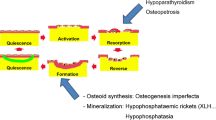
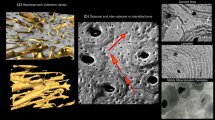
Abstract
Purpose of Review
Metabolic and genetic bone disorders affect not only bone mass but often also the bone material, including degree of mineralization, matrix organization, and lacunar porosity. The quality of juvenile bone is moreover highly influenced by skeletal growth. This review aims to provide a compact summary of the present knowledge on the complex interplay between bone modeling and remodeling during skeletal growth and to alert the reader to the complexity of bone tissue characteristics in children with bone disorders.
Recent Findings
We describe cellular events together with the characteristics of the different tissues and organic matrix organization (cartilage, woven and lamellar bone) occurring during linear growth. Subsequently, we present typical alterations thereof in disorders leading to over-mineralized bone matrix compared to those associated with low or normal mineral content based on bone biopsy studies.
Summary
Growth spurts or growth retardation might amplify or mask disease-related alterations in bone material, which makes the interpretation of bone tissue findings in children complex and challenging.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hunter DJ, Bierma-Zeinstra S. Osteoarthritis Lancet. 2019;393(10182):1745–59. https://doi.org/10.1016/S0140-6736(19)30417-9.
Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393(10169):364–76. https://doi.org/10.1016/S0140-6736(18)32112-3.
Tatangelo G, Watts J, Lim K, Connaughton C, Abimanyi-Ochom J, Borgstrom F, et al. The cost of osteoporosis, osteopenia, and associated fractures in Australia in 2017. J Bone Miner Res. 2019;34(4):616–25. https://doi.org/10.1002/jbmr.3640.
Makitie O, Zillikens MC. Early-onset osteoporosis. Calcif Tissue Int. 2022;110(5):546–61. https://doi.org/10.1007/s00223-021-00885-6.
Sakka SD. Osteoporosis in children and young adults. Best Pract Res Clin Rheumatol. 2022;36(3):101776. https://doi.org/10.1016/j.berh.2022.101776.
Ciancia S, Hogler W, Sakkers RJB, Appelman-Dijkstra NM, Boot AM, Sas TCJ, Renes JS. Osteoporosis in children and adolescents: how to treat and monitor? Eur J Pediatr. 2023;182(2):501–11. https://doi.org/10.1007/s00431-022-04743-x.
Di Marcello F, Di Donato G, d’Angelo DM, Breda L, Chiarelli F. Bone health in children with rheumatic disorders: focus on molecular mechanisms, diagnosis, and management. Int J Mol Sci. 2022;23(10):5725. https://doi.org/10.3390/ijms23105725.
Costantini A, Makitie RE, Hartmann MA, Fratzl-Zelman N, Zillikens MC, Kornak U, et al. Early-onset osteoporosis: rare monogenic forms elucidate the complexity of disease pathogenesis beyond type I collagen. J Bone Miner Res. 2022;37(9):1623–41. https://doi.org/10.1002/jbmr.4668.
Bakkaloglu SA, Bacchetta J, Lalayiannis AD, Leifheit-Nestler M, Stabouli S, Haarhaus M, et al. Bone evaluation in paediatric chronic kidney disease: clinical practice points from the European Society for Paediatric Nephrology CKD-MBD and Dialysis working groups and CKD-MBD working group of the ERA-EDTA. Nephrol Dial Transplant. 2021;36(3):413–25. https://doi.org/10.1093/ndt/gfaa210.
Zheng Y, Rostami Haji Abadi M, Ghafouri Z, Meira Goes S, Johnston JJD, Nour M, Kontulainen S. Bone deficits in children and youth with type 1 diabetes: a systematic review and meta-analysis. Bone. 2022;163:116509. https://doi.org/10.1016/j.bone.2022.116509.
Marini JC, Forlino A, Bachinger HP, Bishop NJ, Byers PH, Paepe A, et al. Osteogenesis imperfecta. Nat Rev Dis Primers. 2017;3:17052. https://doi.org/10.1038/nrdp.2017.52.
Bishop N. Bone material properties in osteogenesis imperfecta. J Bone Miner Res. 2016;31(4):699–708. https://doi.org/10.1002/jbmr.2835.
•• Collins MT, Marcucci G, Anders HJ, Beltrami G, Cauley JA, Ebeling PR, et al. Skeletal and extraskeletal disorders of biomineralization. Nat Rev Endocrinol. 2022;18(8):473–89. https://doi.org/10.1038/s41574-022-00682-7. (This review summarizes current knowledge about principal regulators of mineralization and crystallization and the role of their alteration in disorders of bone mineralization and ectopic mineralization.)
Fratzl P, Weinkamer R. Nature’s hierarchical materials. Prog Mater Sci. 2007;52:1263–334.
Burr DB. Bone quality: understanding what matters. J Musculoskelet Neuronal Interact. 2004;4(2):184–6.
Seeman E, Delmas PD. Bone quality–the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354(21):2250–61. https://doi.org/10.1056/NEJMra053077.
Bala Y, Farlay D, Boivin G. Bone mineralization: from tissue to crystal in normal and pathological contexts. Osteoporos Int. 2013;24(8):2153–66. https://doi.org/10.1007/s00198-012-2228-y.
Fratzl P, Gupta H, Paschalis E, Roschger P. Structure and mechanical quality of the collagen-mineral nano-composite in bone. J Mater Chem. 2004;14:2115–23.
Reznikov N, Shahar R, Weiner S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014;10(9):3815–26. https://doi.org/10.1016/j.actbio.2014.05.024.
Wagermaier W, Klaushofer K, Fratzl P. Fragility of bone material controlled by internal interfaces. Calcif Tissue Int. 2015;97(3):201–12. https://doi.org/10.1007/s00223-015-9978-4.
Bala Y, Seeman E. Bone’s material constituents and their contribution to bone strength in health, disease, and treatment. Calcif Tissue Int. 2015;97(3):308–26. https://doi.org/10.1007/s00223-015-9971-y.
Burr DB. Repair mechanisms for microdamage in bone. J Bone Miner Res. 2014;29(12):2534–6. https://doi.org/10.1002/jbmr.2366.
Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21(2):115–37. https://doi.org/10.1210/edrv.21.2.0395.
Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell … and more. Endocr Rev. 2013;34(5):658–90. https://doi.org/10.1210/er.2012-1026.
Robling AG, Bonewald LF. The osteocyte: new insights. Annu Rev Physiol. 2020;82:485–506. https://doi.org/10.1146/annurev-physiol-021119-034332.
Brockstedt H, Kassem M, Eriksen EF, Mosekilde L, Melsen F. Age- and sex-related changes in iliac cortical bone mass and remodeling. Bone. 1993;14(4):681–91. https://doi.org/10.1016/8756-3282(93)90092-o.
Gamsjaeger S, Hofstetter B, Fratzl-Zelman N, Roschger P, Roschger A, Fratzl P, et al. Pediatric reference Raman data for material characteristics of iliac trabecular bone. Bone. 2014;69:89–97. https://doi.org/10.1016/j.bone.2014.09.012.
Gamsjaeger S, Rauch F, Glorieux FH, Paschalis EP. Cortical bone material / compositional properties in growing children and young adults aged 1.5–23 years, as a function of gender, age, metabolic activity, and growth spurt. Bone. 2022;165:116548. https://doi.org/10.1016/j.bone.2022.116548.
Burr DB. Changes in bone matrix properties with aging. Bone. 2019;120:85–93. https://doi.org/10.1016/j.bone.2018.10.010.
Fratzl P, Groschner M, Vogl G, Plenk H Jr, Eschberger J, Fratzl-Zelman N, et al. Mineral crystals in calcified tissues: a comparative study by SAXS. J Bone Miner Res. 1992;7(3):329–34. https://doi.org/10.1002/jbmr.5650070313.
Fratzl P, Fratzl-Zelman N, Klaushofer K, Vogl G, Koller K. Nucleation and growth of mineral crystals in bone studied by small-angle X-ray scattering. Calcif Tissue Int. 1991;48(6):407–13. https://doi.org/10.1007/BF02556454.
Roschger P, Grabner BM, Rinnerthaler S, Tesch W, Kneissel M, Berzlanovich A, et al. Structural development of the mineralized tissue in the human L4 vertebral body. J Struct Biol. 2001;136(2):126–36. https://doi.org/10.1006/jsbi.2001.4427.
Ruffoni D, Fratzl P, Roschger P, Klaushofer K, Weinkamer R. The bone mineralization density distribution as a fingerprint of the mineralization process. Bone. 2007;40(5):1308–19. https://doi.org/10.1016/j.bone.2007.01.012.
