Abstract
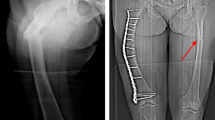
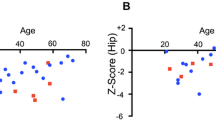
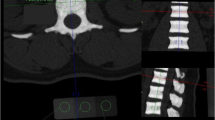
The main hallmark of high bone mass (HBM) disorders is increased bone mineral density, potentially visible in conventional radiographs and quantifiable by other radiographic methods. While one of the most common forms of HBM is CLCN7-related autosomal dominant osteopetrosis type II (ADO II), there is no consensus on diagnostic thresholds. We therefore wanted to assess whether CLCN7-osteopetrosis patients differ from benign HBM cases in terms of (1) bone mineral density, (2) bone structure, and (3) microarchitectural abnormalities. 16 patients meeting the criteria of HBM (DXA T/Z-score ≥ 2.5 at all sites) were included in this retrospective study. Osteologic assessment using dual-energy X-ray absorptiometry (DXA), high-resolution peripheral quantitative computed tomography (HR-pQCT), and serum analyses was performed. The presence of CLCN7 and/or other HBM gene mutations affecting bone mass were tested using a custom designed bone panel. While a DXA threshold for ADO II could be implemented (DXA Z-score ≥ + 6.0), the differences in bone microarchitecture were of lesser extent compared to the benign HBM group. All adult patients with ADO II suffered from elevated fracture rates independent from Z-score. In HR-pQCT, structural alterations, such as bone islets were found only inconsistently. In cases of HBM, a DXA Z-score ≥ 6 may be indicative for an inheritable HBM disorder, such as ADO II. Microarchitectural bone alterations might represent local microfracture repair or accumulation of cartilage remnants due to impaired osteoclast function, but seem not to be correlated with fracture risk.





Similar content being viewed by others
References
Morgan SL, Peace F, Lopez-Ben R, Fineberg N (2010) Distribution of Z-scores in a University cohort with an emphasis on “high” bone mineral density. J Clin Densitom 13(4):385–391. doi:10.1016/j.jocd.2010.07.003
Lomholt S, Amstrup AK, Moser E, Jakobsen NF, Mosekilde L, Vestergaard P, Rejnmark L (2015) Unexplained high BMD in DXA-scanned patients is generalized throughout the skeleton and characterized by thicker cortical and trabecular bone. Calcif Tissue Int 96(4):284–294. doi:10.1007/s00223-015-9955-y
Scane AC, Masud T, Johnson FJ, Francis RM (1994) The reliability of diagnosing osteoporosis from spinal radiographs. Age Ageing 23(4):283–286
Liu G, Peacock M, Eilam O, Dorulla G, Braunstein E, Johnston CC (1997) Effect of osteoarthritis in the lumbar spine and hip on bone mineral density and diagnosis of osteoporosis in elderly men and women. Osteoporos Int 7(6):564–569
Diamond T, Smith A, Schnier R, Manoharan A (2002) Syndrome of myelofibrosis and osteosclerosis: a series of case reports and review of the literature. Bone 30(3):498–501
Gregson CL, Hardcastle SA, Cooper C, Tobias JH (2013) Friend or foe: high bone mineral density on routine bone density scanning, a review of causes and management. Rheumatology (Oxford) 52(6):968–985. doi:10.1093/rheumatology/ket007
