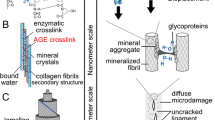
Abstract
Purpose of the review
Bone’s ability to withstand load resisting fracture and adapting to it highly depends on the quality of its matrix and its regulators. This review focuses on the contribution of bone quality to fracture resistance and possible therapeutic targets for skeletal fragility in aging and disease.
Recent findings
The highly organized, hierarchical composite structure of bone extracellular matrix together with its (re)modeling mechanisms and microdamage dynamics determines its stiffness, strength, and toughness. Aging and disease affect the biological processes regulating bone quality, thus resulting in defective extracellular matrix and bone fragility. Targeted therapies are being developed to restore bone’s mechanical integrity. However, their current limitations include low tissue selectivity and adverse side effects.
Summary
Biological and mechanical insights into the mechanisms controlling bone quality, together with advances in drug delivery and studies in animal models, will accelerate the development and translation to clinical application of effective targeted-therapeutics for bone fragility.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Carriero A, Javaheri B, Bassir Kazeruni N, Pitsillides A, Shefelbine S. Age and sex differences in load-induced tibial cortical bone surface strain maps: JBMR Plus; 2021. This article quantifies how the mechanical environment on bone varies with age and sex in C57BL6 mice.
• Ritchie RO. How does human bone resist fracture? Ann N Y Acad Sci. 2010;1192:72.
Skedros JG, Keenan KE, Williams TJ, Kiser CJ. Secondary osteon size and collagen/lamellar organization ("osteon morphotypes") are not coupled, but potentially adapt independently for local strain mode or magnitude. J Struct Biol. 2013;181(2):95–107.
Yeni YN, Brown CU, Wang Z, Norman TL. The influence of bone morphology on fracture toughness of the human femur and tibia. Bone. 1997;21(5):453–9.
Carriero A, Zimmermann EA, Paluszny A, Tang SY, Bale H, Busse B, et al. How tough is brittle bone? Investigating osteogenesis imperfecta in mouse bone. J Bone Miner Res. 2014;29(6):1392–401.
Carriero A, Doube M, Vogt M, Busse B, Zustin J, Levchuk A, et al. Altered lacunar and vascular porosity in osteogenesis imperfecta mouse bone as revealed by synchrotron tomography contributes to bone fragility. Bone. 2014;61:116–24.
Jameson JR, Albert CI, Busse B, Smith PA, Harris GF. 3D micron-scale imaging of the cortical bone canal network in human osteogenesis imperfecta (OI). Medical imaging 2013: Biomedical applications in molecular, structural, and functional imaging; 2013: International Society for Optics and Photonics.
Zimmermann EA, Busse B, Ritchie RO. The fracture mechanics of human bone: influence of disease and treatment. BoneKEy Rep. 2015;4.
Ferguson VL, Ayers RA, Bateman TA, Simske SJ. Bone development and age-related bone loss in male C57BL/6J mice. Bone. 2003;33(3):387–98.
Creecy A, Uppuganti S, Girard MR, Schlunk SG, Amah C, Granke M, et al. The age-related decrease in material properties of BALB/c mouse long bones involves alterations to the extracellular matrix. Bone. 2020;130:115126.
Razi H, Predan J, Fischer FD, Kolednik O, Fratzl P. Damage tolerance of lamellar bone. Bone. 2020;130:115102.
Reznikov N, Shahar R, Weiner S. Three-dimensional structure of human lamellar bone: The presence of two different materials and new insights into the hierarchical organization. Bone. 2014;59:93–104.
• Grandfield K, Vuong V, Schwarcz HP. Ultrastructure of bone: hierarchical features from nanometer to micrometer scale revealed in focused ion beam sections in the TEM. Calcif Tissue Int. 2018;103(6):606–16. This article demonstrates the presence of curved mineral lamellae and presents a better insight into how bone is organized at the nanometer scale.
Carriero A, Zimmermann EA, Shefelbine SJ, Ritchie RO. A methodology for the investigation of toughness and crack propagation in mouse bone. J Mech Behav Biomed Mater. 2014;39:38–47.
• Docaj A, Jeong MS, Zimmermann EA, Ritchie RO, Carriero A. Age effect on bone toughness in osteogenesis imperfecta. Trans Ann Meet Orthopaedic Res Soc; 2020. This conference proceeding abstract presents changes in bone fracture toughness during skeletal growth in healthy and osteogenesis imperfecta mice.
Granke M, Makowski AJ, Uppuganti S, Nyman JS. Prevalent role of porosity and osteonal area over mineralization heterogeneity in the fracture toughness of human cortical bone. J Biomech. 2016;49(13):2748–55.
Shapiro F, Maguire K, Swami S, Zhu H, Flynn E, Wang J, et al. Histopathology of osteogenesis imperfecta bone. Supramolecular assessment of cells and matrices in the context of woven and lamellar bone formation using light, polarization and ultrastructural microscopy. Bone Rep. 2021;14:100734.
Docaj A, Carriero A. Site-specific changes in collagen orientation in osteogenesis imperfecta mouse bone. Eur Soc Biomechan. 2021.
