Abstract
Inflammation has accompanied human beings since the emergence of wounds and infections. In the past decades, numerous efforts have been undertaken to explore the potential role of inflammation in cancer, from tumor development, invasion, and metastasis to the resistance of tumors to treatment. Inflammation-targeted agents not only demonstrate the potential to suppress cancer development, but also to improve the efficacy of other therapeutic modalities. In this review, we describe the highly dynamic and complex inflammatory tumor microenvironment, with discussion on key inflammation mediators in cancer including inflammatory cells, inflammatory cytokines, and their downstream intracellular pathways. In addition, we especially address the role of inflammation in cancer development and highlight the action mechanisms of inflammation-targeted therapies in antitumor response. Finally, we summarize the results from both preclinical and clinical studies up to date to illustrate the translation potential of inflammation-targeted therapies.
Similar content being viewed by others
Background
Among the key factors contributing to the initiation and progression of tumors, inflammation has been intensively investigated for its supporting role in tumor development. Inflammation has accompanied human beings since the emergence of wounds and infections. The ancient Roman physicians Celsus and Galen described the most prominent evidence of inflammation including “redness, swelling, fever, pain, and dysfunction” [1]. The canonical inflammatory process is characterized by a series of vascular changes, the release of chemicals, and the recruitment of white blood cells to inflammatory sites [2]. In addition to the inflammatory response following wounds and infections, inflammation also exists in other pathologies, such as the chronic inflammation which is known to accompany neurodegenerative diseases, diabetes, atherosclerosis, and most importantly cancer.
In the nineteenth century [3], a German pathologist, Rudolf Virchow brought up a theory that there was certain association between tumor and inflammation as evidenced by leukocyte infiltration. Virchow suggested that tumors might originate from chronic inflammation which persisted though no longer needed. The intratumoral leukocyte infiltration has now become a common hallmark of tumors [4]. In the 1970s, Alexander Haddow proposed that tumor might be caused by “overhealing” of wounds [5]. Given that the development of cancer shares similar features with the tissue regeneration process, Harold F. Dvorak suggested that the inflammatory wound-healing processes might facilitate the generation of tumor stroma [6]. Later in the 1990s, some surgeons reported that operational stress induced by resections could promote angiogenesis which favored tumor growth in nude mice [7].
Tumors are not a simple stack of cells, but rather, consist of heterogeneous cancer cells and stromal cells which collectively provide a complex tumor microenvironment (TME) [8]. Tumors are often characterized with the infiltration of immune cells and the upregulation of inflammatory mediators surrounding tumors. This inflammatory microenvironment may impact tumor development varying stages, from tumor initiation to progression. In this review, we discuss the role of inflammation in cancer development, with special focus on the tumor-promoting activities of inflammation. We especially highlight the underlying mechanisms of the antitumor efficacy of inflammation-targeted therapies in cancer, with clinical evidence up to date in relation to inflammation-targeting strategies.
Inflammation mediators in cancer
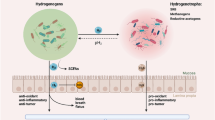
The multi-step cancer development process can be initiated by etiologic factors such as carcinogen irritants or oncogenic infection [9]. Under exposure to such etiologic factors, cells with survival advantages transform into tumor-initiating subpopulations with unlimited growth and self-renewal capacity [10]. As demonstrated by epidemiological studies, the ulcerative colitis and Crohn’s disease could increase the risk of colon cancer, which is one of the best known examples of tumor-associated inflammation [11, 12]. Moreover, oncogenic infection by microbial agents such as Helicobacter pylori [13] and hepatitis B [14] has also been described as risk factors for gastric and hepatic cancer. During the chronic inflammation induced by microbial agents, immune cells such as macrophages at the inflammatory sites produce reactive oxygen species (ROS), leading to persistent DNA damage and subsequent gene mutations [15]. Furthermore, cytokines secreted by immune cells such as tumor necrosis factor-α (TNF-α) and macrophage migration inhibitory factor (MIF), inhibit the activation of p53- and Rb-E2F pathways and thereby promote tumorigenesis [16, 17]. The various components involved in inflammatory processes form a positive feedback loop that supports cancer progression. The inflammatory cytokines and growth factors then activate transcription factors such as NF-κB, collectively contributing to an inflammatory TME [18, 19]. Figure 1 presents a schematic of the crosstalk between major inflammatory cells and inflammatory molecules in the tumor microenvironment.