Hellemans J, Preobrazhenska O, Willaert A, Debeer P, Verdonk PC, Costa T, Janssens K, Menten B, Van Roy N, Vermeulen SJ, Savarirayan R, Van Hul W, Vanhoenacker F, Huylebroeck D, De Paepe A, Naeyaert JM, Vandesompele J, Speleman F, Verschueren K, Coucke PJ, Mortier GR (2004) Loss-of-function mutations in LEMD3 result in osteopoikilosis, Buschke-Ollendorff syndrome and melorheostosis. Nat Genet 36(11):1213–1218. doi:10.1038/ng1453
Whyte MP, Totty WG, Novack DV, Zhang X, Wenkert D, Mumm S (2011) Camurati-Engelmann disease: unique variant featuring a novel mutation in TGFbeta1 encoding transforming growth factor beta 1 and a missense change in TNFSF11 encoding RANK ligand. J Bone Miner Res 26(5):920–933. doi:10.1002/jbmr.283
Benichou O, Cleiren E, Gram J, Bollerslev J, de Vernejoul MC, Van Hul W (2001) Mapping of autosomal dominant osteopetrosis type II (Albers-Schonberg disease) to chromosome 16p13.3. Am J Hum Genet 69(3):647–654. doi:10.1086/323132
Waguespack SG, Hui SL, Dimeglio LA, Econs MJ (2007) Autosomal dominant osteopetrosis: clinical severity and natural history of 94 subjects with a chloride channel 7 gene mutation. J Clin Endocrinol Metab 92(3):771–778. doi:10.1210/jc.2006-1986
Kornak U, Kasper D, Bosl MR, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch TJ (2001) Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104(2):205–215
Cleiren E, Benichou O, Van Hul E, Gram J, Bollerslev J, Singer FR, Beaverson K, Aledo A, Whyte MP, Yoneyama T, deVernejoul MC, Van Hul W (2001) Albers-Schonberg disease (autosomal dominant osteopetrosis, type II) results from mutations in the ClCN7 chloride channel gene. Hum Mol Genet 10(25):2861–2867
Kornak U, Schulz A, Friedrich W, Uhlhaas S, Kremens B, Voit T, Hasan C, Bode U, Jentsch TJ, Kubisch C (2000) Mutations in the a3 subunit of the vacuolar H(+)-ATPase cause infantile malignant osteopetrosis. Hum Mol Genet 9(13):2059–2063
Barvencik F, Kurth I, Koehne T, Stauber T, Zustin J, Tsiakas K, Ludwig CF, Beil FT, Pestka JM, Hahn M, Santer R, Supanchart C, Kornak U, Del Fattore A, Jentsch TJ, Teti A, Schulz A, Schinke T, Amling M (2014) CLCN7 and TCIRG1 mutations differentially affect bone matrix mineralization in osteopetrotic individuals. J Bone Miner Res 29(4):982–991. doi:10.1002/jbmr.2100
Arruda M, Coelho MC, Moraes AB, de Paula Paranhos-Neto F, Madeira M, Farias ML, Neto LV (2016) Bone mineral density and microarchitecture in patients with autosomal dominant osteopetrosis: a report of two cases. J Bone Miner Res 31(3):657–662. doi:10.1002/jbmr.2715
Kamphans T, Krawitz PM (2012) GeneTalk: an expert exchange platform for assessing rare sequence variants in personal genomes. Bioinformatics 28(19):2515–2516. doi:10.1093/bioinformatics/bts462
Zemojtel T, Kohler S, Mackenroth L, Jager M, Hecht J, Krawitz P, Graul-Neumann L, Doelken S, Ehmke N, Spielmann M, Oien NC, Schweiger MR, Kruger U, Frommer G, Fischer B, Kornak U, Flottmann R, Ardeshirdavani A, Moreau Y, Lewis SE, Haendel M, Smedley D, Horn D, Mundlos S, Robinson PN (2014) Effective diagnosis of genetic disease by computational phenotype analysis of the disease-associated genome. Sci Transl Med 6(252):252ra123. doi:10.1126/scitranslmed.3009262
Schwarz JM, Rodelsperger C, Schuelke M, Seelow D (2010) MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods 7(8):575–576. doi:10.1038/nmeth0810-575
