Abstract
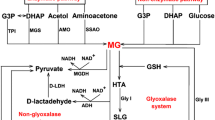
Methylglyoxal (MG) is a potent cytotoxin being produced primarily as result of non-enzymatic reactions in the living systems. The toxicity of MG is believed to be due to its ability to carry out glycation of proteins, nucleic acids and phospholipids. MG can readily modify proteins and nucleic acids, forming advanced glycation end-products which results in cellular dysfunction. In order to limit MG levels, several detoxification enzymes exist in the living systems which can metabolize MG. Of these, glyoxalase pathway is considered to be the primary route of MG metabolism. Here, we have discussed the effects of MG on the functioning of different subcellular compartments and the detoxification machineries existing in these organelles to counter excess MG. We describe how MG exerts its toxic effects at the cellular level and how the cell defends itself from MG. In this context, we have highlighted the important role of glyoxalase pathway in plants. Further, we have also discussed how glycolytic products and enzymes control MG levels and how MG regulates the activity of these glycolytic products as a part of substrate and end-product regulatory mechanisms. Overall, we present a comprehensive overview of MG toxicity and detoxification in the cell and its regulation.


Similar content being viewed by others
References
Alvarez Viveros, M. F., Inostroza-Blancheteau, C., Timmermann, T., Gonzalez, M., & Arce-Johnson, P. (2013). Overexpression of GlyI and GlyII genes in transgenic tomato (Solanum lycopersicum Mill.) plants confers salt tolerance by decreasing oxidative stress. Molecular Biology Reports, 40, 3281–3290.
Amicarelli, F., Colafarina, S., Cesare, P., Aimola, P., DiIlio, C., Miranda, M., et al. (2001). Morphofunctional mitochondrial response to methylglyoxal toxicity in Bufo bufo embryos. The International Journal of Biochemistry and Cell Biology, 33, 1129–1139.
Asada, K. & Takahashi, M. (1987). Production and scavenging of active oxygen in photosynthesis. In D. J. Kyle, C. B. Osmond & C. J. Arntzen (Ed.), Photoinhibition (pp. 228–287). Amsterdam: Elsevier.
Bagga, S., Das, R., & Sopory, S. K. (1987). Inhibition of cell proliferation and glyoxalase-I activity by calmodulin inhibitors and lithium in Brassica oleracea. Journal of Plant Physiology, 129, 149–153.
Banu, M. N., Hoque, M. A., Watanabe-Sugimoto, M., Islam, M. M., Uraji, M., Matsuoka, K., et al. (2010). Proline and glycinebetaine ameliorated NaCl stress via scavenging of hydrogen peroxide and methylglyoxal but not superoxide or nitric oxide in tobacco cultured cells. Bioscience, Biotechnology, and Biochemistry, 74, 2043–2049.
Baskaran, S., & Balasubramanian, K. A. (1990). Effect of methylglyoxal on protein thiol and amino groups in isolated rat enterocytes and colonocytes and activity of various brush border enzymes. Indian Journal of Biochemistry and Biophysics, 27, 13–17.
Baumann, E. (1885). Über Verbindungen der Aldehyde, Ketone und Ketosauren mit den Merkapten. Berichte der Deutschen Chemischen Gesellschaft, 18, 883–892.
Beisswenger, P. J., Howell, S. K., Smith, K., & Szwergold, B. S. (2003). Glyceraldehyde-3-phosphate dehydrogenase activity as an independent modifier of methylglyoxal levels in diabetes. Biochimica et Biophysica Acta-Molecular Basis of Disease, 1637, 98–106.
Bernhauer, K., & Görlich, B. (1929). Über Zuckeroxydationen und -zersetzungen. Biochemische Zeitschrift, 212, 452–465.
Bilova, T., Lukasheva, E., Brauch, D., Greifenhagen, U., Paudel, G., Tarakhovskaya, E., et al. (2016). A snapshot of the plant glycated proteome: Structural, functional, and mechanistic aspects. Journal of Biological Chemistry, 291, 7621–7636.