Heveran CM, Schurman CA, Acevedo C, Livingston EW, Howe D, Schaible EG, et al. Chronic kidney disease and aging differentially diminish bone material and microarchitecture in C57Bl/6 mice. Bone. 2019;127:91–103.
van Hove RP, Nolte PA, Vatsa A, Semeins CM, Salmon PL, Smit TH, et al. Osteocyte morphology in human tibiae of different bone pathologies with different bone mineral density — Is there a role for mechanosensing? Bone. 2009;45(2):321–9.
De Paolis A, Jeong S, Cardoso L, Carriero A. Lacuna morphology affects strains in the cell body and dendrites, and on the bone tissue. Eur Soc Biomechan. 2019.
Ebacher V, Guy P, Oxland TR, Wang R. Sub-lamellar microcracking and roles of canaliculi in human cortical bone. Acta Biomater. 2012;8(3):1093–100.
Poundarik AA, Diab T, Sroga GE, Ural A, Boskey AL, Gundberg CM, et al. Dilatational band formation in bone. Proc Natl Acad Sci U S A. 2012;109(47):19178–83.
Schwarcz H, Binkley D, Luo L, Grandfield K. A search for apatite crystals in the gap zone of collagen fibrils in bone using dark-field illumination. Bone. 2020;135:115304.
Maghsoudi-Ganjeh M, Samuel J, Ahsan AS, Wang X, Zeng X. Intrafibrillar mineralization deficiency and osteogenesis imperfecta mouse bone fragility. J Mechan Behav Biomed Mater. 2021:104377. This article demonstrates a decrease in intrafibrillar mineralization in the osteogenesis imperfecta murine model, and suggest this as one of the causes of its reduced toughness.
Lin L, Samuel J, Zeng X, Wang X. Contribution of extrafibrillar matrix to the mechanical behavior of bone using a novel cohesive finite element model. J Mech Behav Biomed Mater. 2017;65:224–35.
Wang Y, Ural A. A finite element study evaluating the influence of mineralization distribution and content on the tensile mechanical response of mineralized collagen fibril networks. J Mech Behav Biomed Mater. 2019;100:103361.
Nair AK, Gautieri A, Buehler MJ. Role of intrafibrillar collagen mineralization in defining the compressive properties of nascent bone. Biomacromolecules. 2014;15(7):2494–500.
Granke M, Does MD, Nyman JS. The role of water compartments in the material properties of cortical bone. Calcif Tissue Int. 2015;97(3):292–307.
Maghsoudi-Ganjeh M, Wang X, Zeng X. Computational investigation of the effect of water on the nanomechanical behavior of bone. J Mech Behav Biomed Mater. 2020;101:103454.
• Von Euw S, Chan-Chang T-H-C, Paquis C, Haye B, Pehau-Arnaudet G, Babonneau F, et al. Organization of bone mineral: the role of mineral-water interactions. Geosciences. 2018;8:466. This article demonstrates that structural water contained in apatite serve the mineral particles to organize themselves and increase mineralization.
Qin Z, Gautieri A, Nair AK, Inbar H, Buehler MJ. Thickness of hydroxyapatite nanocrystal controls mechanical properties of the collagen–hydroxyapatite interface. Langmuir. 2012;28(4):1982–92.
Shahar R, Weiner S. Open questions on the 3D structures of collagen containing vertebrate mineralized tissues: A perspective. J Struct Biol. 2018;201(3):187–98.
• Xi L, De Falco P, Barbieri E, Karunaratne A, Bentley L, Esapa C, et al. Bone matrix development in steroid-induced osteoporosis is associated with a consistently reduced fibrillar stiffness linked to altered bone mineral quality. Acta Biomater. 2018;76:295–307. This article demonstrates that mineralized fibrils exhibit strain-rate dependent stiffness in healthy mouse bones but not in steroid-induced osteoporotic bone having small mineral platelets.
Zimmermann EA, Gludovatz B, Schaible E, Busse B, Ritchie RO. Fracture resistance of human cortical bone across multiple length-scales at physiological strain rates. Biomaterials. 2014;35(21):5472–81.
Xu M, An B. An analysis of fracture in staggered mineralized collagen fibril arrays. Int J Solids Struct. 2020;193–194:535–49.
Unal M, Akkus O. Raman spectral classification of mineral- and collagen-bound water's associations to elastic and post-yield mechanical properties of cortical bone. Bone. 2015;81:315–26.
• Wang X, Hua R, Ahsan A, Ni Q, Huang Y, Gu S, et al. Age-related deterioration of bone toughness is related to diminishing amount of matrix glycosaminoglycans (Gags). JBMR Plus. 2018;2(3):164–73. This article demonstrates that loss of glycosaminoglycans and the subsequent loss of bound water are one of the origins of age-related deterioration of bone quality.
Fielder M, Nair AK. Effects of hydration and mineralization on the deformation mechanisms of collagen fibrils in bone at the nanoscale. Biomech Model Mechanobiol. 2019;18(1):57–68.
McNerny EM, Gong B, Morris MD, Kohn DH. Bone fracture toughness and strength correlate with collagen cross-link maturity in a dose-controlled lathyrism mouse model. J Bone Miner Res. 2015;30(3):455–64.