Akkus O, Polyakova-Akkus A, Adar F, Schaffler MB. Aging of microstructural compartments in human compact bone. J Bone Miner Res. 2003;18(6):1012–9. https://doi.org/10.1359/jbmr.2003.18.6.1012.
Misof BM, Roschger P, Zhou H, Nieves JW, Bostrom M, Cosman F, et al. No evidence for alteration in early secondary mineralization by either alendronate, teriparatide or combination of both in transiliac bone biopsy samples from postmenopausal osteoporotic patients. Bone Rep. 2020;12:100253. https://doi.org/10.1016/j.bonr.2020.100253.
Zou Z, Tang T, Macias-Sanchez E, Sviben S, Landis WJ, Bertinetti L, Fratzl P. Three-dimensional structural interrelations between cells, extracellular matrix, and mineral in normally mineralizing avian leg tendon. Proc Natl Acad Sci U S A. 2020;117(25):14102–9. https://doi.org/10.1073/pnas.1917932117.
Raguin E, Weinkamer R, Schmitt C, Curcuraci L, Fratzl P. Logistics of bone mineralization in the chick embryo studied by 3D cryo FIB-SEM imaging. Adv Sci (Weinh). 2023;10(22):e2301231. https://doi.org/10.1002/advs.202301231.
Ping H, Wagermaier W, Horbelt N, Scoppola E, Li C, Werner P, et al. Mineralization generates megapascal contractile stresses in collagen fibrils. Science. 2022;376(6589):188–92. https://doi.org/10.1126/science.abm2664.
Paschalis EP, Gamsjaeger S, Klaushofer K. Vibrational spectroscopic techniques to assess bone quality. Osteoporos Int. 2017;28(8):2275–91. https://doi.org/10.1007/s00198-017-4019-y.
Munoz A, Docaj A, Ugarteburu M, Carriero A. Poor bone matrix quality: what can be done about it? Curr Osteoporos Rep. 2021;19(5):510–31. https://doi.org/10.1007/s11914-021-00696-6.
Depalle B, Duarte AG, Fiedler IAK, Pujo-Menjouet L, Buehler MJ, Berteau JP. The different distribution of enzymatic collagen cross-links found in adult and children bone result in different mechanical behavior of collagen. Bone. 2018;110:107–14. https://doi.org/10.1016/j.bone.2018.01.024.
Qiu S, Divine G, Warner E, Rao SD. Reference intervals for bone histomorphometric measurements based on data from healthy premenopausal women. Calcif Tissue Int. 2020;107(6):543–50. https://doi.org/10.1007/s00223-020-00748-6.
Hartmann MA, Blouin S, Misof BM, Fratzl-Zelman N, Roschger P, Berzlanovich A, et al. Quantitative backscattered electron imaging of bone using a thermionic or a field emission electron source. Calcif Tissue Int. 2021;109(2):190–202. https://doi.org/10.1007/s00223-021-00832-5.
Recker RR, Lappe JM, Davies M, Kimmel D. Perimenopausal bone histomorphometry before and after menopause. Bone. 2018;108:55–61. https://doi.org/10.1016/j.bone.2017.12.016.
Szulc P, Seeman E. Thinking inside and outside the envelopes of bone: dedicated to PDD. Osteoporos Int. 2009;20(8):1281–8. https://doi.org/10.1007/s00198-009-0994-y.
Seeman E. Bone modeling and remodeling. Crit Rev Eukaryot Gene Expr. 2009;19(3):219–33. https://doi.org/10.1615/critreveukargeneexpr.v19.i3.40.
Ramchand SK, Seeman E. The influence of cortical porosity on the strength of bone during growth and advancing age. Curr Osteoporos Rep. 2018;16(5):561–72. https://doi.org/10.1007/s11914-018-0478-0.
Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375(9727):1729–36. https://doi.org/10.1016/S0140-6736(10)60320-0.
Paschalis EP, Dempster DW, Gamsjaeger S, Rokidi S, Hassler N, Brozek W, et al. Mineral and organic matrix composition at bone forming surfaces in postmenopausal women with osteoporosis treated with either teriparatide or zoledronic acid. Bone. 2021;145:115848. https://doi.org/10.1016/j.bone.2021.115848.
Paschalis EP, Gamsjaeger S, Klaushofer K, Shane E, Cohen A, Stepan J, et al. Treatment of postmenopausal osteoporosis patients with teriparatide for 24 months reverts forming bone quality indices to premenopausal healthy control values. Bone. 2022;162:116478. https://doi.org/10.1016/j.bone.2022.116478.
Wang B, Vashishth D. Advanced glycation and glycoxidation end products in bone. Bone. 2023;176:116880. https://doi.org/10.1016/j.bone.2023.116880.
Ng AH, Omelon S, Variola F, Allo B, Willett TL, Alman BA, Grynpas MD. Adynamic bone decreases bone toughness during aging by affecting mineral and matrix. J Bone Miner Res. 2016;31(2):369–79. https://doi.org/10.1002/jbmr.2702.
•• Unger S, Ferreira CR, Mortier GR, Ali H, Bertola DR, Calder A, et al. Nosology of genetic skeletal disorders: 2023 revision. Am J Med Genet A. 2023;191(5):1164–209. https://doi.org/10.1002/ajmg.a.63132. (The recently updated nosology of genetic skeletal disorders contains 771 entries associated with 552 genes, comprises cross-references to the MIM catalog, and represents an important tool for differential diagnosis for pediatricians.)
Turner CH. Determinants of skeletal fragility and bone quality. J Musculoskelet Neuronal Interact. 2002;2(6):527–8.
Seeman E. Bone quality. Osteoporos Int. 2003;14(Suppl 5):S3-7. https://doi.org/10.1007/s00198-003-1465-5.
Rauch F. Watching bone cells at work: what we can see from bone biopsies. Pediatr Nephrol. 2006;21(4):457–62. https://doi.org/10.1007/s00467-006-0025-6.
Rauch F. Bone biopsy: indications and methods. Endocr Dev. 2009;16:49–57. https://doi.org/10.1159/000223688.
Mahr M, Blouin S, Misof BM, Paschalis EP, Hartmann MA, Zwerina J, Fratzl-Zelman N. Bone properties in osteogenesis imperfecta: what can we learn from a bone biopsy beyond histology? Wien Med Wochenschr. 2021;171(5–6):111–9. https://doi.org/10.1007/s10354-021-00818-w.
Chavassieux P, Chapurlat R. Interest of bone histomorphometry in bone pathophysiology investigation: foundation, present, and future. Front Endocrinol (Lausanne). 2022;13:907914. https://doi.org/10.3389/fendo.2022.907914.
Bortel EL, Duda GN, Mundlos S, Willie BM, Fratzl P, Zaslansky P. Long bone maturation is driven by pore closing: a quantitative tomography investigation of structural formation in young C57BL/6 mice. Acta Biomater. 2015;22:92–102. https://doi.org/10.1016/j.actbio.2015.03.027.
Zimmermann EA, Riedel C, Schmidt FN, Stockhausen KE, Chushkin Y, Schaible E, et al. Mechanical competence and bone quality develop during skeletal growth. J Bone Miner Res. 2019;34(8):1461–72. https://doi.org/10.1002/jbmr.3730.
Eckstein KN, Thomas SM, Scott AK, Neu CP, Hadley-Miller NA, Payne KA, Ferguson VL. The heterogeneous mechanical properties of adolescent growth plate cartilage: a study in rabbit. J Mech Behav Biomed Mater. 2022;128:105102. https://doi.org/10.1016/j.jmbbm.2022.105102.
Roschger A, Wagermaier W, Gamsjaeger S, Hassler N, Schmidt I, Blouin S, et al. Newly formed and remodeled human bone exhibits differences in the mineralization process. Acta Biomater. 2020;104:221–30. https://doi.org/10.1016/j.actbio.2020.01.004.
Rashid H, Chen H, Javed A. Runx2 is required for hypertrophic chondrocyte mediated degradation of cartilage matrix during endochondral ossification. Matrix Biol Plus. 2021;12:100088. https://doi.org/10.1016/j.mbplus.2021.100088.
Hallett SA, Ono W, Ono N. The hypertrophic chondrocyte: to be or not to be. Histol Histopathol. 2021;36(10):1021–36. https://doi.org/10.14670/HH-18-355.
Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci U S A. 2014;111(33):12097–102. https://doi.org/10.1073/pnas.1302703111.
Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 2014;10(12):e1004820. https://doi.org/10.1371/journal.pgen.1004820.
Matsushita Y, Ono W, Ono N. Growth plate skeletal stem cells and their transition from cartilage to bone. Bone. 2020;136:115359. https://doi.org/10.1016/j.bone.2020.115359.
