in PingDu, GaoTang, and LiJin, respectively, compared with MC. Averaged for 3 positions, the net returns in IC were significantly increased by 15.47 and 62.18% compared with MP and MC, respectively (Table 5).
Nutrient accumulation in soil and plants of peanut and cotton
The N, P, and K accumulation in the soil of peanut and cotton strips were significantly greater in IC than MP or MC in all 3 experimental positions with only one exception where IC decreased the peanut strip K by 9.53% in GaoTang (Table 6). However, for NC treatment, the N, P, and K accumulation in the soil of peanut and cotton strips were significantly lower than IC in all 3 experimental positions with 3 exceptions where N accumulation of peanut strip was increased by 2.77% in PingDu, P accumulation of cotton strip was increased by 6.71% in GaoTang, and K accumulation of peanut strip was increased by 25.50% in GaoTang. In addition, the significantly lower accumulation of N, P, and K in the soil of peanut and cotton strips of SC treatment was recorded compared with IC, suggesting that solid root barriers might inhibited the accumulation of the soil nutrient in both peanut and cotton strips (Table 6).
The accumulation of N in the stem and leaf of peanut did not show significant difference between MP and IC, while IC significantly changed cotton N in the stem and leaf of PingDu and GaoTang compared with MC (Table 7). For the accumulation of N in peanut pod, IC showed significantly higher N accumulation than MP in 3 positions and higher accumulation of N in cotton bud was also found in IC compared with MC in PingDu and GaoTang. Root barrier treatment NC significantly increased the accumulation of N in the stem and leaf of both peanut and cotton compared with IC in GaoTang and LiJin while the changes were not unique in peanut pod and cotton bud when compared between NC and IC. SC significantly reduced the accumulation of peanut pod N in 3 positions and cotton bud N in PingDu and LiJin, compared with IC.
IC significantly increased the accumulation of peanut P in the stem and leaf of GaoTang and LiJin compared with MP and increased the cotton P in the stem and leaf of PingDu and LiJin compared with MC. Additionally, IC showed significantly higher P accumulation in peanut pod and cotton bud of 3 positions, compared with MP and MC. Root barrier treatment NC and SC showed lower P accumulation in the stem, leaf, peanut pod, and cotton bud of the seedlings in 3 positions than IC with a few exceptions, suggesting that root barriers inhibited the accumulation of P in both peanut and cotton plants.
For the K accumulation, intercropping increased the K in the stem, leaf, peanut pod, and cotton bud within a certain range compared with MP and MC. In peanut, NC showed significant higher K in stem and leaf while lower K in the pod, compared with IC. SC showed significant lower K in all of the organs of peanut compared with IC. In cotton, similar results were observed where root barrier treatment NC and SC significantly reduced the K in all of the organs in 3 positions except the SC of stem and leaf in GaoTang and NC of all of the organs in LiJin, compared with IC. These results indicated that root barriers reduced the accumulation of plant K in the organs of both cotton and peanut (Table 7).
Soil bacterial communities
Most of the root barrier intercropping treatments significantly affected diversity indices and affected community structure of soil bacterial community in both peanut strip and cotton strip (Table S1). At blossom-needling stage (0–20 cm), MP showed significantly higher number of OTUs, ACE, and Chao index values than other treatments, while at blossom-needling stage (20–40 cm), NC-C showed significantly higher number of OTUs, Shannon index, ACE, and Chao index values compared with other treatments. Again, NC-C processed higher number of OTUs, ACE, and Chao index values than other treatments, whereas SC-C processed the highest Shannon and Simpson index values at podding stage (0–20 cm). In 20–40 cm top soil of podding stage, however, only NC-C showed significant higher Shannon index values compared with other treatments (Table S1).
