Abstract
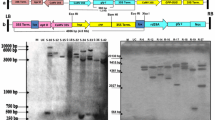
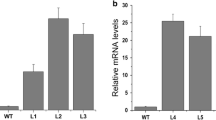
The glyoxalase system plays an important role in various physiological processes in plants, including salt stress tolerance. We report the effects of overexpressing glyoxalase I and glyoxalase II genes in transgenic tomato (Solanum lycopersicum Mill.) cv. Ailsa Craig. Stable expression of both transgenes was detected in the transformed tomato plants under salt stress. The transgenic lines overexpressing GlyI and GlyII under a high NaCl concentration (800 mM) showed reduced lipid peroxidation and the production of H2O2 in leaf tissues. A greater decrease in the chlorophyll a+b content in wild-type (WT) compared with transgenic lines was also observed. These results suggest that the over expression of two genes, GlyI and GlyII, may enhance salt stress tolerance by decreasing oxidative stress in transformed tomato plants. This work will help our understanding of the putative role of the glyoxalase system in the tolerance to abiotic stress in tomato plants.





Similar content being viewed by others
References
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–527
Li D, Zhang Y, Hu X, Shen X, Ma L, Su Z, Wang T, Dong J (2011) Transcriptional profiling of Medicago truncatula under salt stress identified a novel CBF transcription factor MtCBF4 that plays an important role in abiotic stress responses. BMC Plant Biol 11:109
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Miyagawa Y, Tamoi M, Shigeoka S (2001) Overexpression of a cyanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase in tobacco enhances photosynthesis and growth. Nat Biotechnol 19:965–969
Singh GS, Tuteja N (2010) Polyamines and abiotic stress tolerance in plants. Plant Signal Behav 5:26–33
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Seki M, Kamei A, Yamaguchi-Shinozaki K, Shinozaki K (2003) Molecular responses to drought, salinity and frost, common and different paths for plant protection. Curr Opin Biotechnol 14:194–199
Sasaki-Sekimoto Y, Taki N, Obayashi T, Aono M, Matsumoto F, Sakurai N, Suzuki H, Hiraj MY, Noji M, Saito K, Masuda T, Takamiya KI, Shibata D, Ohta H (2005) Coordinated activation of metabolic pathways for antioxidants and defense compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J 44:563–668
Ahuja I, de Vos RCH, Bones AM, Hall RD (2010) Plant molecular stress responses face climate change. Trends Plant Sci 15:1360–1385
Arora A, Sairam RK, Srivastava GC (2002) Oxidative stress and antioxidative system in plants. Curr Sci 82:1227–1238
Gould SK (2003) Free radicals, oxidative stress and antioxidants. In: Thomas B, Murphy DJ, Murray BG (eds) Encyclopedia of applied plant sciences. Elsevier, Amsterdam, pp 9–16
Qiao WH, Zhao XY, Li W, Luo Y, Zhang XS (2007) Overexpression of AeNHX1, a root-specific vacuolar Na+/H+ antiporter from Agropyron elongatum, confers salt tolerance to Arabidopsis and Festuca plants. Plant Cell Rep 26:1663–1672
Singla-Pareek SL, Reddy MK, Sopory SK (2003) Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance. Proc Natl Acad Sci USA 100:14672–14677
Thornalley PJ (1990) The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J 269:1–11
Reddy VSV, Sopory SK (1999) Glyoxalase I from Brassica juncea: molecular cloning, regulation and its over-expression confer tolerance in transgenic tobacco under stress. Plant J 17:385–395
Hossain MA, Hossain MZ, Fujita M (2009) Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust J Crop Sci 3:53–64
Foolad MR, Lin GY (1997) Genetic potential for salt tolerance during germination in Lycopersicon species. HortScience 32:296–300
Kaveh H, Nemati H, Farsi M, Jartoodeh SV (2011) How salinity affect germination and emergence of tomato lines. J Biol Environ Sci 5:159–163
Sun W, Xu X, Zhu H, Liu A, Liu L, Li J, Hua X (2010) Comparative transcriptomic profiling of a salt-tolerant wild tomato species and a salt-sensitive tomato cultivar. Plant Cell Physiol 51:997–1006
Pozueta-Romero JG, Houlne L, Cañas R, Schantz J, Chamarro J (2001) Enhanced regeneration of tomato and pepper seedling explants for Agrobacterium-mediated transformation. Plant Cell Tissue Org 67:173–180
Kyte L, Kleyn J (1996) Plants from test tubes: an introduction to micropropagation, 3rd edn. Timber Press, Inc., Portland
Giorno F, Wolters-Arts M, Grillo S, Scharf KD, Vriezen WH, Mariani C (2010) Developmental and heat stress-regulated expression of HsfA2 and small heat shock proteins in tomato anthers. J Exp Bot 61:453–462
Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK (2008) Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transgenic Res 17:171–180
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Barnes JD, Balaguer L, Manrique E, Elvira S, Davison AW (1992) A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ Exp Bot 32:85–100
Monje OA, Bugbee B (1992) Inherent limitations of nondestructive chlorophyll meters: a comparison of two types of meters. HortScience 27:69–71
Richardson AD, Duigan SP, Berlyn GP (2002) An evaluation of non invasive methods to estimate foliar chlorophyll content. New Phytol 153:185–194
Carvalho LC, Santos S, Velile BJ, Amâncio S (2008) Solanum lycopersicon Mill. and Nicotiana benthamiana L. under high light show distinct responses to anti-oxidative stress. J Plant Physiol 165:1300–1312
Arnon DI (1949) Cooper enzymes in isolated chloroplast. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11:1187–1194
Eherenfeld N, Cañón P, Stange C, Medina C, Arce-Johnson P (2005) Tobamovirus coat protein CPCG induces an HR-like response in sensitive tobacco plants. Mol Cells 19:418–427
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Du Z, Bramlage WJ (1992) Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J Agric Food Chem 40:1556–1570
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Flores P, Botella MA, Cerdá A, Martínez V (2004) Influence of nitrate level on nitrate assimilation in tomato (Lycopersicon esculentum) plants under saline stress. Can J Bot 82:207–213
Zushi K, Matsuzoe N, Kitano M (2009) Developmental and tissue-specific changes in oxidative parameters and antioxidant systems in tomato fruits grown under salt stress. Sci Hortic 122:362–368
Jenks M, Hasegawa P (2005) Plant abiotic stress. Blackwell Publishing, Oxford
Pang CH, Wang BS (2008) Oxidative stress and salt tolerance in plants. In: Luttge U et al (eds) Progress in botany, vol 69., part 3 Springer, Berlin, pp 231–245
Gémes K, Poór P, Horváth E, Kolbert Z, Szopkó D, Szepesi A, Tari I (2011) Cross-talk between salicylic acid and NaCl generated reactive oxygen species and nitric oxide in tomato during acclimation to high salinity. Physiol Plant 142:179–192
Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK (2006) Transgenic tobacco overexpressing glyoxalase pathway enzymes grow and set viable seeds in zinc-spiked soils. Plant Physiol 140:613–623
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afric J Bot 76:167–179
Klimes A, Neumann MJ, Grant SJ, Dobinson KF (2006) Characterization of the glyoxalase I gene from the vascular wilt fungus Verticillium dahliae. Can J Bot 52:816–822
Lin F, Xu J, Shi J, Li H, Li B (2010) Molecular cloning and characterization of a novel glyoxalase I gene TaGlyI in wheat (Triticum aestivum L.). Mol Biol Rep 37:729–735
Saijo Y, Hata S, Kyozuka J, Shimamoto K, Izui K (2000) Overexpression of a single Ca2+ dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J 23:319–327
Yadav SK, Singla-Pareek SL, Sopory SK (2008) An overview on the role of methylglyoxal and glyoxalases in plants. Drug Metabol Drug Interact 23:51–68
Nam MH, Huh SM, Kim KM, Park WJ, Seo JB, Cho K, Kim DY, Kim BG, Yoon IS (2012) Comparative proteomic analysis of early salt stress-responsive proteins in roots of SnRK2 transgenic rice. Proteome Sci 10:25
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Gapinska M, Skłodowska M, Gabara B (2008) Effect of short- and long-term salinity on the activities of antioxidative enzymes and lipid peroxidation in tomato roots. Acta Physiol Plant 30:11–18
Yin L, Mano J, Wang S, Tsuji W, Tanaka K (2010) The involvement of lipid peroxide-derived aldehydes in aluminum toxicity of tobacco roots. Plant Physiol 152:1406–1417
Acknowledgments
We are grateful to Dr. Sudhir K. Sopory, Plant Molecular Biology, International Center for Genetic Engineering & Biotechnology (ICGEB), New Delhi, India for providing the construction with two genes (GlyI and GlyII) of the glyoxalase pathway. This work was financially supported by the Fruit Consortium, Innova Corfo 11IDL2-10423 and the Millennium Nucleus for Plant Functional Genomics (P10-062-F).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Álvarez Viveros, M.F., Inostroza-Blancheteau, C., Timmermann, T. et al. Overexpression of GlyI and GlyII genes in transgenic tomato (Solanum lycopersicum Mill.) plants confers salt tolerance by decreasing oxidative stress. Mol Biol Rep 40, 3281–3290 (2013). https://doi.org/10.1007/s11033-012-2403-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-2403-4