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR (2010) A method and server for predicting damaging missense mutations. Nat Methods 7(4):248–249. doi:10.1038/nmeth0410-248
Doerks T, Copley RR, Schultz J, Ponting CP, Bork P (2002) Systematic identification of novel protein domain families associated with nuclear functions. Genome Res 12(1):47–56. doi:10.1101/gr.203201
Milovanovic P, Adamu U, Simon MJ, Rolvien T, Djuric M, Amling M, Busse B (2015) Age- and sex-specific bone structure patterns portend bone fragility in radii and tibiae in relation to osteodensitometry: a high-resolution peripheral quantitative computed tomography study in 385 individuals. J Gerontol A Biol Sci Med Sci 70(10):1269–1275. doi:10.1093/gerona/glv052
Burrows M, Liu D, Perdios A, Moore S, Mulpuri K, McKay H (2010) Assessing bone microstructure at the distal radius in children and adolescents using HR-pQCT: a methodological pilot study. J Clin Densitom 13(4):451–455. doi:10.1016/j.jocd.2010.02.003
Burt LA, Liang Z, Sajobi TT, Hanley DA, Boyd SK (2016) Sex- and site-specific normative data curves for HR-pQCT. J Bone Miner Res. doi:10.1002/jbmr.2873
Bordier P, Matrajt H, Hioco D, Hepner GW, Thompson GR, Booth CC (1968) Subclinical vitamin-D deficiency following gastric surgery. Histological evidence in bone. Lancet 1(7540):437–440
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28(1):2–17. doi:10.1002/jbmr.1805
Schnitzler CM, Pettifor JM, Mesquita JM, Bird MD, Schnaid E, Smyth AE (1990) Histomorphometry of iliac crest bone in 346 normal black and white South African adults. Bone Miner 10(3):183–199
Koehne T, Vettorazzi E, Kusters N, Luneburg R, Kahl-Nieke B, Puschel K, Amling M, Busse B (2014) Trends in trabecular architecture and bone mineral density distribution in 152 individuals aged 30–90 years. Bone 66:31–38. doi:10.1016/j.bone.2014.05.010
Frattini A, Pangrazio A, Susani L, Sobacchi C, Mirolo M, Abinun M, Andolina M, Flanagan A, Horwitz EM, Mihci E, Notarangelo LD, Ramenghi U, Teti A, Van Hove J, Vujic D, Young T, Albertini A, Orchard PJ, Vezzoni P, Villa A (2003) Chloride channel ClCN7 mutations are responsible for severe recessive, dominant, and intermediate osteopetrosis. J Bone Miner Res 18(10):1740–1747. doi:10.1359/jbmr.2003.18.10.1740
Pangrazio A, Pusch M, Caldana E, Frattini A, Lanino E, Tamhankar PM, Phadke S, Lopez AG, Orchard P, Mihci E, Abinun M, Wright M, Vettenranta K, Bariae I, Melis D, Tezcan I, Baumann C, Locatelli F, Zecca M, Horwitz E, Mansour LS, Van Roij M, Vezzoni P, Villa A, Sobacchi C (2010) Molecular and clinical heterogeneity in CLCN7-dependent osteopetrosis: report of 20 novel mutations. Hum Mutat 31(1):E1071–1080. doi:10.1002/humu.21167
Del Fattore A, Peruzzi B, Rucci N, Recchia I, Cappariello A, Longo M, Fortunati D, Ballanti P, Iacobini M, Luciani M, Devito R, Pinto R, Caniglia M, Lanino E, Messina C, Cesaro S, Letizia C, Bianchini G, Fryssira H, Grabowski P, Shaw N, Bishop N, Hughes D, Kapur RP, Datta HK, Taranta A, Fornari R, Migliaccio S, Teti A (2006) Clinical, genetic, and cellular analysis of 49 osteopetrotic patients: implications for diagnosis and treatment. J Med Genet 43(4):315–325. doi:10.1136/jmg.2005.036673