A schematic of the crosstalk between major inflammatory cells and inflammatory molecules in the tumor microenvironment. The major inflammatory cells include T helper (Th1) cell, regulatory T cells (Tregs), cytotoxic CD8 + T cells, macrophages, neutrophils, myeloid-derived suppressor cells (MDSCs), natural killer (NK) cells, and dendritic cells (DCs). Figures created with BioRender. Abbreviations: CXCR, CXC-chemokine receptor; CXCL, chemokine (C-X-C motif) ligand; TGF-β, transforming growth factor-β; TNF, tumor necrosis factor; IL, interleukin; IFN, interferon
Key inflammatory cells in cancer
The inflammatory TME is highly dynamic and complex, the cell component of which include tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), dendritic cells (DCs), myeloid-derived suppressor cells (MDSCs), and T lymphocytes [19]. These tumor-infiltrating cells collectively maintain an inflammatory environment that allows tumor growth and, moreover, immune suppression during tumor progression. The key inflammatory cells involved in cancer with antitumor or protumoral roles are presented in Table 1.
Tumor-associated neutrophils (TANs)
Neutrophils constitute the largest proportion of blood leukocytes and are the main population of effector cells upon inflammatory stimuli such as pathogen infection. The N1 and N2 polarization of TANs can be induced by type 1 interferon (IFN) and TGF-β, respectively [20]. Tumor-derived factors induce a shift of infiltrating neutrophils toward an antitumor phenotype [21]. Interestingly, the majority of neutrophils in the TME exhibit an N2 phenotype and facilitate tumor metastasis through various mechanisms [22]. For instance, TANs may promote tumor angiogenesis by inducing continuous release of VEGF from peripheral endothelial cells [23]. In addition, TANs may suppress antitumor immunity by producing various proinflammatory and immunosuppressive factors including IL-1β, IL-17, TNF-α, VEGF, CCL4, matrix metallopeptidase (MMP)-9, C-X-C motif chemokine ligand 8 (CXCL8), and angiopoietin-1 (ANG1) [24]. Known tumor-derived cytokines that drive such differentiation of neutrophils include IFN-γ and GM-CSF which upregulate the expression of specific neutrophil activation markers and thereby promote antitumor activity [25]. Tumor-secreted TGF-β facilitates the recruitment of N2 neutrophils which later creates an immunosuppressive microenvironment by producing CCL2 and CCL17 in a paracrine manner [26, 27]. The increased ratio of TANs to lymphocytes is indicative of poor prognosis in many cancer. The infiltration of TANs and their production of chemokines are able to predict the progression of breast cancer [28].
A unique way for neutrophils to combat infection is the release of neutrophil extracellular traps (NETs), a net-like structure primarily composed of DNA-histone complexes from neutrophils, which are identified as a critical type of innate immune response [29]. Compelling evidence recently suggests that neutrophils can be recruited to the site of pre-metastatic niches such as lung [30], liver [31], and omentum [32] where they facilitate the remodeling of local microenvironment that favors tumor cell extravasation through NETs. The IL-8/CXCL8 autocrine signaling in tumor cells could promote the formation of NETs [33, 34]. Other cancer-induced signals that promote NETs release include CXCR1/CXCR2 agonists, G-CSF, and TGF-β [35,36,37]. Clinical evidence that linked NETs with cancer was found in Ewing sarcoma, where the presence of intratumoral NETs indicated poor prognosis of patients [38]. The protumorigenic role of NETs may be attributed to their induction of endothelial-to-mesenchymal transition (EMT), an important mechanism for tumor metastasis [39], as observed in models of ovarian [32], lung [40], pancreatic [41], colorectal [42], and breast cancer [43, 44].
However, based on different status of TME, the role of NETs is variable. NETs can also exert an antitumor effect by directly killing tumor cells and inhibiting tumor growth and metastasis. In colorectal cancer (CRC) and head and neck squamous cell carcinoma, in vitro generated NETs could imped tumor growth by inducing apoptosis and inhibiting proliferation [45, 46]. Furthermore, co-culture of melanoma cells with NETs led to necrosis of melanoma cells [47]. NETosis is associated with the release of protein S100A8/A9, the increased ratio of which to CRP was found to correlate with favorable survival of high-grade serous ovarian cancer (HGSOC) patients [48].