Long F, Ornitz DM. Development of the endochondral skeleton. Cold Spring Harb Perspect Biol. 2013;5(1):a008334. https://doi.org/10.1101/cshperspect.a008334.
Maes C. Signaling pathways effecting crosstalk between cartilage and adjacent tissues: seminars in cell and developmental biology: the biology and pathology of cartilage. Semin Cell Dev Biol. 2017;62:16–33. https://doi.org/10.1016/j.semcdb.2016.05.007.
Tsang KY, Chan D, Cheah KS. Fate of growth plate hypertrophic chondrocytes: death or lineage extension? Dev Growth Differ. 2015;57(2):179–92. https://doi.org/10.1111/dgd.12203.
Wang K, Ma C, Feng JQ, Jing Y. The emerging role of cell transdifferentiation in skeletal development and diseases. Int J Mol Sci. 2022;23(11):5974. https://doi.org/10.3390/ijms23115974.
Odgren PR, Witwicka H, Reyes-Gutierrez P. The cast of clasts: catabolism and vascular invasion during bone growth, repair, and disease by osteoclasts, chondroclasts, and septoclasts. Connect Tissue Res. 2016;57(3):161–74. https://doi.org/10.3109/03008207.2016.1140752.
Khan NM, Clifton KB, Lorenzo J, Hansen MF, Drissi H. Comparative transcriptomic analysis identifies distinct molecular signatures and regulatory networks of chondroclasts and osteoclasts. Arthritis Res Ther. 2020;22(1):168. https://doi.org/10.1186/s13075-020-02259-z.
Tosun B, Wolff LI, Houben A, Nutt S, Hartmann C. Osteoclasts and macrophages-their role in bone marrow cavity formation during mouse embryonic development. J Bone Miner Res. 2022;37(9):1761–74. https://doi.org/10.1002/jbmr.4629.
Fratzl-Zelman N, Valenta A, Roschger P, Nader A, Gelb BD, Fratzl P, Klaushofer K. Decreased bone turnover and deterioration of bone structure in two cases of pycnodysostosis. J Clin Endocrinol Metab. 2004;89(4):1538–47. https://doi.org/10.1210/jc.2003-031055.
Butscheidt S, Rolvien T, Kornak U, Schmidt FN, Schinke T, Amling M, Oheim R. Clinical significance of DXA and HR-pQCT in autosomal dominant osteopetrosis (ADO II). Calcif Tissue Int. 2018;102(1):41–52. https://doi.org/10.1007/s00223-017-0332-x.
Philbrick KA, Martin SA, Colagiovanni AR, Branscum AJ, Turner RT, Iwaniec UT. Effects of hypothalamic leptin gene therapy on osteopetrosis in leptin-deficient mice. J Endocrinol. 2018;236(2):57–68. https://doi.org/10.1530/JOE-17-0524.
Howaldt A, Hennig AF, Rolvien T, Rossler U, Stelzer N, Knaus A, et al. Adult osteosclerotic metaphyseal dysplasia with progressive osteonecrosis of the jaws and abnormal bone resorption pattern due to a LRRK1 splice site mutation. J Bone Miner Res. 2020;35(7):1322–32. https://doi.org/10.1002/jbmr.3995.
Hofstaetter JG, Atkins GJ, Kato H, Kogawa M, Blouin S, Misof BM, et al. A mild case of autosomal recessive osteopetrosis masquerading as the dominant form involving homozygous deep intronic variations in the CLCN7 Gene. Calcif Tissue Int. 2022;111(4):430–44. https://doi.org/10.1007/s00223-022-00988-8.
Whyte MP. Osteopetrosis: discovery and early history of “marble bone disease.” Bone. 2023;171:116737. https://doi.org/10.1016/j.bone.2023.116737.
Gupta HS, Schratter S, Tesch W, Roschger P, Berzlanovich A, Schoeberl T, et al. Two different correlations between nanoindentation modulus and mineral content in the bone-cartilage interface. J Struct Biol. 2005;149(2):138–48. https://doi.org/10.1016/j.jsb.2004.10.010.
Long JT, Leinroth A, Liao Y, Ren Y, Mirando AJ, Nguyen T, et al. Hypertrophic chondrocytes serve as a reservoir for marrow-associated skeletal stem and progenitor cells, osteoblasts, and adipocytes during skeletal development. Elife. 2022;11:e76932. https://doi.org/10.7554/eLife.76932.
Bisseret D, Kaci R, Lafage-Proust MH, Alison M, Parlier-Cuau C, Laredo JD, Bousson V. Periosteum: characteristic imaging findings with emphasis on radiologic-pathologic comparisons. Skeletal Radiol. 2015;44(3):321–38. https://doi.org/10.1007/s00256-014-1976-5.
Seeman E. Periosteal bone formation–a neglected determinant of bone strength. N Engl J Med. 2003;349(4):320–3. https://doi.org/10.1056/NEJMp038101.
Epker BN, Frost HM. Periosteal appositional bone growth from age two to age seventy in man. A tetracycline evaluation. Anat Rec. 1966;154(3):573–7. https://doi.org/10.1002/ar.1091540307.
Rauch F. Bone growth in length and width: the Yin and Yang of bone stability. J Musculoskelet Neuronal Interact. 2005;5(3):194–201.
Currey JD. The many adaptations of bone. J Biomech. 2003;36(10):1487–95. https://doi.org/10.1016/s0021-9290(03)00124-6.
Shapiro F, Wu JY. Woven bone overview: structural classification based on its integral role in developmental, repair and pathological bone formation throughout vertebrate groups. Eur Cell Mater. 2019;38:137–67. https://doi.org/10.22203/eCM.v038a11.
Parfitt AM, Travers R, Rauch F, Glorieux FH. Structural and cellular changes during bone growth in healthy children. Bone. 2000;27(4):487–94. https://doi.org/10.1016/s8756-3282(00)00353-7.
Rauch F, Travers R, Glorieux FH. Cellular activity on the seven surfaces of iliac bone: a histomorphometric study in children and adolescents. J Bone Miner Res. 2006;21(4):513–9. https://doi.org/10.1359/jbmr.060108.
Rauch F, Travers R, Glorieux FH. Intracortical remodeling during human bone development–a histomorphometric study. Bone. 2007;40(2):274–80. https://doi.org/10.1016/j.bone.2006.09.012. (S8756-3282(06)00695-8 [pii]).
Rauch F. Bone accrual in children: adding substance to surfaces. Pediatrics. 2007;119(Suppl 2):S137–40. https://doi.org/10.1542/peds.2006-2023E.
Rauch F, Travers R, Parfitt AM, Glorieux FH. Static and dynamic bone histomorphometry in children with osteogenesis imperfecta. Bone. 2000;26(6):581–9 (S8756-3282(00)00269-6 [pii]).
• Jandl NM, von Kroge S, Sturznickel J, Baranowsky A, Stockhausen KE, Mushumba H, et al. Large osteocyte lacunae in iliac crest infantile bone are not associated with impaired mineral distribution or signs of osteocytic osteolysis. Bone. 2020;135:115324. https://doi.org/10.1016/j.bone.2020.115324. (This work shows the human postnatal development of different aspects of trabecular and cortical bone material up to the age of 25 years in the iliac crest including mineralization and osteocyte lacunae characteristics in primary woven and lamellar bone.)
Fratzl-Zelman N, Roschger P, Misof BM, Pfeffer S, Glorieux FH, Klaushofer K, Rauch F. Normative data on mineralization density distribution in iliac bone biopsies of children, adolescents and young adults. Bone. 2009;44(6):1043–8. https://doi.org/10.1016/j.bone.2009.02.021.
Nawrot-Wawrzyniak K, Varga F, Nader A, Roschger P, Sieghart S, Zwettler E, et al. Effects of tumor-induced osteomalacia on the bone mineralization process. Calcif Tissue Int. 2009;84(4):313–23. https://doi.org/10.1007/s00223-009-9216-z.
Boivin G, Meunier PJ. The mineralization of bone tissue: a forgotten dimension in osteoporosis research. Osteoporos Int. 2003;14(Suppl 3):S19-24. https://doi.org/10.1007/s00198-002-1347-2.
Zizak I, Roschger P, Paris O, Misof BM, Berzlanovich A, Bernstorff S, et al. Characteristics of mineral particles in the human bone/cartilage interface. J Struct Biol. 2003;141(3):208–17. https://doi.org/10.1016/s1047-8477(02)00635-4.
• Mahr M, Blouin S, Behanova M, Misof BM, Glorieux FH, Zwerina J, et al. Increased osteocyte lacunae density in the hypermineralized bone matrix of children with osteogenesis imperfecta type I. Int J Mol Sci. 2021;22(9):4508. https://doi.org/10.3390/ijms22094508. (This is a systematic analysis of osteocyte lacunae characteristics and bone matrix mineralization in a relatively large cohort of young patients with classical type I osteogenesis imperfecta, which serves as a benchmark for comparison to novel forms of OI.)