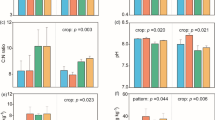
At the phylum level, IC obviously raised the relative abundance of Acidobacteria and Verrucomicrobia, while declined the relative abundance of Gemmatimonadetes, Actinobacteria, and Chloroflexi compared with MP and MC at blossom-needling stage (0–20 cm). NC-P and NC-C did not show obvious changes of the bacterial communities, whereas SC-P and SC-C visibly increased the relative abundance of Proteobacteria and Actinobacteria, and greatly declined the relative abundance of Planctomycetes, and Verrucomicrobia compared with IC (Fig. 2a & Fig. S1). At podding stage (0–20 cm), IC greatly induced the relative abundance of Proteobacteria, Gemmatimonadetes, Actinobacteria, and Bacteroidetes whereas reduced the relative abundance of Acidobacteria, Planctomycetes, and Verrucomicrobia compared with MP and MC. NC-P and NC-C did not show significant changes compared with IC. By contrast, the relative abundance of Proteobacteria, Gemmatimonadetes, Actinobacteria, and Bacteroidetes were obviously declined while the relative abundance of Acidobacteria and Planctomycetes were clearly elevated in SC-P and SC-C compared with IC (Fig. 2d). At blossom-needling stage (0–20 cm), the relative abundance of Proteobacteria, Gemmatimonadetes, and Bacteroidetes were induced while the relative abundance of Nitrospirae and Chloroflexi were reduced in IC compared with MP and MC. Compared NC-P and SC-P with IC, the relative abundance of Planctomycetes, Actinobacteria, and Nitrospirae were increased while Proteobacteria, Gemmatimonadetes, and Bacteroidetes were decreased. Compared NC-C and SC-C with IC, the relative abundance of Proteobacteria and Planctomycetes were induced while Actinobacteria, and Gemmatimonadetes were reduced (Fig. 3a & Fig. S1). At blossom-needling stage (20–40 cm), the relative abundance of Proteobacteria, Actinobacteria, Gemmatimonadetes, Nitrospirae, and Bacteroidetes were higher while Acidobacteria, Planctomycetes, Verrucomicrobia, and Latescibacteria were lower in IC than MP and MC. Additionally, the relative abundance of Planctomycetes and Verrucomicrobia showed higher whereas Actinobacteria, and Gemmatimonadetes showed lower in NC-P and NC-C than IC. SC-P and SC-C processed higher relative abundance of Acidobacteria, Planctomycetes, Verrucomicrobia, and Latescibacteria while lower Proteobacteria, Actinobacteria, Gemmatimonadetes, and Nitrospirae compared with IC (Fig. 3d).
The relative abundance of soil bacterial proportions (0–20 cm) at the phylum level at blossom-needling stage (a) and podding stage (d). The principal coordination analysis (PCoA) in Hellinger distance at blossom-needling stage (b) and podding stage (e) showing changings in soil bacterial community structure. Heatmap based most abundant Kyoto Encyclopedia of Genes and Genomes (KEGG) ortholog (KO) groups showing the relative abundance of top 20 KEGG metabolic pathways across different treatments at blossom-needling stage (c) and podding stage (f) in monocropped peanut (MP), monocropped cotton (MC), peanut intercropped with cotton without barriers (IC), peanut intercropped with cotton with 100 μm nylon mesh barrier: peanut strip (NP) and cotton strip (NC), peanut intercropped with cotton with solid barrier: peanut strip (SP) and cotton strip (SC). The samples were taken from 0 to 20 cm of the top soil
The relative abundance of soil bacterial proportions (20–40 cm) at the phylum level at blossom-needling stage (a) and podding stage (d). The principal coordination analysis (PCoA) in Hellinger distance at blossom-needling stage (b) and podding stage (e) showing changings in soil bacterial community structure. Heatmap based most abundant Kyoto Encyclopedia of Genes and Genomes (KEGG) ortholog (KO) groups showing the relative abundance of top 20 KEGG metabolic pathways across different treatments at blossom-needling stage (c) and podding stage (f) in monocropped peanut (MP), monocropped cotton (MC), peanut intercropped with cotton without barriers (IC), peanut intercropped with cotton with 100 μm nylon mesh barrier: peanut strip (NP) and cotton strip (NC), peanut intercropped with cotton with solid barrier: peanut strip (SP) and cotton strip (SC). The samples were taken from 20 to 40 cm of the top soil
The PCoA analysis revealed evident change in soil community structure of different cropping systems and the bacterial community of all of the treatments grouped well. At blossom-needling stage, no obvious difference was detected among treatments (Figs. 2b & 3b). However, IC, NC-P, and NC-C exerted a distinct difference as compared to the communities of other treatments in both 0–20 and 20–40 cm of the top soil at podding stage (Figs. 2e & 3e).