Gauthier R, Follet H, Langer M, Gineyts E, Rongiéras F, Peyrin F, et al. Relationships between human cortical bone toughness and collagen cross-links on paired anatomical locations. Bone. 2018;112:202–11.
Hunt HB, Pearl JC, Diaz DR, King KB, Donnelly E. Bone tissue collagen maturity and mineral content increase with sustained hyperglycemia in the KK-Ay murine model of type 2 diabetes. J Bone Miner Res. 2018;33(5):921–9.
Taga Y, Kusubata M, Ogawa-Goto K, Hattori S. Site-specific quantitative analysis of overglycosylation of collagen in osteogenesis imperfecta using hydrazide chemistry and SILAC. J Proteome Res. 2013;12(5):2225–32.
Terajima M, Perdivara I, Sricholpech M, Deguchi Y, Pleshko N, Tomer KB, et al. Glycosylation and cross-linking in bone type i collagen. J Biol Chem. 2014;289(33):22636–47.
Poundarik AA, Wu PC, Evis Z, Sroga GE, Ural A, Rubin M, et al. A direct role of collagen glycation in bone fracture. J Mech Behav Biomed Mater. 2015;52:120–30.
• Hudson DM, Archer M, King KB, Eyre DR. Glycation of type I collagen selectively targets the same helical domain lysine sites as lysyl oxidase–mediated cross-linking. J Biol Chem. 2018;293(40):15620–7. This article demonstrates that glycation products and enzymatic cross-link precursors target the same amino acids in the triple helical region of tropocollagen molecules.
Allen MR, Newman CL, Chen N, Granke M, Nyman JS, Moe SM. Changes in skeletal collagen cross-links and matrix hydration in high- and low-turnover chronic kidney disease. Osteoporos Int. 2015;26(3):977–85.
Gautieri A, Passini FS, Silván U, Guizar-Sicairos M, Carimati G, Volpi P, et al. Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue. Matrix Biol. 2017;59:95–108.
• Chen NX, Srinivasan S, O'Neill K, Nickolas TL, Wallace JM, Allen MR, et al. Effect of Advanced Glycation End-Products (AGE) Lowering drug ALT-711 on biochemical, vascular, and bone parameters in a rat model of CKD-MBD. J Bone Miner Res. 2020;35(3):608–17. This article demonstrates that alagebrium administration decreases total AGE cross-link levels, yet the mechanical properties are not normalized in the Cy/+ rat model of chronic kidney disease.
• Allen MR, Wallace J, McNerney E, Nyman J, Avin K, Chen N, et al. N-acetylcysteine (NAC), an anti-oxidant, does not improve bone mechanical properties in a rat model of progressive chronic kidney disease-mineral bone disorder. PLoS One. 2020;15(3):e0230379 This article demonstrates that N-acetylcysteine administration reduces TBARS oxidative stress markers and AGE cross-link levels in the Cy/+ rat model of chronic kidney disease.
Abar O, Dharmar S, Tang SY. The effect of aminoguanidine (AG) and pyridoxamine (PM) on ageing human cortical bone. Bone Joint Res. 2018;7(1):105–10.
Thomas CJ, Cleland TP, Sroga GE, Vashishth D. Accumulation of carboxymethyl-lysine (CML) in human cortical bone. Bone. 2018;110:128–33. This article demonstrates that carboxymethyl-lysine is a non-cross-linking AGE that is abundant in bone and impairs bone mechanical properties.
• Creecy A, Brown KL, Rose KL, Voziyan P, Nyman JS. Post-translational modifications in collagen type I of bone in a mouse model of aging. Bone. 2021;143:115763. This article demonstrates how aging affects post-translational modifications in bone, with particular emphasis on the deamidation of specific residues, and how this relates to bone fracture toughness.
• Morsali R, Dai Z, Wang Y, Qian D, Minary-Jolandan M. Deformation mechanisms of "two-part" natural adhesive in bone interfibrillar nano-interfaces. ACS Biomater Sci Eng. 2019;5(11):5916–24. This article presents new insights into the deformation mechanism between OC and OPN at the interfibrillar interface in bone.
Tavakol M, Vaughan TJ. The structural role of osteocalcin in bone biomechanics and its alteration in type-2 diabetes. Sci Rep. 2020;10(1):17321.
Wang Z, Vashishth D, Picu RC. Bone toughening through stress-induced non-collagenous protein denaturation. Biomech Model Mechanobiol. 2018;17(4):1093–106.
Thurner PJ, Chen CG, Ionova-Martin S, Sun L, Harman A, Porter A, et al. Osteopontin deficiency increases bone fragility but preserves bone mass. Bone. 2010;46(6):1564–73.
Hua R, Ni Q, Eliason TD, Han Y, Gu S, Nicolella DP, et al. Biglycan and chondroitin sulfate play pivotal roles in bone toughness via retaining bound water in bone mineral matrix. Matrix Biol. 2020;94:95–109.
• Sroga GE, Vashishth D. Phosphorylation of extracellular bone matrix proteins and its contribution to bone fragility. J Bone Miner Res. 2018;33(12):2214–29. This article demonstrates that the total phosphorylation of bone matrix proteins decreases with age, with a significant impact on fracture toughness.