Misof BM, Roschger P, Klaushofer K, Rauch F, Ma J, Mack DR, Ward LM. Increased bone matrix mineralization in treatment-naive children with inflammatory bowel disease. Bone. 2017;105:50–6. https://doi.org/10.1016/j.bone.2017.07.011.
Nawrot-Wawrzyniak K, Misof BM, Roschger P, Panczyk-Tomaszewska M, Ziolkowska H, Klaushofer K, Fratzl-Zelman N. Changes in bone matrix mineralization after growth hormone treatment in children and adolescents with chronic kidney failure treated by dialysis: a paired biopsy study. Am J Kidney Dis. 2013;61(5):767–77. https://doi.org/10.1053/j.ajkd.2012.12.010.
Pereira RC, Salusky IB, Roschger P, Klaushofer K, Yadin O, Freymiller EG, et al. Impaired osteocyte maturation in the pathogenesis of renal osteodystrophy. Kidney Int. 2018;94(5):1002–12. https://doi.org/10.1016/j.kint.2018.08.011.
Misof BM, Roschger P, McMillan HJ, Ma J, Klaushofer K, Rauch F, Ward LM. Histomorphometry and bone matrix mineralization before and after bisphosphonate treatment in boys with Duchenne muscular dystrophy: a paired transiliac biopsy study. J Bone Miner Res. 2016;31(5):1060–9. https://doi.org/10.1002/jbmr.2756.
Fratzl-Zelman N, Valta H, Pereira RC, Misof BM, Roschger P, Jalanko H, et al. Abnormally high and heterogeneous bone matrix mineralization after childhood solid organ transplantation: a complex pathology of low bone turnover and local defects in mineralization. J Bone Miner Res. 2017;32(5):1116–25. https://doi.org/10.1002/jbmr.3087.
Harrington J, Holmyard D, Silverman E, Sochett E, Grynpas M. Bone histomorphometric changes in children with rheumatic disorders on chronic glucocorticoids. Pediatr Rheumatol Online J. 2016;14(1):58. https://doi.org/10.1186/s12969-016-0119-z.
Hogler W, Ward L. Osteoporosis in children with chronic disease. Endocr Dev. 2015;28:176–95. https://doi.org/10.1159/000381045.
Ward LM. Glucocorticoid-induced osteoporosis: why kids are different. Front Endocrinol (Lausanne). 2020;11:576. https://doi.org/10.3389/fendo.2020.00576.
Bacchetta J, Schmitt CP, Bakkaloglu SA, Cleghorn S, Leifheit-Nestler M, Prytula A, et al. Diagnosis and management of mineral and bone disorders in infants with CKD: clinical practice points from the ESPN CKD-MBD and Dialysis working groups and the Pediatric Renal Nutrition Taskforce. Pediatr Nephrol. 2023. https://doi.org/10.1007/s00467-022-05825-6.
Cianferotti L, Cipriani C, Corbetta S, Corona G, Defeudis G, Lania AG, et al. Bone quality in endocrine diseases: determinants and clinical relevance. J Endocrinol Invest. 2023. https://doi.org/10.1007/s40618-023-02056-w.
Misof BM, Roschger P, Dempster DW, Zhou H, Bilezikian JP, Klaushofer K, Rubin MR. PTH(1–84) Administration in hypoparathyroidism transiently reduces bone matrix mineralization. J Bone Miner Res. 2016;31(1):180–9. https://doi.org/10.1002/jbmr.2588.
Ovejero D, Misof BM, Gafni RI, Dempster D, Zhou H, Klaushofer K, et al. Bone matrix mineralization in patients with gain-of-function calcium-sensing receptor mutations is distinctly different from that in postsurgical hypoparathyroidism. J Bone Miner Res. 2019;34(4):661–8. https://doi.org/10.1002/jbmr.3638.
Theman TA, Collins MT, Dempster DW, Zhou H, Reynolds JC, Brahim JS, et al. PTH(1–34) replacement therapy in a child with hypoparathyroidism caused by a sporadic calcium receptor mutation. J Bone Miner Res. 2009;24(5):964–73. https://doi.org/10.1359/jbmr.081233.
Theman TA, Collins MT. The role of the calcium-sensing receptor in bone biology and pathophysiology. Curr Pharm Biotechnol. 2009;10(3):289–301. https://doi.org/10.2174/138920109787847538.
Lefevre E, Farlay D, Bala Y, Subtil F, Wolfram U, Rizzo S, et al. Compositional and mechanical properties of growing cortical bone tissue: a study of the human fibula. Sci Rep. 2019;9(1):17629. https://doi.org/10.1038/s41598-019-54016-1.
Rauchenzauner M, Schneider J, Colleselli V, Ruepp M, Cortina G, Hogler W, et al. Comparing modalities of conducting the six-minute walk test in healthy children and adolescents. Minerva Pediatr. 2019;71(3):229–34.
van Coeverden SC, Netelenbos JC, de Ridder CM, Roos JC, Popp-Snijders C, Delemarre-van de Waal HA. Bone metabolism markers and bone mass in healthy pubertal boys and girls. Clin Endocrinol (Oxf). 2002;57(1):107–16. https://doi.org/10.1046/j.1365-2265.2002.01573.x.
Ladang A, Rauch F, Delvin E, Cavalier E. Bone turnover markers in children: from laboratory challenges to clinical interpretation. Calcif Tissue Int. 2023;112(2):218–32. https://doi.org/10.1007/s00223-022-00964-2.
Banica T, Vandewalle S, Zmierczak HG, Goemaere S, De Buyser S, Fiers T, et al. The relationship between circulating hormone levels, bone turnover markers and skeletal development in healthy boys differs according to maturation stage. Bone. 2022;158:116368. https://doi.org/10.1016/j.bone.2022.116368.
Rand MS, Diemar SS, Mollehave LT, Heidemann M, Thuesen BH, Petersen JH, et al. Z-scores of bone turnover markers calculated from new established sex- and age-specific reference curves are associated to future change in BMD in children and adolescents. Bone. 2023;167:116641. https://doi.org/10.1016/j.bone.2022.116641.
Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP. Epidemiology of childhood fractures in Britain: a study using the general practice research database. J Bone Miner Res. 2004;19(12):1976–81. https://doi.org/10.1359/JBMR.040902.
Montero-Lopez R, Laurer E, Tischlinger K, Nagy D, Scala M, Kranewitter W, et al. Spontaneous reshaping of vertebral fractures in an adolescent with osteogenesis imperfecta. Bone Rep. 2022;16:101595. https://doi.org/10.1016/j.bonr.2022.101595.
Roschger P, Paschalis EP, Fratzl P, Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2008;42(3):456–66. https://doi.org/10.1016/j.bone.2007.10.021.
Tauer JT, Robinson ME, Rauch F. Osteogenesis imperfecta: new perspectives from clinical and translational research. JBMR Plus. 2019;3(8):e10174. https://doi.org/10.1002/jbm4.10174.
Etich J, Lessmeier L, Rehberg M, Sill H, Zaucke F, Netzer C, Semler O. Osteogenesis imperfecta-pathophysiology and therapeutic options. Mol Cell Pediatr. 2020;7(1):9. https://doi.org/10.1186/s40348-020-00101-9.
Etich J, Rehberg M, Eckes B, Sengle G, Semler O, Zaucke F. Signaling pathways affected by mutations causing osteogenesis imperfecta. Cell Signal. 2020;76:109789. https://doi.org/10.1016/j.cellsig.2020.109789.
Arshad F, Bishop N. Osteogenesis imperfecta in children. Bone. 2021;148:115914. https://doi.org/10.1016/j.bone.2021.115914.
El-Gazzar A, Hogler W. Mechanisms of bone fragility: from osteogenesis imperfecta to secondary osteoporosis. Int J Mol Sci. 2021;22(2):625. https://doi.org/10.3390/ijms22020625.
Rossi V, Lee B, Marom R. Osteogenesis imperfecta: advancements in genetics and treatment. Curr Opin Pediatr. 2019;31(6):708–15. https://doi.org/10.1097/MOP.0000000000000813.
Marom R, Rabenhorst BM, Morello R. Osteogenesis imperfecta: an update on clinical features and therapies. Eur J Endocrinol. 2020;183(4):R95–106. https://doi.org/10.1530/EJE-20-0299.
Jovanovic M, Guterman-Ram G, Marini JC. Osteogenesis imperfecta: mechanisms and signaling pathways connecting classical and rare OI types. Endocr Rev. 2022;43(1):61–90. https://doi.org/10.1210/endrev/bnab017.