We then used the novel Tax4Fun tool to further explain the predictive functional profiling of microbial communities. At blossom-needling stage, the metabolic functions related to fatty acid biosynthesis, lipoic acid metabolism, peptidoglycan biosynthesis, biosynthesis of ansamycins, D-Alanine metabolism, cell cycle-Caulobacter, sulfur rely system, ribosome, protein export, etc. were significantly higher in the MP group in both 0–20 and 20–40 cm of the top soil especially compared with SC-P and SC-C groups (Figs. 2c & 3c). Strikingly, these putative KEGG pathways were significantly depleted in MP group, but visibly enriched in IC, NC-P, and NC-C groups at podding stage in both 0–20 and 20–40 cm of the top soil (Figs. 2f & 3f). These metabolic results indicate differential regulation of the soil bacterial community functional profiles by the different cropping systems and crop growth stages.
Discussion
The main objective of this paper was to present a peanut/cotton intercropping system that significantly raised the crop productivity and economic returns under non-saline, secondary-saline, and coastal saline soil conditions. The present intercropping system also showed significant advantages over traditional monoculture systems. Based on multi-site field experiments with permeable (100 μm) and impermeable (solid) root barriers, we demonstrated that this phenomenon could be mainly attributed to the altering of the soil bacterial abundance distribution which can enhance the nutrient accumulation including N, P, and K in the soils, peanut pods, and cotton buds. Similarly, Chi et al. examined the advantages of this intercropping system from the point of leaf photosynthesis, dry matter partitioning, and the interactions between border and inner rows [13]; however, our study provides evidence from the perspective of the below-ground interspecific interactions and clear indicate the involvement of soil microbial community in peanut and cotton interaction. These findings highlight the importance of this promising intercropping system and provide an important reference for farmers to lower inputs and obtain more economic returns under various soil conditions.
In the peanut/cotton intercropping system, cotton is a tall crop with a high ground cover from June to September which is in a dominant position; however, peanut is a short crop during the whole co-growth phase except seedling stage which is in a disadvantage position [14]. This could be ascribed to the strong competition of cotton such as light, nutrients, and water [13, 65]. In conformity with earlier reports, we observed that intercropping clearly decreased the peanut pods yield while profoundly increased seed cotton yield compared with their monocultures [13, 49]. Strikingly, the reduction of peanut pods yield were even more aggravated under root barrier treatments especially in solid barrier treatment (SC). Similar results were obtained in seed cotton yield where NC and SC partially eliminated the beneficial effects of intercropping on cotton productivity (Tables 2 & 3). These results have been further demonstrated by land use efficiency of intercropping as quantified by LER where NC and SC dramatically decreased the LER compared with IC (Table 4). Therefore, we speculate that the above observations could be mainly ascribed to the below-ground interactions between the two crop species.
It is well accepted that nutrient translocation from soil to below-ground and from below-ground to above-ground components of crops could further influence the concentrations of these nutrients in peanut pods and cotton buds [66,67,68]. Consistent with previous reports, we observed that intercropping accelerated the accumulations of N, P, and K at various degrees in both peanut and cotton strips of soil compared with their monocultures (Table 6). It can be presumed that this intercropping system could promote the adequate availability and improve the acquisition of these nutrients in soil [4]. Indeed, crops are known to benefit from intercropping with legumes such as peanut for the symbiotic relationship between rhizobia and legume roots could provide more available N for their neighboring crop species [69,70,71]. It is worth noting that the enhanced accumulation of N was only observed in stems, leaves, and buds of cotton of IC treatment; however, although intercropping stimulated the uptake of N in the above-ground parts of peanut, it failed to increase this nutrient’s concentration in peanut pods (Table 7). These discrepancies might result from the reduced allocation of N from the vegetative tissues to the reproductive organs which warrant further investigation. By contrast, the accumulation of P and K in vegetative tissues of peanut and cotton showed similar trends as those in the soil where IC dramatically increased these nutrient contents compared with their monocultures (Table 7). Particularly, the induction was more pronounced in cotton than peanut plants while the reduction by SC was more pronounced than NC. On the one hand, this phenomenon might be due to the competition effects of the above-ground parts where higher light interception and leaf transpiration at the top of cotton than peanut could enhance the absorption capacity of nutrients [11, 72]. On the other hand, the interactions of the below-ground part of the two species prompted us to further elucidate the underlying mechanisms.