Tumor-associated macrophages (TAMs)
The wide spectrum of immune functions of TAMs in inflammatory processes such as wound healing has been well documented [49]. Similar to neutrophils, macrophage can also be divided into proinflammatory M1 and anti-inflammatory M2 subtypes [50]. The expression profile of M1 macrophages includes high levels of MHC class II, CD80, and CD86, whereas M2 macrophages highly express CD163 and CD206 [51]. Upon exposure to cytokines such as IL-4, M-CSF/CSF1, IL-10, IL-33, IL-21, and TGF-β, TAMs switch to M2 phenotype, whereas M1 TAMs can be activated by TNF-α or granulocyte–macrophage colony-stimulating factor (GM-CSF), M1 TAMs facilitate the recruitment and antitumor activities of cytotoxic CD8 + T cells and natural killer (NK) cells.
In the inflammatory TME, macrophages account for 30%-50% of cell populations and are believed to provide “soil” for tumor growth. The switch of TAMs between M1 and M2 status largely depends on the molecules present in the TME where tumor cells take advantage of macrophage plasticity to its own benefit ADDIN EN.CITE [52]. At the early stage of the tumor, macrophages polarize to M1 to initiate antitumor responses. When tumors progress to advanced stage, the anti-inflammatory characteristics of TAMs are controlled by tumor cells and polarize to M2 phenotype that promotes tumor progression [53]. M1 macrophages have long been identified as antitumor macrophages, by identifying and directly killing tumor cells. M1macrophage-mediated tumor cell killing is based on its secretion of cytotoxic molecules such as ROS and NO, which is a rather slow process [54]. Another mechanism for M1macrophage-mediated killing of tumor cells is antibody-dependent cell-mediated cytotoxicity (ADCC), which occurs within a few hours and relies on the presence of antitumor antibodies [55]. On the contrary, M2 TAMs are protumoral macrophages that adversely affect the activities of immune effector cells. For tumor healing, the proinflammatory M1 macrophages repolarize into anti-inflammatory M2 TAMs to control inflammation, which unfortunately promote tumor progression [56]. Thus, it is not surprising that a lower M1/M2 ratio of TAMs was significantly related to the progression and poor prognosis of cancer patients [16, 57, 58].
One underlying mechanism for the M2 TAM-induced cancer progression is the direct increase in angiogenesis, mainly by upregulating angiogenesis-associated genes such as VEGF, PDGF, and PGE2 [59]. The indirect proangiogenic effect of the M2 TAMs is mediated by CXCL12, IL-1β, IL-8, and Sema4d which activate the response of endothelial cells to growth factor signaling [60, 61]. M2 TAMs also facilitate the invasion and metastasis of tumors by expressing proteinase, cathepsin, urokinase, and matrix remodeling enzymes which degrade tumor extracellular matrix (ECM) [49]. On the other hand, it was recently reported that miRNAs-containing exosomes released from M2 TAMs could upregulate TGF-β that promotes EMT and causes the imbalance between regulatory T cells (Tregs) and T helper 17 (Th17) cells [62,63,64]. Moreover, during tumor progression, the presence of M2 TAMs was associated with the malignant potential of tumors and a higher programmed cell death 1 ligand 1 (PD-L1) expression level on tumor and immune cells [65, 66].
Dendritic cells (DCs)
DCs are bone marrow-derived cells that detect danger signal in the environment and transmit the signal to adaptive immune cells such as T lymphocytes [67]. Thus, DCs function as a messenger between innate and adaptive immunity. The non-activated DCs are referred to as immature DCs which present self-antigens to T cells, inducing immune tolerance by enhancing the activities of regulatory T cells [68]. DC maturation can be initiated by various signals leading to distinct phenotypes to induce different immune responses, such as fms-related tyrosine kinase receptor 3 (FLT3) [69]. The initial signal for the antitumor response of CD8 + T cells relies on the presentation of tumor-associated antigens (TAAs) on MHC molecules by DCs [70]. In the TME however, the functions of tumor-infiltrating DCs are often suppressed by tumor cells, leading to T cell tolerance rather than antitumor immune response [71]. Presentation of TAAs by DCs in the absence of costimulatory signals may lead to T cell anergy [72]. Tumor-derived factors also modulate the maturation status of DCs, inducing inflammation that favors tumor growth. For instance, tumor-derived IL-6 and M-CSF convert immature DCs into macrophages and prevent the priming of tumor-specific T cells [73]. Furthermore, PD-L1 and PD-L2 expressed on DCs may also inhibit the proliferation and functional cytokine production of activated T cells [74].