Song IW, Nagamani SC, Nguyen D, Grafe I, Sutton VR, Gannon FH, et al. Targeting TGF-beta for treatment of osteogenesis imperfecta. J Clin Invest. 2022;132(7):e152571. https://doi.org/10.1172/JCI152571.
Kang H, Aryal Ac S, Barnes AM, Martin A, David V, Crawford SE, Marini JC. Antagonism between PEDF and TGF-beta contributes to type VI osteogenesis imperfecta bone and vascular pathogenesis. J Bone Miner Res. 2022;37(5):925–37. https://doi.org/10.1002/jbmr.4540.
Claeys L, Storoni S, Eekhoff M, Elting M, Wisse L, Pals G, et al. Collagen transport and related pathways in osteogenesis imperfecta. Hum Genet. 2021;140(8):1121–41. https://doi.org/10.1007/s00439-021-02302-2.
El-Gazzar A, Voraberger B, Rauch F, Mairhofer M, Schmidt K, Guillemyn B, et al. Bi-allelic mutation in SEC16B alters collagen trafficking and increases ER stress. EMBO Mol Med. 2023;15(4):e16834. https://doi.org/10.15252/emmm.202216834.
El-Gazzar A, Kang H, Fratzl-Zelman N, Webb E, Barnes AM, Jovanovic M, et al. SMAD3 mutation in LDS3 causes bone fragility by impairing the TGF-beta pathway and enhancing osteoclastogenesis. Bone Rep. 2022;17:101603. https://doi.org/10.1016/j.bonr.2022.101603.
Wang JS, Tokavanich N, Wein MN. SP7: from bone development to skeletal disease. Curr Osteoporos Rep. 2023;21(2):241–52. https://doi.org/10.1007/s11914-023-00778-7.
Lui JC, Raimann A, Hojo H, Dong L, Roschger P, Kikani B, et al. A neomorphic variant in SP7 alters sequence specificity and causes a high-turnover bone disorder. Nat Commun. 2022;13(1):700. https://doi.org/10.1038/s41467-022-28318-4.
Ludwig K, Ward LM, Khan N, Robinson ME, Miranda V, Bardai G, et al. Dominant osteogenesis imperfecta with low bone turnover caused by a heterozygous SP7 variant. Bone. 2022;160:116400. https://doi.org/10.1016/j.bone.2022.116400.
El-Gazzar A, Mayr JA, Voraberger B, Brugger K, Blouin S, Tischlinger K, et al. A novel cryptic splice site mutation in COL1A2 as a cause of osteogenesis imperfecta. Bone Rep. 2021;15:101110. https://doi.org/10.1016/j.bonr.2021.101110.
Eyre DR, Weis MA. Bone collagen: new clues to its mineralization mechanism from recessive osteogenesis imperfecta. Calcif Tissue Int. 2013;93(4):338–47. https://doi.org/10.1007/s00223-013-9723-9.
Gistelinck C, Kwon RY, Malfait F, Symoens S, Harris MP, Henke K, et al. Zebrafish type I collagen mutants faithfully recapitulate human type I collagenopathies. Proc Natl Acad Sci U S A. 2018;115(34):E8037–46. https://doi.org/10.1073/pnas.1722200115.
Gistelinck C, Weis M, Rai J, Schwarze U, Niyazov D, Song KM, et al. Abnormal bone collagen cross-linking in osteogenesis imperfecta/Bruck syndrome caused by compound heterozygous PLOD2 mutations. JBMR Plus. 2021;5(3):e10454. https://doi.org/10.1002/jbm4.10454.
Daponte V, Tonelli F, Masiero C, Syx D, Exbrayat-Heritier C, Biggiogera M, et al. Cell differentiation and matrix organization are differentially affected during bone formation in osteogenesis imperfecta zebrafish models with different genetic defects impacting collagen type I structure. Matrix Biol. 2023. https://doi.org/10.1016/j.matbio.2023.06.003.
Van Dijk FS, Sillence DO. Osteogenesis imperfecta: clinical diagnosis, nomenclature and severity assessment. Am J Med Genet A. 2014;164A(6):1470–81. https://doi.org/10.1002/ajmg.a.36545.
Fratzl-Zelman N, Misof BM, Klaushofer K, Roschger P. Bone mass and mineralization in osteogenesis imperfecta. Wien Med Wochenschr. 2015;165(13–14):271–7. https://doi.org/10.1007/s10354-015-0369-2.
Roschger P, Fratzl-Zelman N, Misof BM, Glorieux FH, Klaushofer K, Rauch F. Evidence that abnormal high bone mineralization in growing children with osteogenesis imperfecta is not associated with specific collagen mutations. Calcif Tissue Int. 2008;82(4):263–70. https://doi.org/10.1007/s00223-008-9113-x.
Fratzl-Zelman N, Morello R, Lee B, Rauch F, Glorieux FH, Misof BM, et al. CRTAP deficiency leads to abnormally high bone matrix mineralization in a murine model and in children with osteogenesis imperfecta type VII. Bone. 2010;46(3):820–6. https://doi.org/10.1016/j.bone.2009.10.037.
Fratzl-Zelman N, Barnes AM, Weis M, Carter E, Hefferan TE, Perino G, et al. Non-lethal type VIII osteogenesis imperfecta has elevated bone matrix mineralization. J Clin Endocrinol Metab. 2016;101(9):3516–25. https://doi.org/10.1210/jc.2016-1334.
Kok DH, Sakkers RJ, Pruijs HE, Joosse P, Castelein RM. Bone mineral density in developing children with osteogenesis imperfecta: a longitudinal study with 9 years of follow-up. Acta Orthop. 2013;84(4):431–6. https://doi.org/10.3109/17453674.2013.831321.
Robinson ME, Trejo P, Palomo T, Glorieux FH, Rauch F. Osteogenesis imperfecta: skeletal outcomes after bisphosphonate discontinuation at final height. J Bone Miner Res. 2019;34(12):2198–204. https://doi.org/10.1002/jbmr.3833.
Indermaur M, Casari D, Kochetkova T, Peruzzi C, Zimmermann E, Rauch F, et al. Compressive strength of iliac bone ECM is not reduced in osteogenesis imperfecta and increases with mineralization. J Bone Miner Res. 2021;36(7):1364–75. https://doi.org/10.1002/jbmr.4286.
Misof BM, Roschger P, Mahr M, Fratzl-Zelman N, Glorieux FH, Hartmann MA, et al. Accelerated mineralization kinetics in children with osteogenesis imperfecta type 1. Bone. 2023;166:116580. https://doi.org/10.1016/j.bone.2022.116580.
Paschalis EP, Gamsjaeger S, Fratzl-Zelman N, Roschger P, Masic A, Brozek W, et al. Evidence for a role for nanoporosity and pyridinoline content in human mild osteogenesis imperfecta. J Bone Miner Res. 2016;31(5):1050–9. https://doi.org/10.1002/jbmr.2780.
Fratzl-Zelman N, Schmidt I, Roschger P, Glorieux FH, Klaushofer K, Fratzl P, et al. Mineral particle size in children with osteogenesis imperfecta type I is not increased independently of specific collagen mutations. Bone. 2014;60:122–8. https://doi.org/10.1016/j.bone.2013.11.023.
Fratzl-Zelman N, Schmidt I, Roschger P, Roschger A, Glorieux FH, Klaushofer K, et al. Unique micro- and nano-scale mineralization pattern of human osteogenesis imperfecta type VI bone. Bone. 2015;73:233–41. https://doi.org/10.1016/j.bone.2014.12.023.
Shapiro F, Maguire K, Swami S, Zhu H, Flynn E, Wang J, Wu JY. Histopathology of osteogenesis imperfecta bone. Supramolecular assessment of cells and matrices in the context of woven and lamellar bone formation using light, polarization and ultrastructural microscopy. Bone Rep. 2021;14:100734. https://doi.org/10.1016/j.bonr.2020.100734.
Glorieux FH, Rauch F, Plotkin H, Ward L, Travers R, Roughley P, et al. Type V osteogenesis imperfecta: a new form of brittle bone disease. J Bone Miner Res. 2000;15(9):1650–8. https://doi.org/10.1359/jbmr.2000.15.9.1650.
Blouin S, Fratzl-Zelman N, Glorieux FH, Roschger P, Klaushofer K, Marini JC, Rauch F. Hypermineralization and high osteocyte lacunar density in osteogenesis imperfecta type V bone indicate exuberant primary bone formation. J Bone Miner Res. 2017;32(9):1884–92. https://doi.org/10.1002/jbmr.3180.
Glorieux FH, Ward LM, Rauch F, Lalic L, Roughley PJ, Travers R. Osteogenesis imperfecta type VI: a form of brittle bone disease with a mineralization defect. J Bone Miner Res. 2002;17(1):30–8. https://doi.org/10.1359/jbmr.2002.17.1.30.