It has been well documented that high biodiversity increases ecosystem functions [15, 56, 73]. Unexpectedly, limited changes of soil bacterial community diversity and richness in the peanut/cotton intercropping system were detected compared with their monocultures (Table S1). By contrast, the bacterial community composition of soil changed dramatically in various treatments (Figs. 2 & 3). The discrepancies between the previous researches and ours might resulted from the two major reasons. First, rhizosphere soil has been utilized to detect the soil bacterial community diversity whereas we collected the soil between root zoon and the connection part of the two crops for our sampling method could take into account both root exudates and soil. Second, our work was the first time to reveal the below-ground interactions between legume (peanut) and fiber (cotton) crop species, thus, this interspecific interactions might be different from the previous reports. Therefore, it is expected that the profound role of this intercropping system could be attributed to the changes of the bacterial community composition rather than diversity. To further unravel the role of interspecific interactions, PCoA analysis was carried out and IC, NC-P, and NC-C exerted a distinct difference as compared to MP, MC, SC-P, and SC-C in both 0–20 and 20–40 cm of the top soil at podding stage (Figs. 2e & 3e). Consistent with the data of plant nutrient accumulation (Table 7), these results could be interpreted by the application of root barriers and clearly showed that permeable (100 μm) nylon mesh root barrier performed better than impermeable (solid) root barrier, thus, further confirmed the importance of the below-ground interactions in this intercropping system. The PCoA analysis further indicated that the changes of bacterial community composition at podding stage might play a more vital role in overyielding and enhancing economic returns of this intercropping system. The heatmap based KEGG analysis found that some crucial metabolic pathways such as lipoic acid metabolism, fatty acid biosynthesis, and protein export were significantly enriched in IC, NC-P, and NC-C compared with other treatments (Figs. 2f & 3f). Lipoic acid has been reported to recover metabolic distortions through modulating ion homeostasis such as K+ [74] which was consistent with our findings that intercropping significantly accelerated the allocation of K from soil to plants, and then from vegetative tissues to the reproductive organs especially in cotton. Similarly, the facilitation of fatty acid biosynthesis by intercropping would also provide basis for the growth and survival of soil bacteria [75, 76] which in turn promoted the seed cotton yield; however, further investigation are still needed to explore the detailed mechanisms concerning the reduction of peanut pods yield in this intercropping system.
Conclusions
In summary, the present study leads us to conclude that peanut/cotton intercropping system could induce the seed cotton yield while reduce the peanut pods yield compared with their monocultures. Moreover, the economic returns have been induced under both normal and salinity soil conditions. Using root barrier treatments, we found that the bacterial community composition between monoculture and intercropping were dramatically changed at peanut podding stage and blossom-needling stage. The changes of soil microbial communities could further attribute to the accumulation of soil nutrients such as N, P, and K in both crop species, promotion of the translocation of these nutrients from vegetative tissues to reproductive organs, and finally contribution to the enhanced economic returns. Our findings fill a gap of knowledge on the below-ground interactions in peanut/cotton intercropping system and propose a previously unidentified mechanism combining crop nutrient allocation with soil bacterial community composition. Additionally, this study provides guidance for farmers to select the best cropping system under various soil conditions. Future work could be extended by evaluating the soil bacterial community structures in salinity soil and carrying out multiple year experiments to gain a more general understanding of the microbial-driven below-ground processes in this intercropping system.
Availability of data and materials
The datasets generated during and/or analyses during the current study are available in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database under Submission ID of SUB10633613 and Accession of PRJNA779843.