In recent decades, immunogenic cell death (ICD) has received considerable research attention. ICD is accompanied by the release and chronic exposure of damage-associated molecular patterns (DAMPs), conferring a potent adjuvanticity to dying cancer cells. ROS production and endoplasmic reticulum (ER) stress are required for the emission of DAMPs which bind to the pattern recognition receptors (PRRs) expressed on immune cells, especially DCs [75]. This recognition and binding process is often associated with the generation of immunological memory [76, 77]. Multiple studies have described the critical role of DCs in the immune response triggered by tumor cells undergoing ICD [78], which demonstrated that the robust antitumor T cell response induced by ICD largely relied on DCs in the TME. It is thus conceivable that manipulating DCs in the TME holds great potential as anticancer strategies. Whereas ICD contributes to the success of many anticancer treatments including chemotherapy, radiotherapy, and target therapies, the immunogenicity varies among cells with different death modalities. A recent study suggested that cancer cells undergoing ferroptosis would impede the maturation of DCs, with poor engulfment and antigen presentation capacity, adding concerns to the applications of ferroptosis-inducing therapeutics [79].
Myeloid-derived suppressor cells (MDSCs)
Mouse myeloid-derived suppressor cells (MDSCs) are immature myeloid cells and can be divided into monocytic-myeloid-derived suppressor cells (M-MDSCs) with surface expression of CD11b + Ly6G-Ly6C-high and polymorphonuclear-myeloid-derived suppressor cells (PMN-MDSCs) with CD11b + Ly6G + Ly6C-low [77]. In contrast, the identification of expression profile of human MDSCs is lacking as human leukocytes do not express Gr-1. Given the potent immune-suppressive activities of MDSCs and their similarities with neutrophils and monocytes, it is of paramount importance to identify robust marker combinations and gating parameters for MDSC subsets. A multicenter study identified 10 putative subsets of MDSCs in peripheral blood mononuclear cells (PBMC) obtained from healthy donors to examine the identification marker combinations for circulating MDSCs [80]
The multiple mechanisms for the suppression on antitumor immunity by M-MDSCs have been intensively documented. MDSCs either directly interact with T cells or reshape the TME through the cellular and molecular immunosuppressive network, interfering the normal functions of T cells. M-MDSCs are rapidly recruited to the inflammatory tumor tissues upon exposure to chemokines such as CCL2, CCL5, CXCL8, and CXCL12 and produce multiple immunosuppressive cytokines such as ARG1, nitric oxide (NO), TGF-β, and IL-10 [81, 82]. For example, the upregulation of ARG1 in MDSCs results in L-arginine starvation that leads to T cell dysfunction by decreasing the expression of T cell receptor (TCR) ζ-chain [83]. In addition, MDSC-induced tumor progression is also mediated by tumor angiogenesis. Tumor-derived factors such as VEGF, IL-6, and IL-10 recruit MDSCs which in turn produce more VEGF via STAT3 signaling, thereby establishing a positive feedback loop that potentiates tumor angiogenesis [84, 85]. Apart from the VEGF/VEGFR axis that stimulates MDSCs, the proangiogenic MMPs produced by MDSCs serve as a secondary angiogenetic signals [86]. MMPs are a family of ECM enzymes that facilitate the invasion of tumor cells, and among them MMP9 is perceived as a key regulator for tumor angiogenesis induced by PMN-MDSCs [87].
Given that high M-MDSC fraction is correlated with decreased expansion and activation of tumor-specific T cells [88], MDSCs have now become a novel marker for predicting patients’ response to immune checkpoint blockade (ICB) therapy. For instance, patients with lower fractions of circulating MDSCs are more sensitive to ipilimumab treatment [89], especially melanoma patients [90, 91]. Upon CTLA-4 blockade, tumor-infiltrating MDSCs exhibit increased expression of colony-stimulating factor-1 receptor (CSF-1R), which in turn is correlated with increased MDSC infiltration in tumors. CSF-1/CSF-1R signaling blockade could not only be used to decrease the numbers of MDSCs, but also convert the immune-suppressive MDSCs toward an antitumor phenotype [92, 93]. Likewise, IL-10 secreted by DCs in the TME could increase the number of tumor-infiltrating MDSCs, conferring adaptive resistance to PD-1 antibody treatment [94]. Targeting MDSCs via CSF-1/CSF-1R inhibitors thus becomes a potential strategy to overcome tumor resistance to ICBs. Though a large number of agents targeting the upstream factors or receptors of MDSC accumulation are being tested to potentiate ICB efficacy, it has to be addressed that the majority of MDSC-recruiting chemokines can also act on other immune cells with antitumor activities such as T lymphocytes [95] and NK cells [96]. Thus, such chemokine blockades would possibly yield both positive and negative effect on tumors.