Biswas, S., Ray, M., Misra, S., Dutta, D. P., & Ray, S. (1997). Selective inhibition of mitochondrial respiration and glycolysis in human leukaemic leucocytes by methylglyoxal. Biochemical Journal, 323, 343–348.
Bito, A., Haider, M., Hadler, I., & Breitenbach, M. (1997). Identification and phenotypic analysis of two glyoxalase II encoding genes from Saccharomyces cerevisiae, GLO2 and GLO4, and intracellular localization of the corresponding proteins. Journal of Biological Chemistry, 272, 21509–21519.
Blackinton, J., Lakshminarasimhan, M., Thomas, K. J., Ahmad, R., Greggio, E., Raza, A. S., et al. (2009). Formation of a stabilized cysteine sulfinic acid is critical for the mitochondrial function of the parkinsonism protein DJ-1. Journal of Biological Chemistry, 284, 6476–6485.
Canet-Avilés, R. M., Wilson, M. A., Miller, D. W., Ahmad, R., McLendon, C., Bandyopadhyay, S., et al. (2004). The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proceedings of the National Academy of Sciences USA, 101, 9103–9108.
Chan, C. M., Huang, D. Y., Huang, Y. P., Hsu, S. H., Kang, L. Y., Shen, C. M., et al. (2016). Methylglyoxal induces cell death through endoplasmic reticulum stress-associated ROS production and mitochondrial dysfunction. Journal of Cellular and Molecular Medicine, 20, 1749–1760.
Chang, K. C., Paek, K. S., Kim, H. J., Lee, Y. S., Yabe-Nishimura, C., & Seo, H. G. (2002). Substrate-induced up-regulation of aldose reductase by methylglyoxal, a reactive oxoaldehyde elevated in diabetes. Molecular Pharmacology, 61, 1184–1191.
Chen, Z. Y., Brown, R. L., Damann, K. E., & Cleveland, T. E. (2004). Identification of a maize kernel stress-related protein and its effect on aflatoxin accumulation. Phytopathology, 94, 938–945.
Cooper, R. A. (1984). Metabolism of methylglyoxal in microorganisms. Annual Reviews of Microbiology, 38, 49–68.
Cordell, P. A., Futers, T. S., Grant, P. J., & Pease, R. J. (2004). The Human hydroxyacylglutathione hydrolase (HAGH) gene encodes both cytosolic and mitochondrial forms of glyoxalase II. Journal of Biological Chemistry, 279, 28653–28661.
Crook, E. M., & Law, K. (1952). Glyoxalase: The role of the components. Biochemical Journal, 52, 492–499.
Crowder, M. W., Maiti, M. K., Banovic, L., & Makaroff, C. A. (1997). Glyoxalase II from A. thaliana requires Zn(II) for catalytic activity. FEBS Letters, 418, 351–354.
Deswal, R., Chakaravarty, T. N., & Sopory, S. K. (1993). The glyoxalase system in higher-plants—Regulation in growth and differentiation. Biochemical Society Transactions, 21, 527–530.
Espartero, J., Sanchez-Aguayo, I., & Pardo, J. M. (1995). Molecular characterization of glyoxalase-I from a higher plant: Upregulation by stress. Plant Molecular Biology, 29, 1223–1233.
Fabiano, C. C., Tezotto, T., Favarin, J. L., Polacco, J. C., & Mazzafera, P. (2014). Essentiality of nickel in plants: A role in plant stresses. Frontiers in Plant Science, 6, 754.
Ghosh, A., & Islam, T. (2016). Genome-wide analysis and expression profiling of glyoxalase gene families in soybean (Glycine max) indicate their development and abiotic stress specific response. BMC Plant Biology, 16, 87.