Kläning E, Christensen B, Sørensen ES, Vorup-Jensen T, Jensen JK. Osteopontin binds multiple calcium ions with high affinity and independently of phosphorylation status. Bone. 2014;66:90–5.
Morgan S, Poundarik AA, Vashishth D. Do Non-collagenous Proteins Affect Skeletal Mechanical Properties? Calcif Tissue Int. 2015;97(3):281–91.
Depalle B, McGilvery CM, Nobakhti S, Aldegaither N, Shefelbine SJ, Porter AE. Osteopontin regulates type I collagen fibril formation in bone tissue. Acta Biomater. 2021;120:194–202.
Kram V, Shainer R, Jani P, Meester JAN, Loeys B, Young MF. Biglycan in the Skeleton. J Histochem Cytochem. 2020;68(11):747–62.
Zheng HX, Chen J, Zu YX, Wang EZ, Qi SS. Chondroitin sulfate prevents STZ Induced diabetic osteoporosis through decreasing blood glucose, antioxidative stress, anti-inflammation and OPG/RANKL expression regulation. Int J Mol Sci. 2020;21(15).
Boskey AL, Imbert L. Bone quality changes associated with aging and disease: a review. Ann N Y Acad Sci. 2017;1410(1):93–106.
Busse B, Djonic D, Milovanovic P, Hahn M, Püschel K, Ritchie RO, et al. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell. 2010;9(6):1065–75.
Adele B. Bone mineral crystal size. Osteoporos Int. 2003;14(5):16–21.
Burr DB. Changes in bone matrix properties with aging. Bone. 2019;120:85–93.
Aido M, Kerschnitzki M, Hoerth R, Checa S, Spevak L, Boskey AL, et al. Effect of in vivo loading on bone composition varies with animal age. Exp Gerontol. 2015;63:48–58.
McCreadie BR, Morris MD, Chen T-C, Sudhaker Rao D, Finney WF, Widjaja E, et al. Bone tissue compositional differences in women with and without osteoporotic fracture. Bone. 2006;39(6):1190–5.
Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21(2):195–214.
Zimmermann EA, Schaible E, Gludovatz B, Schmidt FN, Riedel C, Krause M, et al. Intrinsic mechanical behavior of femoral cortical bone in young, osteoporotic and bisphosphonate-treated individuals in low- and high energy fracture conditions. Sci Rep. 2016;6(1):21072.
Brennan O, Kuliwaba JS, Lee TC, Parkinson IH, Fazzalari NL, McNamara LM, et al. Temporal changes in bone composition, architecture, and strength following estrogen deficiency in osteoporosis. Calcif Tissue Int. 2012;91(6):440–9.
Turunen MJ, Kaspersen JD, Olsson U, Guizar-Sicairos M, Bech M, Schaff F, et al. Bone mineral crystal size and organization vary across mature rat bone cortex. J Struct Biol. 2016;195(3):337–44.
Comelekoglu U, Bagis S, Yalin S, Ogenler O, Yildiz A, Sahin NO, et al. Biomechanical evaluation in osteoporosis: ovariectomized rat model. Clin Rheumatol. 2007;26(3):380–4.
Mathavan N, Turunen MJ, Tägil M, Isaksson H. Characterising bone material composition and structure in the ovariectomized (OVX) rat model of osteoporosis. Calcif Tissue Int. 2015;97(2):134–44.
Peng Z, Tuukkanen J, Zhang H, Jämsä T, Väänänen H. The mechanical strength of bone in different rat models of experimental osteoporosis. Bone. 1994;15(5):523–32.
Morello R. Osteogenesis imperfecta and therapeutics. Matrix Biol. 2018;71:294–312.
Imbert L, Aurégan J-C, Pernelle K, Hoc T. Microstructure and compressive mechanical properties of cortical bone in children with osteogenesis imperfecta treated with bisphosphonates compared with healthy children. J MechanBehav Biomed Mater. 2015;46:261–70.
Vardakastani V, Saletti D, Skalli W, Marry P, Allain J-M, Adam C. Increased intra-cortical porosity reduces bone stiffness and strength in pediatric patients with osteogenesis imperfecta. Bone. 2014;69:61–7.
Wronski TJ, Dann LM, Scott KS, Cintrón M. Long-term effects of ovariectomy and aging on the rat skeleton. Calcif Tissue Int. 1989;45(6):360–6.
Enderli TA, Burtch SR, Templet JN, Carriero A. Animal models of osteogenesis imperfecta: applications in clinical research. Orthop Res Rev. 2016;8:41–55.
Busse B, Bale HA, Zimmermann EA, Panganiban B, Barth HD, Carriero A, et al. Vitamin D deficiency induces early signs of aging in human bone, increasing the risk of fracture. Sci Transl Med. 2013;5(193):193ra88–8.
Zimmermann EA, Köhne T, Bale HA, Panganiban B, Gludovatz B, Zustin J, et al. Modifications to nano-and microstructural quality and the effects on mechanical integrity in Paget's disease of bone. J Bone Miner Res. 2015;30(2):264–73.