Abbreviations
- ANOVA:
-
Analysis of variance
- DNA:
-
Deoxyribonucleic acid
- IC:
-
Intercropping
- K:
-
Potassium
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- LER:
-
Land equivalent ratio
- LERc:
-
Land equivalent ratio of cotton
- LERp:
-
Land equivalent ratio of peanut
- MC:
-
Monoculture of cotton
- Mc:
-
The yields of cotton in monocropping systems
- Mp:
-
The yields of peanut in monocropping systems
- MP:
-
Monoculture of peanut
- N:
-
Nitrogen
- NCBI:
-
National Center for Biotechnology Information
- NC-C:
-
Permeable root barrier treatments cotton side
- NC-P:
-
Permeable root barrier treatments peanut side
- SC-C:
-
Impermeable root barrier treatments cotton side
- SC-P:
-
Impermeable root barrier treatments peanut side
- SRA:
-
Sequence Read Archive
- P:
-
Phosphorus
- PCR:
-
Polymerase chain reaction
- PCoA:
-
The principal coordinates analysis
- Yc:
-
The yields of cotton in intercropping systems
- Yp:
-
The yields of peanut in intercropping systems
References
Brooker RW, Bennett AE, Cong W-F, Daniell TJ, George TS, Hallett PD, et al. Improving intercropping: a synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015;206(1):107–17.
Gou F, van Ittersum MK, Simon E, Leffelaar PA, van der Putten PEL, Zhang L, et al. Intercropping wheat and maize increases total radiation interception and wheat RUE but lowers maize RUE. Eur J Agron. 2017;84:125–39.
Li R, Chen N, Zhang X, Zeng L, Wang X, Tang S, et al. Quantitative analysis of agricultural drought propagation process in the Yangtze River basin by using cross wavelet analysis and spatial autocorrelation. Agric For Meteorol. 2020;280:107809.
Xue Y, Xia H, Christie P, Zhang Z, Li L, Tang C. Crop acquisition of phosphorus, iron and zinc from soil in cereal/legume intercropping systems: a critical review. Ann Bot. 2016;117(3):363–77.
Curtright AJ, Tiemann LK. Intercropping increases soil extracellular enzyme activity: a meta-analysis. Agric Ecosyst Environ. 2021;319:107489.
Dowling A, Sadras VO, Roberts P, Doolette A, Zhou Y, Denton MD. Legume-oilseed intercropping in mechanised broadacre agriculture-a review. Field Crops Res. 2021;260:107980.
Hauggaard-Nielsen H, Jensen ES. Facilitative root interactions in intercrops. Plant Soil. 2005;274(1):237–50.
Cong W, Hoffland E, Li L, Six J, Sun J, Bao X, et al. Intercropping enhances soil carbon and nitrogen. Glob Chang Biol. 2015;21(4):1715–26.
Duchene O, Vian J-F, Celette F. Intercropping with legume for agroecological cropping systems: complementarity and facilitation processes and the importance of soil microorganisms. A review. Agric Ecosyst Environ. 2017;240:148–61.
Wang R, Sun Z, Zhang L, Yang N, Feng L, Bai W, et al. Border-row proportion determines strength of interspecific interactions and crop yields in maize/peanut strip intercropping. Field Crops Res. 2020;253:107819.
Raza MA, Feng LY, van der Werf W, Cai GR, Khalid MHB, Iqbal N, et al. Narrow-wide-row planting pattern increases the radiation use efficiency and seed yield of intercrop species in relay-intercropping system. Food Energy Secur. 2019;8(3):e170.
Zhang D, Sun Z, Feng L, Bai W, Yang N, Zhang Z, et al. Maize plant density affects yield, growth and source-sink relationship of crops in maize/peanut intercropping. Field Crops Res. 2020;257:107926.
Chi B, Zhang Y, Zhang D, Zhang X, Dai J, Dong H. Wide-strip intercropping of cotton and peanut combined with strip rotation increases crop productivity and economic returns. Field Crops Res. 2019;243:107617.
Singh RJ, Ahlawat IPS. Growth behaviour of transgenic cotton with peanut intercropping system using modified fertilization technique. Proc Natl Acad Sci India Sec B Biol Sci. 2014;84(1):19–30.
Li B, Li Y-Y, Wu H-M, Zhang F-F, Li C-J, Li X-X, et al. Root exudates drive interspecific facilitation by enhancing nodulation and N2 fixation. Proc Natl Acad USA. 2016;113(23):6496–501.
Ehrmann J, Ritz K. Plant: soil interactions in temperate multi-cropping production systems. Plant Soil. 2014;376(1):1–29.
Li S, Wu F. Diversity and co-occurrence patterns of soil bacterial and fungal communities in seven intercropping systems. Front Microbiol. 2018;9:1521.