Pang Q, Chi Y, Zhao Z, Xing X, Li M, Wang O, Jiang Y, Liao R, Sun Y, Dong J, Xia W (2016) Novel mutations of CLCN7 cause autosomal dominant osteopetrosis type II (ADO-II) and intermediate autosomal recessive osteopetrosis (IARO) in Chinese patients. Osteoporos Int 27(3):1047–1055. doi:10.1007/s00198-015-3320-x
Gregson CL, Wheeler L, Hardcastle SA, Appleton LH, Addison KA, Brugmans M, Clark GR, Ward KA, Paggiosi M, Stone M, Thomas J, Agarwal R, Poole KE, McCloskey E, Fraser WD, Williams E, Bullock AN, Davey Smith G, Brown MA, Tobias JH, Duncan EL (2016) Mutations in known monogenic high bone mass loci only explain a small proportion of high bone mass cases. J Bone Miner Res 31(3):640–649. doi:10.1002/jbmr.2706
Whyte MP, Chines A, Silva DP Jr, Landt Y, Ladenson JH (1996) Creatine kinase brain isoenzyme (BB-CK) presence in serum distinguishes osteopetroses among the sclerosing bone disorders. J Bone Miner Res 11(10):1438–1443. doi:10.1002/jbmr.5650111010
Hahn M, Vogel M, Amling M, Ritzel H, Delling G (1995) Microcallus formations of the cancellous bone: a quantitative analysis of the human spine. J Bone Miner Res 10(9):1410–1416. doi:10.1002/jbmr.5650100919
Busse B, Bale HA, Zimmermann EA, Panganiban B, Barth HD, Carriero A, Vettorazzi E, Zustin J, Hahn M, Ager JW 3rd, Puschel K, Amling M, Ritchie RO (2013) Vitamin D deficiency induces early signs of aging in human bone, increasing the risk of fracture. Sci Transl Med 5(193):188. doi:10.1126/scitranslmed.3006286
Everts V, de Vries TJ, Helfrich MH (2009) Osteoclast heterogeneity: lessons from osteopetrosis and inflammatory conditions. Biochim Biophys Acta 1792(8):757–765. doi:10.1016/j.bbadis.2009.05.004
Neutzsky-Wulff AV, Sims NA, Supanchart C, Kornak U, Felsenberg D, Poulton IJ, Martin TJ, Karsdal MA, Henriksen K (2010) Severe developmental bone phenotype in ClC-7 deficient mice. Dev Biol 344(2):1001–1010. doi:10.1016/j.ydbio.2010.06.018
Neutzsky-Wulff AV, Karsdal MA, Henriksen K (2008) Characterization of the bone phenotype in ClC-7-deficient mice. Calcif Tissue Int 83(6):425–437. doi:10.1007/s00223-008-9185-7
Roschger P, Paschalis EP, Fratzl P, Klaushofer K (2008) Bone mineralization density distribution in health and disease. Bone 42(3):456–466. doi:10.1016/j.bone.2007.10.021
Acknowledgements
We thank Elke Leicht for expert technical assistance in preparing the histological sections. Furthermore, Felix Schmidt acknowledges the Joachim Herz Stiftung for a PhD Scholarship in cooperation with the PIER Helmholtz Graduate School, University of Hamburg and DESY Hamburg.
Funding
This project has received funding from the European Community’s Seventh Framework Programme under grant agreement no. 602300 (SYBIL) and the German Federal Ministry of Education and Research (BMBF) within the project “Detection and Individualized Management of Early Onset Osteoporosis (DIMEOS)”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Sebastian Butscheidt, Tim Rolvien, Uwe Kornak, Felix Schmidt, Thorsten Schinke, Michael Amling, and Ralf Oheim declare that they have no conflict of interest.
Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Butscheidt, S., Rolvien, T., Kornak, U. et al. Clinical Significance of DXA and HR-pQCT in Autosomal Dominant Osteopetrosis (ADO II). Calcif Tissue Int 102, 41–52 (2018). https://doi.org/10.1007/s00223-017-0332-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0332-x