Seitz S, Priemel M, Zustin J, Beil FT, Semler J, Minne H, et al. Paget's disease of bone: histologic analysis of 754 patients. J Bone Miner Res. 2009;24(1):62–9.
Lekkala S, Taylor EA, Hunt HB, Donnelly E. Effects of diabetes on bone material properties. Curr Osteoporos Rep. 2019;17(6):455–64.
Hunt HB, Miller NA, Hemmerling KJ, Koga M, Lopez KA, Taylor EA, et al. Bone tissue composition in postmenopausal women varies with glycemic control from normal glucose tolerance to type 2 diabetes mellitus. J Bone Miner Res. 2020.
•• Daniele G, Winnier D, Mari A, Bruder J, Fourcaudot M, Pengou Z, et al. The potential role of the osteopontin–osteocalcin–osteoprotegerin triad in the pathogenesis of prediabetes in humans. Acta Diabetol. 2018;55(2):139–48 This article demonstrates that hormones playing a role in bone remodeling also affect glucose metabolism.
Creecy A, Uppuganti S, Merkel AR, O’Neal D, Makowski AJ, Granke M, et al. Changes in the fracture resistance of bone with the progression of type 2 diabetes in the ZDSD rat. Calcif Tissue Int. 2016;99(3):289–301.
Cardoso L, Herman BC, Verborgt O, Laudier D, Majeska RJ, Schaffler MB. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res. 2009;24(4):597–605.
Tami AE, Nasser P, Verborgt O, Schaffler MB, Tate MLK. The role of interstitial fluid flow in the remodeling response to fatigue loading. J Bone Miner Res. 2002;17(11):2030–7.
•• McDonald MM, Khoo WH, Ng PY, Xiao Y, Zamerli J, Thatcher P, et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell. 2021;184(5):1330–1347.e13 This article demonstrates the existence of osteomorphs, new bone cells that originate trough osteoclasts fission and capable to fuse back into osteoclasts for bone repair purposes.
•• Shi C, Wu T, He Y, Zhang Y, Fu D. Recent advances in bone-targeted therapy. Pharmacol Ther. 2020;207:107473 This review presents bone-targeting moieties and strategies for the development of novel therapies applicable to bone-related pathologies.
Bessueille L, Briolay A, Como J, Mebarek S, Mansouri C, Gleizes M, et al. Tissue-nonspecific alkaline phosphatase is an anti-inflammatory nucleotidase. Bone. 2020;133:115262.
Alliston T. Biological regulation of bone quality. Curr Osteoporos Rep. 2014;12(3):366–75.
Vashishth D, Verborgt O, Divine G, Schaffler M, Fyhrie DP. Decline in osteocyte lacunar density in human cortical bone is associated with accumulation of microcracks with age. Bone. 2000;26(4):375–80.
Seref-Ferlengez Z, Kennedy OD, Schaffler MB. Bone microdamage, remodeling and bone fragility: how much damage is too much damage? BoneKEy Rep. 2015;4.
•• Alvandi LM, Chen D, Majeska RJ, Florencio-Silva R, Seref-Ferlengez Z, Schaffler MB. Mineral deposition is required to repair diffuse damage in bone in vivo. Amer Soc Bone Mineral Res; 2019. This conference proceeding abstract demonstrates new insights into the process of spontaneous repair of diffuse damage through a physicochemical remineralization process without the need of osteocytes activity.
Tsourdi E, Jähn K, Rauner M, Busse B, Bonewald LF. Physiological and pathological osteocytic osteolysis. J Musculoskelet Neuronal Interact. 2018;18(3):292.
Poulet B, Liu K, Plumb D, Vo P, Shah M, Staines K, et al. Overexpression of TIMP-3 in chondrocytes produces transient reduction in growth plate length but permanently reduces adult bone quality and quantity. PLoS One. 2016;11(12):e0167971.
Miller B, Spevak L, Lukashova L, Javaheri B, Pitsillides AA, Boskey A, et al. Altered bone mechanics, architecture and composition in the skeleton of TIMP-3-deficient mice. Calcif Tissue Int. 2017;100(6):631–40.
Kaya S, Basta-Pljakic J, Seref-Ferlengez Z, Majeska RJ, Cardoso L, Bromage TG, et al. Lactation-induced changes in the volume of osteocyte lacunar-canalicular space alter mechanical properties in cortical bone tissue. J Bone Miner Res. 2017;32(4):688–97.
Jáuregui EJ, Akil O, Acevedo C, Hall-Glenn F, Tsai BS, Bale HA, et al. Parallel mechanisms suppress cochlear bone remodeling to protect hearing. Bone. 2016;89:7–15.
• De Paolis A, Miller BJ, Doube M, Bodey AJ, Rau C, Richter C-P, et al. Increased cochlear otic capsule thickness and intracortical canal porosity in the oim mouse model of osteogenesis imperfecta. J Struct Biol. 2021:107708. This article quantifies the morphological changes of the otic capsule in mice with osteogenesis imperfecta, and how it might be involved in their hearing loss.