Ghosh, A., Kushwaha, H. R., Hasan, M. R., Pareek, A., Sopory, S. K., & Singla-Pareek, S. L. (2016). Presence of unique glyoxalase III proteins in plants indicates the existence of shorter route for methylglyoxal detoxification. Scientific Reports, 6, 18358.
Ghosh, A., Pareek, A., Sopory, S. K., & Singla-Pareek, S. L. (2014). A glutathione responsive rice glyoxalase II, OsGLYII-2, functions in salinity adaptation by maintaining better photosynthesis efficiency and anti-oxidant pool. The Plant Journal, 80, 93–105.
Gomes, R. A., Vicente, Miranda. H., Sousa, Silva. M., Graça, G., Coelho, A. V., do Nascimento Ferreira, A. E., et al. (2008). Protein glycation and methylglyoxal metabolism in yeast: Finding peptide needles in protein haystacks. FEMS Yeast Research, 8, 174–181.
Gómez Ojeda, A., Corrales Escobosa, A. R., Wrobel, K., Yanez Barrientos, E., & Wrobel, K. (2013). Effect of Cd(II) and Se(IV) exposure on cellular distribution of both elements and concentration levels of glyoxal and methylglyoxal in Lepidium sativum. Metallomics, 5, 1254–1261.
Grüning, N. M., Du, D., Keller, M. A., Luisi, B. F., & Ralser, M. (2014). Inhibition of triosephosphate isomerase by phosphoenolpyruvate in the feedback-regulation of glycolysis. Open Biology, 4, 130–232.
Hopper, D. J., & Cooper, R. A. (1971). The regulation of Escherichia coli methylglyoxal synthase: A new control site in glycolysis? FEBS Letters, 13, 213–216.
Hopper, D. J., & Cooper, R. A. (1972). The purification and properties of Escherichia coli methylglyoxal synthase. Biochemical Journal, 128, 321–329.
Hoque, M. A., Uraji, M., Banu, M. N. A., Mori, I. C., Nakamura, Y., & Murata, Y. (2010). The effect of methylglyoxal on glutathione S-transferase from Nicotiana tabacum. Bioscience, Biotechnology, and Biochemistry, 74, 2124–2126.
Hoque, M. A., Uraji, M., Torii, A., Banu, M. N., Mori, I. C., Nakamura, Y., et al. (2012a). Methylglyoxal inhibition of cytosolic ascorbate peroxidase from Nicotiana tabacum. Journal of Biochemical and Molecular Toxicology, 26, 315–321.
Hoque, T. S., Uraji, M., Tuya, A., Nakamura, Y., & Murata, Y. (2012b). Methylglyoxal inhibits seed germination and root elongation and up-regulates transcription of stress-responsive genes in ABA-dependent pathway in Arabidopsis. Plant Biology, 14, 854–858.
Horton, P., Park, K. J., Obayashi, T., Fujita, N., Harada, H., Adams-Collier, C. J., et al. (2007). WoLF PSORT: Protein localization predictor. Nucleic Acids Research, 35(Web Server issue), W585–W587.
Hossain, M. A., Hossain, M. Z., & Fujita, M. (2009). Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Australian Journal of Crop Science, 3, 53–64.
Huang, S. M., Chuang, H. C., Wu, C. H., & Yen, G. C. (2008). Cytoprotective effects of phenolic acids on methylglyoxal-induced apoptosis in Neuro-2A cells. Molecular Nutrition and Food Research, 52, 940–949.
Jain, D., Khandal, H., Khurana, J. P., & Chattopadhyay, D. (2016). A pathogenesis related-10 protein CaARP functions as aldo/keto reductase to scavenge cytotoxic aldehydes. Plant Molecular Biology, 90, 171–187.
Kalapos, M. P. (1999). Methylglyoxal in living organisms: Chemistry, biochemistry, toxicology and biological implications. Toxicology Letters, 110, 145–175.
Kalapos, M. P. (2008a). Methylglyoxal and glucose metabolism: A historical perspective and future avenues for research. Drug Metabolism and Drug Interactions, 23, 69–91.