Callaway RM. Positive interactions among plants. Bot Rev. 1995;61(4):306–49.
Singh RJ, Ahlawat IPS, Kumar K. Productivity and profitability of the transgenic cotton–wheat production system through peanut intercropping and FYM addition. Exp Agric. 2013;49(3):321–35.
Liang J, Shi W. Cotton/halophytes intercropping decreases salt accumulation and improves soil physicochemical properties and crop productivity in saline-alkali soils under mulched drip irrigation: a three-year field experiment. Field Crops Res. 2021;262:108027.
Meena HN, Meena M, Yadav RS. Comparative performance of seed types on yield potential of peanut (Arachis hypogaea L.) under saline irrigation. Field Crops Res. 2016;196:305–10.
Qin W, Yan H, Zou B, Guo R, Ci D, Tang Z, et al. Arbuscular mycorrhizal fungi alleviate salinity stress in peanut: evidence from pot-grown and field experiments. Food Energy Secur. 2021;10(4):e314.
Deinlein U, Stephan AB, Horie T, Luo W, Xu G, Schroeder JI. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014;19(6):371–9.
Zhang X, Chen N, Li J, Chen Z, Niyogi D. Multi-sensor integrated framework and index for agricultural drought monitoring. Remote Sens Environ. 2017;188:141–63.
Khorsandi F, Anagholi A. Reproductive compensation of cotton after salt stress relief at different growth stages. J Agron Crop Sci. 2009;195(4):278–83.
Hasegawa PM. Sodium (Na+) homeostasis and salt tolerance of plants. Environ Exp Bot. 2013;92(8):19–31.
Tanji KK. Salinity in the soil environment. In: Läuchli A, Lüttge U, editors. Salinity: Environment - Plants - Molecules. Dordrecht: Springer Netherlands; 2002. p. 21–51.
Naveed M, Ditta A, Ahmad M, Mustafa A, Ahmad Z, Conde-Cid M, et al. Processed animal manure improves morpho-physiological and biochemical characteristics of Brassica napus L. under nickel and salinity stress. Environ Sci Pollut Res. 2021;28(33):45629–45.
Shahzad H, Ullah S, Iqbal M, Bilal HM, Shah GM, Ahmad S, et al. Salinity types and level-based effects on the growth, physiology and nutrient contents of maize (Zea mays). Ital J Agron. 2019;14(4):199–207.
Hanin M, Ebel C, Ngom M, Laplaze L, Masmoudi K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front Plant Sci. 2016;7:1787.
Puniran-Hartley N, Hartley J, Shabala L, Shabala S. Salinity-induced accumulation of organic osmolytes in barley and wheat leaves correlates with increased oxidative stress tolerance: in planta evidence for cross-tolerance. Plant Physiol Biochem. 2014;83:32–9.
Zarza X, Atanasov KE, Marco F, Arbona V, Carrasco P, Kopka J, et al. Polyamine oxidase 5 loss-of-function mutations in Arabidopsis thaliana trigger metabolic and transcriptional reprogramming and promote salt stress tolerance. Plant Cell Environ. 2016;40(4):527–42.
Wani AS, Ahmad A, Hayat S, Tahir I. Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant Physiol Biochem. 2019;135:385–94.
Zhao Y, Dong W, Zhang N, Ai X, Wang M, Huang Z, et al. A wheat Allene oxide Cyclase gene enhances salinitytolerance via jasmonate signaling. Plant Physiol. 2013;164:1068–76.
Li X, Yu B, Cui Y, Yin Y. Melatonin application confers enhanced salt tolerance by regulating Na+ and cl− accumulation in rice. Plant Growth Regul. 2017;83(3):441–54.
Zhu Y, Guo J, Feng R, Jia J, Han W, Gong H. The regulatory role of silicon on carbohydrate metabolism in Cucumis sativus L. under salt stress. Plant Soil. 2016;406(1):231–49.
Tian S, Guo R, Zou X, Zhang X, Yu X, Zhan Y, et al. Priming with the green leaf volatile (Z)-3-Hexeny-1-yl acetate enhances salinity stress tolerance in peanut (Arachis hypogaea L.) seedlings. Front Plant Sci. 2019;10:785.
Greenway H, Munns R. Mechanisms of salt tolerance in nonhalophytes. Annu Rev Plant Physiol. 1980;31(1):149–90.