Brister EY, Vasi Z, Antipova O, Robinson A, Tan X, Agarwal A, et al. X-ray fluorescence microscopy: a method of measuring ion concentrations in the ear. Hear Res. 2020;391:107948.
Morikawa M, Derynck R, Miyazono K. TGF-β and the TGF-β family: context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol. 2016;8(5):a021873.
Zhao Y-p, Tian Q-y, Frenkel S, Liu C-j. The promotion of bone healing by progranulin, a downstream molecule of BMP-2, through interacting with TNF/TNFR signaling. Biomaterials. 2013;34(27):6412–21.
Eguchi K, Akiba Y, Akiba N, Nagasawa M, Cooper LF, Uoshima K. Insulin-like growth factor binding Protein-3 suppresses osteoblast differentiation via bone morphogenetic protein-2. Biochem Biophys Res Commun. 2018;507(1):465–70.
Turner C, Garetto L, Dunipace A, Zhang W, Wilson M, Grynpas M, et al. Fluoride treatment increased serum IGF-1, bone turnover, and bone mass, but not bone strength, in rabbits. Calcif Tissue Int. 1997;61(1):77–83.
Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, et al. Recombinant human leptin in women with hypothalamic amenorrhea. New Engl J Med. 2004;351(10):987–97.
Kawai M, Rosen CJ. The insulin-like growth factor system in bone: basic and clinical implications. Endocrinol Metab Clin N Am. 2012;41(2):323–vi.
Tang S, Alliston T. Regulation of postnatal bone homeostasis by TGFbeta. Bonekey Rep. 2013;2:255.
Balooch G, Balooch M, Nalla RK, Schilling S, Filvaroff EH, Marshall GW, et al. TGF-β regulates the mechanical properties and composition of bone matrix. ProcNat Acad Sci. 2005;102(52):18813–8.
Eckardt H, Bundgaard KG, Christensen KS, Lind M, Hansen ES, Hvid I. Effects of locally applied vascular endothelial growth factor (VEGF) and VEGF-inhibitor to the rabbit tibia during distraction osteogenesis. J Orthop Res. 2003;21(2):335–40.
Chindamo G, Sapino S, Peira E, Chirio D, Gonzalez MC, Gallarate M. Bone diseases: current approach and future perspectives in drug delivery systems for bone targeted therapeutics. Nanomaterials. 2020;10(5):875.
Paiva KBS, Granjeiro JM. Matrix Metalloproteinases in Bone Resorption, Remodeling, and Repair. Prog Mol Biol Transl Sci. 2017;148:203–303.
Shay G, Tauro M, Loiodice F, Tortorella P, Sullivan DM, Hazlehurst LA, et al. Selective inhibition of matrix metalloproteinase-2 in the multiple myeloma-bone microenvironment. Oncotarget. 2017;8(26):41827.
Nyman JS, Lynch CC, Perrien DS, Thiolloy S, O'Quinn EC, Patil CA, et al. Differential effects between the loss of MMP-2 and MMP-9 on structural and tissue-level properties of bone. J Bone Miner Res. 2011;26(6):1252–60.
Tokuhara CK, Santesso MR, Oliveira GSN, Ventura TMDS, Doyama JT, Zambuzzi WF, et al. Updating the role of matrix metalloproteinases in mineralized tissue and related diseases. J Appl Oral Sci. 2019;27:e20180596.
Paiva KB, Granjeiro JM. Bone tissue remodeling and development: focus on matrix metalloproteinase functions. Arch Biochem Biophys. 2014;561:74–87.
Tang SY, Herber RP, Ho SP, Alliston T. Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. J Bone Miner Res. 2012;27(9):1936–50.
Lin T-H, Pajarinen J, Lu L, Nabeshima A, Cordova L, Yao Z, et al. NF-κB as a therapeutic target in inflammatory-associated bone diseases. Adv Protein Chem Struct Biol. 2017;107:117–54.
D'Oronzo S, Coleman R, Brown J, Silvestris F. Metastatic bone disease: Pathogenesis and therapeutic options: Up-date on bone metastasis management. J Bone Oncol. 2019;15:004–4.
Polyzos SA, Makras P, Tournis S, Anastasilakis AD. Off-label uses of denosumab in metabolic bone diseases. Bone. 2019;129:115048.
Jähn-Rickert K, Wölfel EM, Jobke B, Riedel C, Hellmich M, Werner M, et al. Elevated bone hardness under denosumab treatment, with persisting lower osteocyte viability during discontinuation. Front Endocrinol (Lausanne). 2020;11:250.
Plotkin LI, Bellido T. Osteocytic signalling pathways as therapeutic targets for bone fragility. Nat Rev Endocrinol. 2016;12(10):593–605.
Wang X, Yamauchi K, Mitsunaga T. A review on osteoclast diseases and osteoclastogenesis inhibitors recently developed from natural resources. Fitoterapia. 2020;142:104482.
Gourion-Arsiquaud S, Allen MR, Burr DB, Vashishth D, Tang SY, Boskey AL. Bisphosphonate treatment modifies canine bone mineral and matrix properties and their heterogeneity. Bone. 2010;46(3):666–72.
Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures: lessons from materials research. Bone. 2013;55(2):495–500.