Kalapos, M. P. (2008b). The tandem of free radicals and methylglyoxal. Chemico-Biological Interactions, 171, 251–271.
Kaur, C., Kushwaha, H. R., Pareek, A., Sopory, S. K., & Singla-Pareek, S. L. (2015a). Analysis of global gene expression profiles of rice in response to methylglyoxal indicates its possible role as a stress signal molecule. Frontiers in Plant Science, 6, 682.
Kaur, C., Sharma, S., Singla-Pareek, S. L., & Sopory, S. K. (2015b). Methylglyoxal, Triose Phosphate Isomerase, and Glyoxalase pathway: Implications in abiotic stress and signaling in plants. In Pandey, G. K., (Ed.) Elucidation of abiotic stress signaling in plants (pp. 347–366). New York: Springer.
Kaur, C., Singla-Pareek, S. L., & Sopory, S. K. (2014). Glyoxalase and methylglyoxal as biomarkers for plant stress tolerance. Critical Reviews in Plant Sciences, 33, 429–456.
Kaur, C., Tripathi, A. K., Nutan, K. K., Sharma, S., Ghosh, A., Tripathi, J. K., Pareek, A., Singla-Pareek, S. L., & Sopory, S. K. (2016). A nucleus-localized rice glyoxalase I enzyme, OsGLYI-8 functions in the detoxification of methylglyoxal in the nucleus. The Plant Journal. doi: 10.1111/tpj.13407.
Kaur, C., Vishnoi, A., Ariyadasa, T. U., Bhattacharya, A., Singla-Pareek, S. L., & Sopory, S. K. (2013). Episodes of horizontal gene-transfer and gene-fusion led to co-existence of different metal-ion specific glyoxalase I. Scientific Reports, 3, 3076.
Kwak, M. K., Lee, M. H., Park, S. J., Shin, S. M., Liu, R., & Kang, S. O. (2016). Polyamines regulate cell growth and cellular methylglyoxal in high-glucose medium independently of intracellular glutathione. FEBS Letters, 590, 739–749.
Kwon, K., Choi, D., Hyun, J. K., Jung, H. S., Baek, K., & Park, C. (2013). Novel glyoxalases from Arabidopsis thaliana. FEBS Journal, 280, 3328–3339.
Leoncini, G. (1979). The role of alpha-oxoaldehydes in biological systems. The Italian Journal of Biochemistry, 28, 285–294.
Leoncini, G., Maresca, M., & Bonsignore, A. (1980). The effect of methylglyoxal on the glycolytic enzymes. FEBS Letters, 117, 17–18.
Leoncini, G., Maresca, M., & Buzzi, E. (1989). Inhibition of the glycolytic pathway by methylglyoxal in human platelets. Cell Biochemistry and Function, 7, 65–70.
Lin, J., Nazarenus, T. J., Frey, J. L., Liang, X., Wilson, M. A., & Stone, J. M. (2011). A plant DJ–1 homolog is essential for Arabidopsis thaliana chloroplast development. PLoS ONE, 6, e23731.
Liu, J., Desai, K., Wang, R., & Wu, L. (2013). Up-regulation of aldolase A and methylglyoxal production in adipocytes. British Journal of Pharmacology, 168, 1639–1646.
Liu, J., Mak, T. C.-P., Banigesh, A., Desai, K., Wang, R., & Wu, L. (2012). Aldolase B knockdown prevents high glucose-induced methylglyoxal overproduction and cellular dysfunction in endothelial cells. PLoS ONE, 7, e41495.
Liu, J., Wang, R., Desai, K., & Wu, L. (2011). Upregulation of aldolase B and overproduction of methylglyoxal in vascular tissues from rats with metabolic syndrome. Cardiovascular Research, 92, 494–503.
Maiti, M. K., Krishnasamy, S., Owen, H. A., & Makaroff, C. A. (1997). Molecular characterization of glyoxalase II from Arabidopsis thaliana. Plant Molecular Biology, 35, 471–481.