Guo R, Yan H, Li X, Zou X, Zhang X, Yu X, et al. Green leaf volatile (Z)-3-hexeny-1-yl acetate reduces salt stress in peanut by affecting photosynthesis and cellular redox homeostasis. Physiol Plant. 2020;170(1):75–92.
El-Akhal MR, Rincón A, Coba de la Peña T, Lucas MM, El Mourabit N, Barrijal S, et al. Effects of salt stress and rhizobial inoculation on growth and nitrogen fixation of three peanut cultivars. Plant Biol. 2012;15(2):415–21.
Wang Z, Yan L, Wan L, Huai D, Kang Y, Shi L, et al. Genome-wide systematic characterization of bZIP transcription factors and their expression profiles during seed development and in response to salt stress in peanut. BMC Genomics. 2019;20(1):51.
Ci D, Tang Z, Ding H, Cui L, Zhang G, Li S, et al. The synergy effect of arbuscular mycorrhizal fungi symbiosis and exogenous calcium on bacterial community composition and growth performance of peanut (Arachis hypogaea L.) in saline alkali soil. J Microbiol. 2021;59(1):51–63.
Zhang X, Chen N, Sheng H, Ip C, Yang L, Chen Y, et al. Urban drought challenge to 2030 sustainable development goals. Sci Total Environ. 2019;693:133536.
Lazof DB, Bernstein N. The NaCl induced inhibition of shoot growth: The case for disturbed nutrition with special consideration of calcium. In: Callow JA, editor. Advances in Botanical Research, vol. 29: Academic; 1998. p. 113–89. https://www.sciencedirect.com/science/article/pii/S0065229608603110.
Feghhenabi F, Hadi H, Khodaverdiloo H, van Genuchten MT. Seed priming alleviated salinity stress during germination and emergence of wheat (Triticum aestivum L.). Agric Water Manag. 2020;231:106022.
Dong Y, Hu G, Yu J, Thu SW, Grover CE, Zhu S, et al. Salt-tolerance diversity in diploid and polyploid cotton (Gossypium) species. Plant J. 2020;101(5):1135–51.
Rodriguez-Uribe L, Higbie SM, Stewart JM, Wilkins T, Lindemann W, Sengupta-Gopalan C, et al. Identification of salt responsive genes using comparative microarray analysis in upland cotton (Gossypium hirsutum L.). Plant Sci. 2011;180(3):461–9.
Li X-F, Wang Z-G, Bao X-G, Sun J-H, Yang S-C, Wang P, et al. Long-term increased grain yield and soil fertility from intercropping. Nat Sustainability. 2021;4(11):943–50.
Singh RJ, Ahlawat IPS, Sharma NK. Resource use efficiency of transgenic cotton and peanut intercropping system using modified fertilization technique. Int J Plant Prod. 2015;9(4):523–40.
Dong H, Xin C, Li W. Soil salinity grading of cotton field in coastal saline area (in Chinese with English abstract). Shandong Agric Sci. 2012;44(3):36–9.
Barbano DM, Lynch JM, Fleming JR. Direct and indirect determination of true protein content of milk by Kjeldahl analysis: collaborative study. J Assoc Official Anal Chem. 1991;74(2):281–8.
Khashi u Rahman M, Wang X, Gao D, Zhou X, Wu F. Root exudates increase phosphorus availability in the tomato/potato onion intercropping system. Plant Soil. 2021;464(1):45–62.
Chakraborty K, Bhaduri D, Meena HN, Kalariya K. External potassium (K+) application improves salinity tolerance by promoting Na+-exclusion, K+-accumulation and osmotic adjustment in contrasting peanut cultivars. Plant Physiol Biochem. 2016;103:143–53.
Rao MR, Willey RW. Evaluation of yield stability in intercropping: studies on sorghum/pigeonpea. Exp Agric. 1980;16(2):105–16.
Cheng H, Wu B, Wei M, Wang S, Rong X, Du D, et al. Changes in community structure and metabolic function of soil bacteria depending on the type restoration processing in the degraded alpine grassland ecosystems in northern Tibet. Sci Total Environ. 2021;755:142619.