Fioramonti M, Santini D, Iuliani M, Ribelli G, Manca P, Papapietro N, et al. Cabozantinib targets bone microenvironment modulating human osteoclast and osteoblast functions. Oncotarget. 2017;8(12):20113.
Nakai Y, Okamoto K, Terashima A, Ehata S, Nishida J, Imamura T, et al. Efficacy of an orally active small-molecule inhibitor of RANKL in bone metastasis. Bone Res. 2019;7:1.
Marom R, Rabenhorst BM, Morello R. Osteogenesis imperfecta: an update on clinical features and therapies. Eur J Endocrinol. 2020;183(4):R95–106.
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, et al. Romosozumab treatment in postmenopausal women with osteoporosis. New Engl J Med. 2016;375(16):1532–43.
Zhao H, Bernardo MM, Osenkowski P, Sohail A, Pei D, Nagase H, et al. Differential inhibition of membrane type 3 (MT3)-matrix metalloproteinase (MMP) and MT1-MMP by tissue inhibitor of metalloproteinase (TIMP)-2 and TIMP-3 rgulates pro-MMP-2 activation. J Biol Chem. 2004;279(10):8592–601.
Jiang C, Xia W, Wu T, Pan C, Shan H, Wang F, et al. Inhibition of microRNA-222 up-regulates TIMP3 to promotes osteogenic differentiation of MSCs from fracture rats with type 2 diabetes mellitus. J Cell Mol Med. 2020;24(1):686–94.
Sleeman A, Clements JN. Abaloparatide: A new pharmacological option for osteoporosis. Am J Health Syst Pharm. 2019;76(3):130–5.
Bernhardsson M, Aspenberg P. Abaloparatide versus teriparatide: a head to head comparison of effects on fracture healing in mouse models. Acta Orthop. 2018;89(6):674–7.
Ueland T, Stilgren L, Bollerslev J. Bone matrix levels of Dickkopf and Sclerostin are positively correlated with bone mass and strength in postmenopausal osteoporosis. Int J Mol Sci. 2019;20(12).
Ross RD, Mashiatulla M, Robling AG, Miller LM, Sumner DR. Bone matrix composition following PTH treatment is not dependent on sclerostin status. Calcif Tissue Int. 2016;98(2):149–57.
Gardinier JD, Al-Omaishi S, Rostami N, Morris MD, Kohn DH. Examining the influence of PTH(1-34) on tissue strength and composition. Bone. 2018;117:130–7.
Makowski AJ, Uppuganti S, Wadeer SA, Whitehead JM, Rowland BJ, Granke M, et al. The loss of activating transcription factor 4 (ATF4) reduces bone toughness and fracture toughness. Bone. 2014;62:1–9.
Wang X, Guo B, Li Q, Peng J, Yang Z, Wang A, et al. miR-214 targets ATF4 to inhibit bone formation. Nat Med. 2013;19(1):93–100.
Molstad DH, Mattson AM, Begun DL, Westendorf JJ, Bradley EW. Hdac3 regulates bone modeling by suppressing osteoclast responsiveness to RANKL. J Biol Chem. 2020;295(51):17713–23.
McGee-Lawrence ME, Bradley EW, Dudakovic A, Carlson SW, Ryan ZC, Kumar R, et al. Histone deacetylase 3 is required for maintenance of bone mass during aging. Bone. 2013;52(1):296–307.
Razidlo DF, Whitney TJ, Casper ME, McGee-Lawrence ME, Stensgard BA, Li X, et al. Histone deacetylase 3 depletion in osteo/chondroprogenitor cells decreases bone density and increases marrow fat. PLoS One. 2010;5(7):e11492.
Carpio LR, Bradley EW, McGee-Lawrence ME, Weivoda MM, Poston DD, Dudakovic A, et al. Histone deacetylase 3 supports endochondral bone formation by controlling cytokine signaling and matrix remodeling. Sci Signal. 2016;9(440):ra79–ra.
Kim J-M, Yang Y-S, Park KH, Ge X, Xu R, Li N, et al. A RUNX2 stabilization pathway mediates physiologic and pathologic bone formation. Nat Commun 2020;11(1):1–17.
Valenti MT, Mottes M, Cheri S, Deiana M, Micheletti V, Cosaro E, et al. Runx2 overexpression compromises bone quality in acromegalic patients. Endocr Relat Cancer. 2018;25(3):269–77.
Kim W-J, Shin H-L, Kim B-S, Kim H-J, Ryoo H-M. RUNX2-modifying enzymes: therapeutic targets for bone diseases. Exp Mol Med. 2020;52(8):1178–84.
Schroeder TM, Westendorf JJ. Histone deacetylase inhibitors promote osteoblast maturation. J Bone Miner Res. 2005;20(12):2254–63.
Ouyang N, Li H, Wang M, Shen H, Si J, Shen G. The transcription factor Foxc1 promotes osteogenesis by directly regulating Runx2 in response of intermittent parathyroid hormone (1–34) treatment. Front Pharmacol. 2020;11:592.