Farber CR, Reich A, Barnes AM, Becerra P, Rauch F, Cabral WA, et al. A novel IFITM5 mutation in severe atypical osteogenesis imperfecta type VI impairs osteoblast production of pigment epithelium-derived factor. J Bone Miner Res. 2014;29(6):1402–11. https://doi.org/10.1002/jbmr.2173.
Hedjazi G, Guterman-Ram G, Blouin S, Schemenz V, Wagermaier W, Fratzl P, et al. Alterations of bone material properties in growing Ifitm5/BRIL p.S42 knock-in mice, a new model for atypical type VI osteogenesis imperfecta. Bone. 2022;162:116451. https://doi.org/10.1016/j.bone.2022.116451.
Albert C, Jameson J, Smith P, Harris G. Reduced diaphyseal strength associated with high intracortical vascular porosity within long bones of children with osteogenesis imperfecta. Bone. 2014;66:121–30. https://doi.org/10.1016/j.bone.2014.05.022.
Carriero A, Doube M, Vogt M, Busse B, Zustin J, Levchuk A, et al. Altered lacunar and vascular porosity in osteogenesis imperfecta mouse bone as revealed by synchrotron tomography contributes to bone fragility. Bone. 2014;61:116–24. https://doi.org/10.1016/j.bone.2013.12.020.
Carriero A, Zimmermann EA, Paluszny A, Tang SY, Bale H, Busse B, et al. How tough is brittle bone? Investigating osteogenesis imperfecta in mouse bone. J Bone Miner Res. 2014;29(6):1392–401. https://doi.org/10.1002/jbmr.2172.
Zebaze R, Ebeling PR. Disorganization and musculoskeletal diseases: novel insights into the enigma of unexplained bone abnormalities and fragility fractures. Curr Osteoporos Rep. 2023;21(2):154–66. https://doi.org/10.1007/s11914-022-00759-2.
Bergen DJM, Maurizi A, Formosa MM, McDonald GLK, El-Gazzar A, Hassan N, et al. High bone mass disorders: new insights from connecting the clinic and the bench. J Bone Miner Res. 2023;38(2):229–47. https://doi.org/10.1002/jbmr.4715.
Whyte MP. Carbonic anhydrase II deficiency. Bone. 2023;169:116684. https://doi.org/10.1016/j.bone.2023.116684.
Pillai NR, Aggarwal A, Orchard P. Phenotype-autosomal recessive osteopetrosis. Bone. 2022;165:116577. https://doi.org/10.1016/j.bone.2022.116577.
Polgreen LE, Imel EA, Econs MJ. Autosomal dominant osteopetrosis. Bone. 2023;170:116723. https://doi.org/10.1016/j.bone.2023.116723.
Stauber T, Wartosch L, Vishnolia S, Schulz A, Kornak U. CLCN7, a gene shared by autosomal recessive and autosomal dominant osteopetrosis. Bone. 2023;168:116639. https://doi.org/10.1016/j.bone.2022.116639.
Hald JD, Beck-Nielsen S, Gregersen PA, Gjorup H, Langdahl B. Pycnodysostosis in children and adults. Bone. 2023;169:116674. https://doi.org/10.1016/j.bone.2023.116674.
Helfrich MH, Aronson DC, Everts V, Mieremet RH, Gerritsen EJ, Eckhardt PG, et al. Morphologic features of bone in human osteopetrosis. Bone. 1991;12(6):411–9. https://doi.org/10.1016/8756-3282(91)90030-m.
Barvencik F, Kurth I, Koehne T, Stauber T, Zustin J, Tsiakas K, et al. CLCN7 and TCIRG1 mutations differentially affect bone matrix mineralization in osteopetrotic individuals. J Bone Miner Res. 2014;29(4):982–91. https://doi.org/10.1002/jbmr.2100.
Nakayama H, Takakuda K, Matsumoto HN, Miyata A, Baba O, Tabata MJ, et al. Effects of altered bone remodeling and retention of cement lines on bone quality in osteopetrotic aged c-Src-deficient mice. Calcif Tissue Int. 2010;86(2):172–83. https://doi.org/10.1007/s00223-009-9331-x.
Gelb BD, Shi GP, Chapman HA, Desnick RJ. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science. 1996;273(5279):1236–8. https://doi.org/10.1126/science.273.5279.1236.
Li B, Wang Y, Fan Y, Ouchi T, Zhao Z, Li L. Cranial suture mesenchymal stem cells: insights and advances. Biomolecules. 2021;11(8):1129. https://doi.org/10.3390/biom11081129.
Chai W, Hao W, Liu J, Han Z, Chang S, Cheng L, et al. Visualizing cathepsin K-Cre expression at the single-cell level with GFP reporters. JBMR Plus. 2023;7(1):e10706. https://doi.org/10.1002/jbm4.10706.
Mijanovic O, Jakovleva A, Brankovic A, Zdravkova K, Pualic M, Belozerskaya TA, et al. Cathepsin K in pathological conditions and new therapeutic and diagnostic perspectives. Int J Mol Sci. 2022;23(22):13762. https://doi.org/10.3390/ijms232213762.
Dai R, Wu Z, Chu HY, Lu J, Lyu A, Liu J, Zhang G. Cathepsin K: the action in and beyond bone. Front Cell Dev Biol. 2020;8:433. https://doi.org/10.3389/fcell.2020.00433.
Bizaoui V, Michot C, Baujat G, Amouroux C, Baron S, Capri Y, et al. Pycnodysostosis: natural history and management guidelines from 27 French cases and a literature review. Clin Genet. 2019;96(4):309–16. https://doi.org/10.1111/cge.13591.
Doherty MA, Langdahl BL, Vogel I, Haagerup A. Clinical and genetic evaluation of Danish patients with pycnodysostosis. Eur J Med Genet. 2021;64(2):104135. https://doi.org/10.1016/j.ejmg.2021.104135.
Sait H, Srivastava P, Gupta N, Kabra M, Kapoor S, Ranganath P, et al. Phenotypic and genotypic spectrum of CTSK variants in a cohort of twenty-five Indian patients with pycnodysostosis. Eur J Med Genet. 2021;64(7):104235. https://doi.org/10.1016/j.ejmg.2021.104235.
Taka TM, Lung B, Stepanyan H, So D, Yang S. Orthopedic treatment of pycnodysostosis: a systematic review. Cureus. 2022;14(4):e24275. https://doi.org/10.7759/cureus.24275.
Omer Sulaiman H, Thalange NKS. Pycnodysostosis: a growth hormone responsive skeletal dysplasia. AACE Clin Case Rep. 2021;7(4):231–5. https://doi.org/10.1016/j.aace.2021.02.006.
Drake MT, Clarke BL, Oursler MJ, Khosla S. Cathepsin K inhibitors for osteoporosis: biology, potential clinical utility, and lessons learned. Endocr Rev. 2017;38(4):325–50. https://doi.org/10.1210/er.2015-1114.
Nishi Y, Atley L, Eyre DE, Edelson JG, Superti-Furga A, Yasuda T, et al. Determination of bone markers in pycnodysostosis: effects of cathepsin K deficiency on bone matrix degradation. J Bone Miner Res. 1999;14(11):1902–8. https://doi.org/10.1359/jbmr.1999.14.11.1902.
Everts V, Delaisse JM, Korper W, Jansen DC, Tigchelaar-Gutter W, Saftig P, Beertsen W. The bone lining cell: its role in cleaning Howship’s lacunae and initiating bone formation. J Bone Miner Res. 2002;17(1):77–90. https://doi.org/10.1359/jbmr.2002.17.1.77.
Garnero P, Ferreras M, Karsdal MA, Nicamhlaoibh R, Risteli J, Borel O, et al. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J Bone Miner Res. 2003;18(5):859–67. https://doi.org/10.1359/jbmr.2003.18.5.859.
Delaisse JM, Andersen TL, Engsig MT, Henriksen K, Troen T, Blavier L. Matrix metalloproteinases (MMP) and cathepsin K contribute differently to osteoclastic activities. Microsc Res Tech. 2003;61(6):504–13. https://doi.org/10.1002/jemt.10374.
Pirapaharan DC, Olesen JB, Andersen TL, Christensen SB, Kjaersgaard-Andersen P, Delaisse JM, Soe K. Catabolic activity of osteoblast lineage cells contributes to osteoclastic bone resorption in vitro. J Cell Sci. 2019;132(10):jcs229351. https://doi.org/10.1242/jcs.229351.
Zhu L, Tang Y, Li XY, Keller ET, Yang J, Cho JS, et al. Osteoclast-mediated bone resorption is controlled by a compensatory network of secreted and membrane-tethered metalloproteinases. Sci Transl Med. 2020;12(529):eaaw6143. https://doi.org/10.1126/scitranslmed.aaw6143.