Vascular endothelial cells
In addition to immune cells, vascular endothelial cells are also considered a key participant during the inflammatory process in tumors. In direct contact with the cellular and molecular components of blood, vascular endothelial cells form a barrier between blood and the subcutaneous tissue, regulating the permeability of blood vessels and tissue infiltration of blood components. The proinflammatory phenotypes of endothelial cells can be induced by TNF-α and IL-1 released from leukocytes via the TNFR/IL-1 and NF-κB pathway [97]. The activated endothelial cells then express increased luminal endothelial adhesion molecules and produce various chemokines such as CXCL8, CXCL2, complement C5a, leucine, and platelet-activating factor (PAF), mediating the process called vascular inflammation that facilitates leukocyte recruitment into tissues [98]. Due to decreased adhesion molecules upon vascular inflammation, the weakened endothelial junctions make it easier for leukocytes to migrate through vascular walls.
The intricate tumor metastasis process is orchestrated by both cancer and normal cells such as endothelial cells. In the TME, the migration and invasion of cancer cells into tissues are similar to those of leukocytes. However, tumor cells are larger in size and may be mechanically trapped in the blood vessels [99]. To cross endothelial barriers, a large number of molecules such as selectins are required to facilitate leukocyte transmigration [100, 101]. The selectin-mediated rolling of tumor cells represents one of these machinery. For instance the expression of E-selectin on bone marrow endothelial cells and its ligands expression on prostate cancer cells are fundamental for the bone metastasis of prostate cancer [102]. Similarly, E-selectin-mediated rolling of cancer cells on endothelium was observed in breast, pancreatic, and colon cancer [103,104,105].
Key inflammatory cytokines in cancer
Cytokines are polypeptides or glycoproteins with molecular weights of less than 30 kDa and could transduce inflammatory or anti-inflammatory signals to cells in the TME. Many of the inflammatory cytokines are associated with the onset and progression of tumors [106], and these cancer-related are often upregulated in the TME [107]. Table 2 presents the key inflammatory cytokines involved in cancer. Understanding the action mechanisms of these cytokines on tumors would facilitate the development of corresponding anticancer therapeutics.
Tumor necrosis factor alpha (TNF-α)
The regulatory activities of TNF-α in the innate immune system have been reviewed extensively throughout time. TNF-α can be produced by macrophages, T lymphocytes, NK cells, neutrophils, mast cells, eosinophils, and neurons and is involved in a wide range of inflammatory signaling [108]. As a proinflammatory cytokine, the aberrant expression of TNF-α was also identified in multiple malignancies including prostate, ovarian, liver, and breast cancer [109,110,111,112]. For instance, the mRNA and protein levels of TNF-α were both upregulated in tumor and stromal cells of breast cancers with worse prognosis [113]. TNF-α is also involved in resistance to anticancer therapy, as evidenced by the decreased sensitivity of gastric cancer to trastuzumab following TNF-α exposure [114]. Strategies targeting TNF-α have been proved effective in pancreatic cancer models [115].
By binding to its receptors TNF-αR-1 and TNF-αR-2, TNF-α promotes tumor proliferation and angiogenesis and induces the EMT of tumor cells [116].
TNF-α may play contrary roles in carcinogenesis depending on its concentrations. The antitumor effect of high concentrations of TNF-α was observed in a murine sarcoma model, whereas low levels of TNF-α led to a protumorigenic phenotype [117].