Besio R, Garibaldi N, Leoni L, Cipolla L, Sabbioneda S, Biggiogera M, et al. Cellular stress due to impairment of collagen prolyl hydroxylation complex is rescued by the chaperone 4-phenylbutyrate. Dis Model Mech. 2019;12(6).
Besio R, Iula G, Garibaldi N, Cipolla L, Sabbioneda S, Biggiogera M, et al. 4-PBA ameliorates cellular homeostasis in fibroblasts from osteogenesis imperfecta patients by enhancing autophagy and stimulating protein secretion. Biochimica et Biophysica Acta (BBA)-Molecul Basis Dis. 2018;1864(5):1642–52.
Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank A-M, Bocian C, et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20(6):771–84 e6.
Yang Y, Zhao C, Liang J, Yu M, Qu X. Effect of dipeptidyl peptidase-4 inhibitors on bone metabolism and the possible underlying mechanisms. Front Pharmacol. 2017;8:487.
Yoshida T, Wang J, Stern PH. Gonadal hormones and bone. Handb Exp Pharmacol. 2020;262:65–91.
Powell KM, Skaggs C, Pulliam A, Berman A, Allen MR, Wallace JM. Zoledronate and Raloxifene combination therapy enhances material and mechanical properties of diseased mouse bone. Bone. 2019;127:199–206.
Allen MR, Hogan HA, Hobbs WA, Koivuniemi AS, Koivuniemi MC, Burr DB. Raloxifene enhances material-level mechanical properties of femoral cortical and trabecular bone. Endocrinology. 2007;148(8):3908–13.
Yoon SH, Grynpas M, Mitchell J. Intermittent PTH treatment improves bone and muscle in glucocorticoid treated Mdx mice: a model of duchenne muscular dystrophy. Bone. 2019;121:232–42.
Chiang CY, Zebaze RMD, Ghasem-Zadeh A, Iuliano-Burns S, Hardidge A, Seeman E. Teriparatide improves bone quality and healing of atypical femoral fractures associated with bisphosphonate therapy. Bone. 2013;52(1):360–5.
Varela A, Chouinard L, Lesage E, Guldberg R, Smith SY, Kostenuik PJ, et al. One year of abaloparatide, a selective peptide activator of the PTH1 receptor, increased bone mass and strength in ovariectomized rats. Bone. 2017;95:143–50.
Javaheri B, Carriero A, Staines KA, Chang Y-M, Houston D, Oldknow KJ, et al. Phospho1 deficiency transiently modifies bone architecture yet produces consistent modification in osteocyte differentiation and vascular porosity with ageing. Bone. 2015;81:277–91.
Carriero A, Bruse JL, Oldknow KJ, Millán JL, Farquharson C, Shefelbine SJ. Reference point indentation is not indicative of whole mouse bone measures of stress intensity fracture toughness. Bone. 2014;69:174–9.
Kiffer-Moreira T, Yadav MC, Zhu D, Narisawa S, Sheen C, Stec B, et al. Pharmacological inhibition of PHOSPHO1 suppresses vascular smooth muscle cell calcification. J Bone Miner Res. 2013;28(1):81–91.
Terkeltaub RA. Inorganic pyrophosphate generation and disposition in pathophysiology. Am J Physiol-Cell Physiol. 2001;281(1):C1–C11.
Rodriguez-Florez N, Carriero A, Shefelbine SJ. The use of XFEM to assess the influence of intra-cortical porosity on crack propagation. Comput Meth Biomechan Biomed Eng. 2017;20(4):385–92.
Cipriani C, Colangelo L, Santori R, Renella M, Mastrantonio M, Minisola S, et al. The interplay between bone and glucose metabolism. Front Endocrinol. 2020;11:122.
Mu C-F, Shen J, Liang J, Zheng H-S, Xiong Y, Wei Y-H, et al. Targeted drug delivery for tumor therapy inside the bone marrow. Biomaterials. 2018;155:191–202.
Skjødt MK, Frost M, Abrahamsen B. Side effects of drugs for osteoporosis and metastatic bone disease. Br J Clin Pharmacol. 2019;85(6):1063–71.
Feng Q, Xu J, Zhang K, Yao H, Zheng N, Zheng L, et al. Dynamic and cell-infiltratable hydrogels as injectable carrier of therapeutic cells and drugs for treating challenging bone defects. ACS Cent Sci. 2019;5(3):440–50.
Cai M, Yang L, Zhang S, Liu J, Sun Y, Wang X. A bone-resorption surface-targeting nanoparticle to deliver anti-miR214 for osteoporosis therapy. Int J Nanomedicine. 2017;12:7469.
Lavrador P, Gaspar VM, Mano JF. Stimuli-responsive nanocarriers for delivery of bone therapeutics–Barriers and progresses. J Control Release. 2018;273:51–67.
Acknowledgements
This work was supported by the National Science Foundation (CBET-1829310).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors state that they have no conflicts of interest.
Human and animal rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Biomechanics
Rights and permissions
About this article
Cite this article
Muñoz, A., Docaj, A., Ugarteburu, M. et al. Poor bone matrix quality: What can be done about it?. Curr Osteoporos Rep 19, 510–531 (2021). https://doi.org/10.1007/s11914-021-00696-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-021-00696-6