Jansen IDC, Papapoulos SE, Bravenboer N, de Vries TJ, Appelman-Dijkstra NM. Increased bone resorption during lactation in pycnodysostosis. Int J Mol Sci. 2021;22(4):1810. https://doi.org/10.3390/ijms22041810.
Everts V, Jansen IDC, de Vries TJ. Mechanisms of bone resorption. Bone. 2022;163:116499. https://doi.org/10.1016/j.bone.2022.116499.
Kaur P, Panigrahi I, Kaur H, Singh T, Chaudhry C. Overlapping phenotypes in osteopetrosis and pycnodysostosis in Asian-Indians. Case Rep Genet. 2021;2021:7133508. https://doi.org/10.1155/2021/7133508.
Zhao D, Sun L, Zheng W, Hu J, Zhou B, Wang O, et al. Novel mutation in LRP5 gene cause rare osteosclerosis: cases studies and literature review. Mol Genet Genomics. 2023;298(3):683–92. https://doi.org/10.1007/s00438-023-02008-2.
Whyte MP, McAlister WH, Zhang F, Bijanki VN, Nenninger A, Gottesman GS, et al. New explanation for autosomal dominant high bone mass: mutation of low-density lipoprotein receptor-related protein 6. Bone. 2019;127:228–43. https://doi.org/10.1016/j.bone.2019.05.003.
van Lierop AH, Appelman-Dijkstra NM, Papapoulos SE. Sclerostin deficiency in humans. Bone. 2017;96:51–62. https://doi.org/10.1016/j.bone.2016.10.010.
Whyte MP, Mumm S, Baker JC, Zhang F, Sedighi H, Duan S, Cundy T. LRP6 high bone mass characterized in two generations harboring a unique mutation of low-density lipoprotein receptor-related protein 6. JBMR Plus. 2023;7(4):e10717. https://doi.org/10.1002/jbm4.10717.
van Bezooijen RL, Bronckers AL, Gortzak RA, Hogendoorn PC, van der Wee-Pals L, Balemans W, et al. Sclerostin in mineralized matrices and van Buchem disease. J Dent Res. 2009;88(6):569–74. https://doi.org/10.1177/0022034509338340.
van Lierop AH, Hamdy NA, Hamersma H, van Bezooijen RL, Power J, Loveridge N, Papapoulos SE. Patients with sclerosteosis and disease carriers: human models of the effect of sclerostin on bone turnover. J Bone Miner Res. 2011;26(12):2804–11. https://doi.org/10.1002/jbmr.474.
Roetzer KM, Uyanik G, Brehm A, Zwerina J, Zandieh S, Czech T, et al. Novel familial mutation of LRP5 causing high bone mass: genetic analysis, clinical presentation, and characterization of bone matrix mineralization. Bone. 2018;107:154–60. https://doi.org/10.1016/j.bone.2017.12.002.
Stein SA, Witkop C, Hill S, Fallon MD, Viernstein L, Gucer G, et al. Sclerosteosis: neurogenetic and pathophysiologic analysis of an American kinship. Neurology. 1983;33(3):267–77. https://doi.org/10.1212/wnl.33.3.267.
Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet. 2001;10(5):537–43. https://doi.org/10.1093/hmg/10.5.537.
Hassler N, Roschger A, Gamsjaeger S, Kramer I, Lueger S, van Lierop A, et al. Sclerostin deficiency is linked to altered bone composition. J Bone Miner Res. 2014;29(10):2144–51. https://doi.org/10.1002/jbmr.2259.
Kotwal A, Clarke BL. Melorheostosis: a rare sclerosing bone dysplasia. Curr Osteoporos Rep. 2017;15(4):335–42. https://doi.org/10.1007/s11914-017-0375-y.
Kang H, Jha S, Deng Z, Fratzl-Zelman N, Cabral WA, Ivovic A, et al. Somatic activating mutations in MAP2K1 cause melorheostosis. Nat Commun. 2018;9(1):1390. https://doi.org/10.1038/s41467-018-03720-z.
Jha S, Fratzl-Zelman N, Roschger P, Papadakis GZ, Cowen EW, Kang H, et al. Distinct clinical and pathological features of melorheostosis associated with somatic MAP2K1 mutations. J Bone Miner Res. 2019;34(1):145–56. https://doi.org/10.1002/jbmr.3577.
Jha S, Cowen EW, Lehky TJ, Alter K, Flynn L, Reynolds JC, et al. Clinical evaluation of melorheostosis in the context of a natural history clinical study. JBMR Plus. 2019;3(8):e10214. https://doi.org/10.1002/jbm4.10214.
Kang H, Jha S, Ivovic A, Fratzl-Zelman N, Deng Z, Mitra A, et al. Somatic SMAD3-activating mutations cause melorheostosis by up-regulating the TGF-beta/SMAD pathway. J Exp Med. 2020;217(5):e20191499. https://doi.org/10.1084/jem.20191499.
Fratzl-Zelman N, Roschger P, Kang H, Jha S, Roschger A, Blouin S, et al. Melorheostotic bone lesions caused by somatic mutations in MAP2K1 have deteriorated microarchitecture and periosteal reaction. J Bone Miner Res. 2019;34(5):883–95. https://doi.org/10.1002/jbmr.3656.
Carpenter TO, Shaw NJ, Portale AA, Ward LM, Abrams SA, Pettifor JM. Rickets. Nat Rev Dis Primers. 2017;3:17101. https://doi.org/10.1038/nrdp.2017.101.
Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101(2):394–415. https://doi.org/10.1210/jc.2015-2175.
Cianferotti L. Osteomalacia is not a single disease. Int J Mol Sci. 2022;23(23):14896. https://doi.org/10.3390/ijms232314896.
Haffner D, Leifheit-Nestler M, Grund A, Schnabel D. Rickets guidance: part I-diagnostic workup. Pediatr Nephrol. 2022;37(9):2013–36. https://doi.org/10.1007/s00467-021-05328-w.
•• Fischer PR, Sempos CT, Pettifor JM, Fraser DR, Munns CF, Durazo-Arvizu RA, Thacher TD. Serum 1,25-dihydroxyvitamin D levels in the diagnosis and pathogenesis of nutritional rickets - a multivariable re-analysis of a case-control study. Am J Clin Nutr. 2023;117(5):998–1004. https://doi.org/10.1016/j.ajcnut.2023.02.011. (This study shows the metabolic link between low-calcium intake, rachitic bone lesions, and serum levels of FGF23 and phosphate.)
Thacher TD, Pettifor JM, Tebben PJ, Creo AL, Skrinar A, Mao M, et al. Rickets severity predicts clinical outcomes in children with X-linked hypophosphatemia: utility of the radiographic Rickets Severity Score. Bone. 2019;122:76–81. https://doi.org/10.1016/j.bone.2019.02.010.
Rothenbuhler A, Linglart A. Hypophosphatasia in children and adolescents: clinical features and treatment. Arch Pediatr. 2017;24(5S2):5S66–70. https://doi.org/10.1016/S0929-693X(18)30017-4.
•• Schnitzler CM, Pettifor JM. Calcium deficiency rickets in African adolescents: cortical bone histomorphometry. JBMR Plus. 2019;3(6):e10169. https://doi.org/10.1002/jbm4.10169. (This study demonstrates that at the bone tissue level, calcium deficiency results in a similar histologic picture as in X-linked hypophosphatamia with peri-osteocytic lesions due to osteocytic osteolysis.)
Beck-Nielsen SS, Mughal Z, Haffner D, Nilsson O, Levtchenko E, Ariceta G, et al. FGF23 and its role in X-linked hypophosphatemia-related morbidity. Orphanet J Rare Dis. 2019;14(1):58. https://doi.org/10.1186/s13023-019-1014-8.
Uday S, Manaseki-Holland S, Bowie J, Mughal MZ, Crowe F, Hogler W. The effect of vitamin D supplementation and nutritional intake on skeletal maturity and bone health in socio-economically deprived children. Eur J Nutr. 2021;60(6):3343–53. https://doi.org/10.1007/s00394-021-02511-5.
Uday S, Fratzl-Zelman N, Roschger P, Klaushofer K, Chikermane A, Saraff V, et al. Cardiac, bone and growth plate manifestations in hypocalcemic infants: revealing the hidden body of the vitamin D deficiency iceberg. BMC Pediatr. 2018;18(1):183. https://doi.org/10.1186/s12887-018-1159-y.
Uday S, Hogler W. The burden of vitamin D deficiency in household members of children presenting with symptomatic vitamin D deficiency. Front Endocrinol (Lausanne). 2022;13:958422. https://doi.org/10.3389/fendo.2022.958422.