Mano, J. I. (2012). Reactive carbonyl species: their production from lipid peroxides, action in environmental stress, and the detoxification mechanism. Plant Physiology and Biochemistry, 59, 90–97.
Marasinghe, G. P. K., Sander, I. M., Bennett, B., Periyannan, G., Yang, K.-W., Makaroff, C. A., et al. (2005). Structural studies on a mitochondrial glyoxalase II. Journal of Biological Chemistry, 280, 40668–40675.
Martins, A. M., Cordeiro, C. A., & Ponces Freire, A. M. (2001). In situ analysis of methylglyoxal metabolism in Saccharomyces cerevisiae. FEBS Letters, 499, 41–44.
Maurino, V. G., Welchen, E., García, L., Schmitz, J., Fuchs, P., Wagner, S., et al. (2016). d-Lactate dehydrogenase links methylglyoxal degradation and electron transport through cytochrome c. Plant Physiology, 172, 901–912.
Meyerhof, O., & Lohmann, K. (1934). Über die enzymatische Gleichgewicthsreaktion zwischen Hexosediphosphosaure und Dioxyacetonphosphosaure. Biochemische Zeitschrift, 271, 89–110.
Monder, C. (1967). α-Keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate. Journal of Biological Chemistry, 242, 4603–4609.
Morcos, M., Du, X., Pfisterer, F., Hutter, H., Sayed, A. A., Thornalley, P., et al. (2008). Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell, 7, 260–269.
Murata-Kamiya, N., & Kamiya, H. (2001). Methylglyoxal, an endogenous aldehyde, crosslinks DNA polymerase and the substrate DNA. Nucleic Acids Research, 29, 3433–3438.
Mustafiz, A., Ghosh, A., Tripathi, A. K., Kaur, C., Ganguly, A. K., Bhavesh, N. S., et al. (2014). A unique Ni2+-dependent and methylglyoxal-inducible rice glyoxalase I possesses a single active site and functions in abiotic stress response. The Plant Journal, 78, 951–963.
Mustafiz, A., Singh, A. K., Pareek, A., Sopory, S. K., & Singla-Pareek, S. L. (2011). Genome-wide analysis of rice and Arabidopsis identifies two glyoxalase genes that are highly expressed in abiotic stresses. Functional and Integrative Genomics, 11, 293–305.
Nahar, K., Hasanuzzaman, M., Alam, M. M., & Fujita, M. (2015). Glutathione-induced drought stress tolerance in mung bean: coordinated roles of the antioxidant defence and methylglyoxal detoxification systems. AoB Plants, 7, plv069.
Nakadate, Y., Uchida, K., Shikata, K., Yoshimura, S., Azuma, M., Hirata, T., et al. (2009). The formation of argpyrimidine, a methylglyoxal-arginine adduct, in the nucleus of neural cells. Biochemical and Biophysical Research Communications, 378, 209–212.
Noy, T., Vergnolle, O., Hartman, T. E., Rhee, K. Y., Jacobs, W. R., Berney, M., et al. (2016). Central role of pyruvate kinase in carbon co-catabolism of Mycobacterium tuberculosis. Journal of Biological Chemistry, 291, 7060–7069.
Pallotta, M. L. (2012). Mitochondrial involvement to methylglyoxal detoxification: d-Lactate/malate antiporter in Saccharomyces cerevisiae. Antonie van Leeuwenhoek, 102, 163–175.
Pallotta, M. L., Valenti, D., Iacovino, M., & Passarella, S. (2004). Two separate pathways for d-lactate oxidation by Saccharomyces cerevisiae mitochondria which differ in energy production and carrier involvement. Biochimica et Biophysica Acta, 1608, 104–113.
Paulus, C., Kollner, B., & Jacobsen, H. J. (1993). Physiological and biochemical-characterization of glyoxalase-I, a general marker for cell-proliferation, from a soybean cell-suspension. Planta, 189, 561–566.