Ali A, Imran Ghani M, Li Y, Ding H, Meng H, Cheng Z. Hiseq base molecular characterization of soil microbial community, diversity structure, and predictive functional profiling in continuous cucumber planted soil affected by diverse cropping systems in an intensive greenhouse region of northern China. Int J Mol Sci. 2019;20(11):2619.
Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–63.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6.
Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods. 2013;10(1):57–9.
Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10(10):996–8.
Wang Q, Garrity George M, Tiedje James M, Cole James R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–7.
Kõljalg U, Larsson K-H, Abarenkov K, Nilsson RH, Alexander IJ, Eberhardt U, et al. UNITE: a database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol. 2005;166(3):1063–8.
Aßhauer KP, Wemheuer B, Daniel R, Meinicke P. Tax4Fun: predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics. 2015;31(17):2882–4.
Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31(9):814–21.
Raza MA, Feng LY, van der Werf W, Iqbal N, Khan I, Khan A, et al. Optimum strip width increases dry matter, nutrient accumulation, and seed yield of intercrops under the relay intercropping system. Food Energy Secur. 2020;9(2):e199.
Wang X, Deng X, Pu T, Song C, Yong T, Yang F, et al. Contribution of interspecific interactions and phosphorus application to increasing soil phosphorus availability in relay intercropping systems. Field Crops Res. 2017;204:12–22.
Malézieux E, Crozat Y, Dupraz C, Laurans M, Makowski D, Ozier-Lafontaine H, et al. Mixing plant species in cropping systems: concepts, tools and models. A review. Agronomy Sustainable Dev. 2009;29(1):43–62.
Wu K, Fullen MA, An T, Fan Z, Zhou F, Xue G, et al. Above- and below-ground interspecific interaction in intercropped maize and potato: a field study using the ‘target’ technique. Field Crops Res. 2012;139:63–70.
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol. 2006;57:233–66.
Mulder L, Hogg B, Bersoult A, Cullimore JV. Integration of signalling pathways in the establishment of the legume-rhizobia symbiosis. Physiol Plant. 2005;123(2):207–18.
Guo F, Wang M, Si T, Wang Y, Zhao H, Zhang X, et al. Maize-peanut intercropping led to an optimization of soil from the perspective of soil microorganism. Arch Agron Soil Sci. 2021;67(14):1986–99.
Yang F, Liao D, Wu X, Gao R, Fan Y, Raza MA, et al. Effect of aboveground and belowground interactions on the intercrop yields in maize-soybean relay intercropping systems. Field Crops Res. 2017;203:16–23.
Yu R-P, Lambers H, Callaway RM, Wright AJ, Li L. Belowground facilitation and trait matching: two or three to tango? Trends Plant Sci. 2021;26(12):1227–35.
Gorcek Z, Erdal S. Lipoic acid mitigates oxidative stress and recovers metabolic distortions in salt-stressed wheat seedlings by modulating ion homeostasis, the osmo-regulator level and antioxidant system. J Sci Food Agric. 2015;95(14):2811–7.
Paulucci NS, Medeot DB, Dardanelli MS, de Lema MG. Growth temperature and salinity impact fatty acid composition and degree of unsaturation in peanut-nodulating rhizobia. Lipids. 2011;46(5):435–41.
Paulucci NS, Medeot DB, Woelke M, Dardanelli MS, de Lema MG. Monounsaturated fatty acid aerobic synthesis in Bradyrhizobium TAL1000 peanut-nodulating is affected by temperature. J Appl Microbiol. 2013;114(5):1457–67.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (31901461), the Shandong Provincial Modern Agriculture Industrial Technology (SDAIT-04-05), and the Opening Foundation of Shandong Provincial Crop Varieties Improvement (2020LZGC001).
Author information
Authors and Affiliations
Contributions
TS, WX, and KZ conceived and designed the experiments. XW, XXZ, XJZ, XY, and YW performed the experiments. TS, WX, and KZ contributed to the writing of the manuscript and performed the final editing of the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were carried out in compliance with local and national regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xie, W., Zhang, K., Wang, X. et al. Peanut and cotton intercropping increases productivity and economic returns through regulating plant nutrient accumulation and soil microbial communities. BMC Plant Biol 22, 121 (2022). https://doi.org/10.1186/s12870-022-03506-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-022-03506-y