Taylor SN. Vitamin D in toddlers, preschool children, and adolescents. Ann Nutr Metab. 2020;76(Suppl 2):30–41. https://doi.org/10.1159/000505635.
Millan JL, Whyte MP. Alkaline phosphatase and hypophosphatasia. Calcif Tissue Int. 2016;98(4):398–416. https://doi.org/10.1007/s00223-015-0079-1.
Whyte MP, Leung E, Wilcox WR, Liese J, Argente J, Martos-Moreno GA, et al. Natural history of perinatal and infantile hypophosphatasia: a retrospective study. J Pediatr. 2019;209:116–214. https://doi.org/10.1016/j.jpeds.2019.01.049.
• Rolvien T, Schmidt T, Schmidt FN, von Kroge S, Busse B, Amling M, Barvencik F. Recovery of bone mineralization and quality during asfotase alfa treatment in an adult patient with infantile-onset hypophosphatasia. Bone. 2019;127:67–74. https://doi.org/10.1016/j.bone.2019.05.036. (This study shows the direct effect from asfotase alfa on osteomalacia in a bone biopsy sample in a patient with hypophosphatasia.)
Whyte MP, Simmons JH, Moseley S, Fujita KP, Bishop N, Salman NJ, et al. Asfotase alfa for infants and young children with hypophosphatasia: 7 year outcomes of a single-arm, open-label, phase 2 extension trial. Lancet Diabetes Endocrinol. 2019;7(2):93–105. https://doi.org/10.1016/S2213-8587(18)30307-3.
Mannes I, Rothenbuhler A, Merzoug V, Di Rocco F, Linglart A, Adamsbaum C. Imaging patterns in pediatric hypophosphatasia. Pediatr Radiol. 2022;52(5):998–1006. https://doi.org/10.1007/s00247-021-05232-3.
Ward LM, Glorieux FH, Whyte MP, Munns CF, Portale AA, Hogler W, et al. Effect of burosumab compared with conventional therapy on younger vs older children with X-linked hypophosphatemia. J Clin Endocrinol Metab. 2022;107(8):e3241–53. https://doi.org/10.1210/clinem/dgac296.
Ewert A, Rehberg M, Schlingmann KP, Hiort O, John-Kroegel U, Metzing O, et al. Effects of burosumab treatment on mineral metabolism in children and adolescents with X-linked hypophosphatemia. J Clin Endocrinol Metab. 2023. https://doi.org/10.1210/clinem/dgad223.
Kishnani PS, Rockman-Greenberg C, Rauch F, Bhatti MT, Moseley S, Denker AE, et al. Five-year efficacy and safety of asfotase alfa therapy for adults and adolescents with hypophosphatasia. Bone. 2019;121:149–62. https://doi.org/10.1016/j.bone.2018.12.011.
Agoro R, White KE. Regulation of FGF23 production and phosphate metabolism by bone-kidney interactions. Nat Rev Nephrol. 2023;19(3):185–93. https://doi.org/10.1038/s41581-022-00665-x.
• Meaux MN, Alioli C, Linglart A, Lemoine S, Vignot E, Bertholet-Thomas A, et al. X-linked hypophosphatemia, not only a skeletal disease but also a chronic inflammatory state. J Clin Endocrinol Metab. 2022;107(12):3275–86. https://doi.org/10.1210/clinem/dgac543. (Although no data are given on bone material properties, this study demonstrates that X-linked hypophosphatemia is a systemic disorder and not solely a defect of bone mineralization. Thus, bone tissue abnormalities should in future also be considered as potentially resulting from chronic inflammation.)
Buss DJ, Reznikov N, McKee MD. Crossfibrillar mineral tessellation in normal and Hyp mouse bone as revealed by 3D FIB-SEM microscopy. J Struct Biol. 2020;212(2):107603. https://doi.org/10.1016/j.jsb.2020.107603.
Beck-Nielsen SS, Brixen K, Gram J, Molgaard C. High bone mineral apparent density in children with X-linked hypophosphatemia. Osteoporos Int. 2013;24(8):2215–21. https://doi.org/10.1007/s00198-013-2286-9.
Cheung M, Roschger P, Klaushofer K, Veilleux LN, Roughley P, Glorieux FH, Rauch F. Cortical and trabecular bone density in X-linked hypophosphatemic rickets. J Clin Endocrinol Metab. 2013;98(5):E954–61. https://doi.org/10.1210/jc.2012-4133.
Robinson ME, AlQuorain H, Murshed M, Rauch F. Mineralized tissues in hypophosphatemic rickets. Pediatr Nephrol. 2020;35(10):1843–54. https://doi.org/10.1007/s00467-019-04290-y.
Nguyen-Khac V, Bonnet-Lebrun A, Skalli W, Adamsbaum C, Linglart A, Wicart P. Changes in adipose bone marrow and bone morphology in X-linked hypophosphatemic rickets. Orthop Traumatol Surg Res. 2023;109(3):103529. https://doi.org/10.1016/j.otsr.2022.103529.
Fratzl-Zelman N, Gamsjaeger S, Blouin S, Kocijan R, Plasenzotti P, Rokidi S, et al. Alterations of bone material properties in adult patients with X-linked hypophosphatemia (XLH). J Struct Biol. 2020;211(3):107556. https://doi.org/10.1016/j.jsb.2020.107556.
Fratzl-Zelman N, Hartmann MA, Gamsjaeger S, Rokidi S, Paschalis EP, Blouin S, Zwerina J. Bone matrix mineralization and response to burosumab in adult patients with X-linked hypophosphatemia: results from the phase 3, single-arm international trial. J Bone Miner Res. 2022;37(9):1665–78. https://doi.org/10.1002/jbmr.4641.
Marie PJ, Glorieux FH. Histomorphometric study of bone remodeling in hypophosphatemic vitamin D-resistant rickets. Metab Bone Dis Relat Res. 1981;3(1):31–8.
Marie PJ, Glorieux FH. Relation between hypomineralized periosteocytic lesions and bone mineralization in vitamin D-resistant rickets. Calcif Tissue Int. 1983;35(4–5):443–8.
Boukpessi T, Hoac B, Coyac BR, Leger T, Garcia C, Wicart P, et al. Osteopontin and the dento-osseous pathobiology of X-linked hypophosphatemia. Bone. 2017;95:151–61. https://doi.org/10.1016/j.bone.2016.11.019.
Prentice A, Ceesay M, Nigdikar S, Allen SJ, Pettifor JM. FGF23 is elevated in Gambian children with rickets. Bone. 2008;42(4):788–97. https://doi.org/10.1016/j.bone.2007.11.014.
Murali SK, Andrukhova O, Clinkenbeard EL, White KE, Erben RG. Excessive osteocytic Fgf23 secretion contributes to pyrophosphate accumulation and mineralization defect in Hyp mice. PLoS Biol. 2016;14(4):e1002427. https://doi.org/10.1371/journal.pbio.1002427.
Teti A, Zallone A. Do osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisited. Bone. 2009;44(1):11–6. https://doi.org/10.1016/j.bone.2008.09.017.
Yuan Y, Jagga S, Martins JS, Rana R, Pajevic PD, Liu ES. Impaired 1,25 dihydroxyvitamin D3 action and hypophosphatemia underlie the altered lacuno-canalicular remodeling observed in the Hyp mouse model of XLH. PLoS One. 2021;16(5):e0252348. https://doi.org/10.1371/journal.pone.0252348.
Insogna KL, Rauch F, Kamenicky P, Ito N, Kubota T, Nakamura A, et al. Burosumab improved histomorphometric measures of osteomalacia in adults with X-linked hypophosphatemia: a phase 3, single-arm, international trial. J Bone Miner Res. 2019;34(12):2183–91. https://doi.org/10.1002/jbmr.3843.
Fratzl-Zelman N, Wesseling-Perry K, Makitie RE, Blouin S, Hartmann MA, Zwerina J, et al. Bone material properties and response to teriparatide in osteoporosis due to WNT1 and PLS3 mutations. Bone. 2021;146:115900. https://doi.org/10.1016/j.bone.2021.115900.
Acknowledgements
This work was supported by the Austrian Social Health Insurance Fund (OEGK), the Austrian Workers’ Compensation Board (AUVA), and the Vienna Bone and Growth Center (VBGC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
AR received research funding and non-related honoraria for consultancy and scientific presentations from Kyowa Kirin. BMM, PF, and NFZ received no funding and declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raimann, A., Misof, B.M., Fratzl, P. et al. Bone Material Properties in Bone Diseases Affecting Children. Curr Osteoporos Rep 21, 787–805 (2023). https://doi.org/10.1007/s11914-023-00822-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-023-00822-6