Pietkiewicz, J., Gamian, A., Staniszewska, M., & Danielewicz, R. (2009). Inhibition of human muscle-specific enolase by methylglyoxal and irreversible formation of advanced glycation end products. Journal of Enzyme Inhibition and Medicinal Chemistry, 24, 356–364.
Qiu, Q. S., Huber, J. L., Booker, F. L., Jain, V., Leakey, A. D. B., Fiscus, E. L., et al. (2008). Increasing protein carbonylation in leaves of Arabidopsis and soybean in response to elevated [CO2]. Photosynthesis Research, 97, 155–166.
Quan, S., Switzenberg, R., Reumann, S., & Hu, J. (2010). In vivo subcellular targeting analysis validates a novel peroxisome targeting signal type 2 and the peroxisomal localization of two proteins with putative functions in defense in Arabidopsis. Plant Signaling Behaviour, 5, 151–153.
Rabbani, N., & Thornalley, P. J. (2008). Dicarbonyls linked to damage in the powerhouse: Glycation of mitochondrial proteins and oxidative stress. Biochemical Society Transactions, 36, 1045–1050.
Rabbani, N., & Thornalley, P. J. (2011). Glyoxalase in diabetes, obesity and related disorders. Seminars in Cell and Developmental Biology, 22, 309–317.
Rabbani, N., & Thornalley, P. J. (2012). Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids, 42, 1133–1142.
Racker, E. (1951). The mechanism of action of glyoxalase. Journal of Biological Chemistry, 190, 685–696.
Ramaswamy, O., Guha-Mukherjee, S., & Sopory, S. K. (1983). Presence of glyoxalase I in pea. International Journal of Biochemistry, 7, 307–318.
Ramaswamy, O., Guha-Mukherjee, S., & Sopory, S. K. (1984). Correlation of glyoxalase I activity with cell proliferation in Datura callus culture. Plant Cell Reports, 3, 121–124.
Ray, S., & Ray, M. (1981). Isolation of methylglyoxal synthase from goat liver. Journal of Biological Chemistry, 256, 6230–6233.
Ray, A., Ray, S., Mukhopadhyay, S., & Ray, M. (2013). Methylglyoxal with glycine or succinate enhances differentiation and shoot morphogenesis in Nicotiana tabacum callus. Biologia Plantarum, 57, 219–223.
Richard, J. P. (1984). Acid–base catalysis of the elimination and isomerization-reactions of triose phosphates. Journal of the American Chemical Society, 106, 4926–4936.
Richard, J. P. (1991). Kinetic parameters for the elimination reaction catalyzed by triosephosphate isomerase and an estimation of the reaction’s physiological significance. Biochemistry, 30, 4581–4585.
Richard, J. P. (1993). Mechanism for the formation of methylglyoxal from triosephosphates. Biochemical Society Transactions, 21, 549–553.
Roy, A., Hashmi, S., Li, Z., Dement, A. D., Cho, K. H., & Kim, J. H. (2016). The glucose metabolite methylglyoxal inhibits expression of the glucose transporter genes by inactivating the cell surface glucose sensors Rgt2 and Snf3 in yeast. Molecular Biology of the Cell, 27, 862–871.
Saito, R., Shimakawa, G., Nishi, A., Iwamoto, T., Sakamoto, K., Yamamoto, H., et al. (2013). Functional analysis of the AKR4C subfamily of Arabidopsis thaliana: model structures, substrate specificity, acrolein toxicity, and responses to light and [CO2]. Bioscience, Biotechnology, and Biochemistry, 77, 2038–2045.
Saito, R., Yamamoto, H., Makino, A., Sugimoto, T., & Miyake, C. (2011). Methylglyoxal functions as hill oxidant and stimulates the photoreduction of O2 at photosystem I: A symptom of plant diabetes. Plant, Cell and Environment, 34, 1454–1464.
Sankaranarayanan, S., Jamshed, M., & Samuel, M. A. (2015). Degradation of glyoxalase I in Brassica napus stigma leads to self-incompatibility response. Nature Plants, 1, 15185.
Sartori, A., Mano, C. M., Mantovani, M. C., Dyszy, F. H., Massari, J., Tokikawa, R., et al. (2013). Ferricytochrome c directly oxidizes aminoacetone to methylglyoxal: A catabolite accumulated in carbonyl stress. PLoS ONE, 8, e57790.
Scheckhuber, C. Q. (2015). Penicillium chrysogenum as a model system for studying cellular effects of methylglyoxal. BMC Microbiology, 15, 1.
Sengupta, D., Naik, D., & Reddy, A. R. (2015). Plant aldo-keto reductases (AKRs) as multi-tasking soldiers involved in diverse plant metabolic processes and stress defense: A structure–function update. Journal of Plant Physiology, 179, 40–55.
Sethi, U., Basu, A., & Guha-mukherjee, S. (1988). Control of cell-proliferation and differentiation by regulating polyamine biosynthesis in cultures of Brassica and its correlation with glyoxalase-I activity. Plant Science, 56, 167–175.
Sharma, S., Mustafiz, A., Singla-Pareek, S. L., Shankar-Srivastava, P., & Sopory, S. K. (2012). Characterization of stress and methylglyoxal inducible triose phosphate isomerase (OscTPI) from rice. Plant Signaling Behavior, 7, 1337–1345.
Shimakawa, G., Suzuki, M., Yamamoto, E., Saito, R., Iwamoto, T., Nishi, A., et al. (2014). Why don’t plants have diabetes? Systems for scavenging reactive carbonyls in photosynthetic organisms. Biochemical Society Transactions, 42, 543–547.
Singla-Pareek, S. L., Reddy, M. K., & Sopory, S. K. (2003). Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance. Proceedings of the National Academy of Sciences USA, 100, 14672–14677.
Singla-Pareek, S. L., Yadav, S. K., Pareek, A., Reddy, M. K., & Sopory, S. K. (2006). Transgenic tobacco overexpressing glyoxalase pathway enzymes grow and set viable seeds in zinc-spiked soils. Plant Physiology, 140, 613–623.
Singla-Pareek, S. L., Yadav, S. K., Pareek, A., Reddy, M. K., & Sopory, S. K. (2008). Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transgenic Research, 17, 171–180.
Subedi, K. P., Choi, D., Kim, I., Min, B., & Park, C. (2011). Hsp31 of Escherichia coli K-12 is glyoxalase III. Molecular Microbiology, 81, 926–936.
Suh, K. S., Choi, E. M., Rhee, S. Y., & Kim, Y. S. (2014). Methylglyoxal induces oxidative stress and mitochondrial dysfunction in osteoblastic MC3T3-E1 cells. Free Radical Research, 48, 206–217.
Szent-Gyorgyi, A., Egyud, L. G., & McLaughlin, J. A. (1967). Keto-aldehydes and cell division. Science, 155, 539–541.
Takagi, D., Inoue, H., Odawara, M., Shimakawa, G., & Miyake, C. (2014). The Calvin cycle inevitably produces sugar-derived reactive carbonyl methylglyoxal during photosynthesis: A potential cause of plant diabetes. Plant and Cell Physiology, 55, 333–340.
Thornalley, P. J. (1990). The glyoxalase system: New developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochemical Journal, 269, 1–11.
Thornalley, P. J. (1998). Glutathione-dependent detoxification of α-oxoaldehyde by the glyoxalase system: Involvement in disease mechanisms and anti-proliferative activity of glyoxalase I inhibitors. Chemico-Biological Interactions, 111–112, 137–151.
Thornalley, P. J. (2008). Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems—Role in ageing and disease. Drug Metabolism and Drug Interactions, 23, 125–150.
Thornalley, P. J., Waris, S., Fleming, T., Santarius, T., Larkin, S. J., Winklhofer-Roob, B. M., et al. (2010). Imidazopurinones are markers of physiological genomic damage linked to DNA instability and glyoxalase 1-associated tumour multidrug resistance. Nucleic Acids Research, 38, 5432–5442.
Turk, Z., Nemet, I., Varga-Defteardarovic, L., & Car, N. (2006). Elevated level of methylglyoxal during diabetic ketoacidosis and its recovery phase. Diabetes and Metabolism, 32, 176–180.
Untereiner, A. A., Dhar, A., Liu, J., & Wu, L. (2011). Increased renal methylglyoxal formation with down-regulation of PGC-1α-FBPase pathway in cystathionine γ-lyase knockout mice. PLoS ONE, 6, e29592.
Upadhyaya, C. P., Venkatesh, J., Gururani, M. A., Asnin, L., Sharma, K., Ajappala, H., et al. (2011). Transgenic potato overproducing l-ascorbic acid resisted an increase in methylglyoxal under salinity stress via maintaining higher reduced glutathione level and glyoxalase enzyme activity. Biotechnology Letters, 33, 2297–2307.
Veena, Reddy, V. S., & Sopory, S. K. (1999). Glyoxalase I from Brassica juncea: Molecular cloning, regulation and its over-expression confer tolerance in transgenic tobacco under stress. The Plant Journal, 17, 385–395.
Von Pechmann, H. (1887). Über die Spaltung der Nitrosoketone. Berichte der Deutschen Chemischen Gesellschaft, 20, 3213–3214.
Wani, S. H., & Gosal, S. S. (2011). Introduction of OsglyII gene into Oryza sativa for increasing salinity tolerance. Biologia Plantarum, 55, 536–540.
Xu, X. M., Lin, H., Maple, J., Bjorkblom, B., Alves, G., Larsen, J. P., et al. (2010). The Arabidopsis DJ-1a protein confers stress protection through cytosolic SOD activation. Journal of Cell Science, 123, 1644–1651.
Yadav, S. K., Singla-Pareek, S. L., Ray, M., Reddy, M. K., & Sopory, S. K. (2005). Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochemical and Biophysical Research Communications, 337, 61–67.
Yamauchi, Y., Hasegawa, A., Mizutani, M., & Sugimoto, Y. (2012). Chloroplastic NADPH-dependent alkenal/one oxidoreductase contributes to the detoxification of reactive carbonyls produced under oxidative stress. FEBS Letters, 586, 1208–1213.
Yamauchi, Y., Hasegawa, A., Taninaka, A., Mizutani, M., & Sugimoto, Y. (2011). NADPH-dependent reductases involved in the detoxification of reactive carbonyls in plants. Journal of Biological Chemistry, 286, 6999–7009.
Yim, H. S., Kang, S. O., Hah, Y. C., Chock, P. B., & Yim, M. B. (1995). Free radicals generated during the glycation reaction of amino acids by methylglyoxal. A model study of protein-cross-linked free radicals. Journal Biological Chemistry, 270, 28228–28233.
Zang, T. M., Hollman, D. A., Crawford, P. A., Crowder, M. W., & Makaroff, C. A. (2001). Arabidopsis glyoxalase II contains a zinc/iron binuclear metal center that is essential for substrate binding and catalysis. Journal of Biological Chemistry, 276, 4788–4795.
Acknowledgements
CK acknowledges Department of Science and Technology, Government of India for the Grant received as DST-INSPIRE Award. SS thanks University Grants Commission for Dr. D.S. Kothari Postdoctoral Fellowship. IUABC grant from IUSSTF is gratefully acknowledged by SLS-P.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, C., Sharma, S., Singla-Pareek, S.L. et al. Methylglyoxal detoxification in plants: Role of glyoxalase pathway. Ind J Plant Physiol. 21, 377–390 (2016). https://doi.org/10.1007/s40502-016-0260-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-016-0260-1