In melanoma, TNF-α not only induces tumor metastasis ADDIN EN.CITE [118], but also inhibits CD8 T lymphocytes accumulation in the TME ADDIN EN.CITE [119], leading to further evaluation of a TNF-α blockade in pre-clinical models. TNF-α also augments TGF-β signals and promotes TGF-β-induced EMT ADDIN EN.CITE [116]. A recent study suggested that TNF-α upregulates the level of prion protein (PrP) in cancer cells and promotes cancer cell migration ADDIN EN.CITE [120]. TNF-α only exhibits inhibitory effect on Treg functions when in co-culture with effector T cells, but also promotes Treg survival [121]. Several reports suggested that TNF-neutralizing antibodies could increase the Treg frequency in the peripheral blood of patients with rheumatoid arthritis [122, 123]. However, some reports suggested that TNF is able to increase expansion, stability, and possibly function of Tregs via TNFR2 [124]. TNFR2 is highly expressed on Tregs supporting the proliferation and suppressive activities of Tregs [125]. TNFR2 was identified as a expression biomarker for the highly suppressive subset of Tregs [125]. The antagonistic TNFR2 antibodies are thus potential treatment for tumors. TNFR2 antagonists were capable of targeting surface TNFR2 on ovarian cancer cells, inhibiting NF-κB pathway activation and proliferation of tumor cells [126].
Transforming growth factor-beta (TGF-β)
Produced by inflammatory cells such as neutrophils and macrophages, TGF-β has long been identified as a pleiotropic cytokine involved in tumor initiation and progression [127]. Three isoforms mammalian TGF-β ligands have been identified so far: TGF-β1, TGF-β2, and TGF-β3, which, by binding to their receptors type I (TGF-βRI) and type II (TGF-βRII), stimulate downstream signaling via phosphorylation of Smads and regulate the transcription of target genes[128]. In addition to tumor cells, the bone matrix is also an important source of TGF-β, linking TGF-β to the bone metastasis of tumors [129].
Interestingly, in the context of tumors, the role of TGF-β may vary according to the stage. In normal condition and early stages of tumorigenesis, TGF-β potently inhibits the growth and development of tumors at the early stage, whereas it induces the proliferation, invasion, metastasis, and angiogenesis of tumors at the later stage [127, 130,131,132]. The aberrant expression of TGF-β signaling has been found in multiple tumor types including hepatocellular carcinoma, colon, prostate, lung, and breast cancer [133]. Known mechanisms for the TGF-β-mediated tumor support include increased EMT, immune escape, angiogenesis, and suppressed tumor apoptosis [134, 135], whereas the tumor-suppressive role of TGF-β may be mediated by apoptosis induction and immune cell modulation [128]. TGF-β mediates the EMT of tumors potentially by promoting the secretion of MMP2 and MMP9 and suppressing the activity of tissue inhibitors of MMPs (TIMPs) [136]. TGF-β also increases the formation of blood vessels in breast tumors by upregulating VEGF and MCP-1 [137]. It was recently reported that Treg cells work in synergy with tumor cells to create an immunosuppressive TME by secreting TGF-β [138]. Thus, inhibiting TGF-β significantly holds great potential to enhance the efficacy of anticancer treatments.
Interferons (IFNs)
IFNs can be classified in type I, type II, and type III based on their structures and receptors and are widely involved in tumor and inflammatory responses. Among them, type I interferons (IFN-Is) consist of 13 isoforms and are widely recognized for their antipathogen and proinflammatory activities. The type I IFN receptor is composed of the IFNAR1 and IFNAR2 subunits. The most important source of type I IFN is plasmacytoid DCs (pDCs) which are also referred to as the natural “IFN-producing cells.” In addition, B cells are also able to produce type I IFN in vivo, and fibroblasts can produce IFNβ upon after viral infections [139, 140]. In recent decades, emerging data suggest that IFN I is implicated in many aspects of antitumor immunity such as antigen presentation, tumor cell apoptosis, and immunosuppression.
During chronic inflammation, the feedback protective processes induced by IFN-Is provide tumor cells with supportive microenvironment for tumor growth and progression [141, 142]. Alongside the proinflammatory signals for tumor progression, IFN-Is may also facilitate the immune evasion of tumor cells by upregulating immune-suppressive pathways ranging from danger sensing to cytokine production [143, 144]. For instance in head and neck squamous cell carcinoma (HNSCC), cancer-specific IFN-I activation attenuates the expansion and functions of CD8 + T effector cells and is associated with poor clinical outcomes [145].
In addition, IFN-I was reported to promote cancer stemness by triggering the epigenetic regulator KDM1B [146]. IFN-stimulated genes (ISGs) are overexpressed in epithelial cells which spontaneously trigger EMT of tumor cells, thereby regulating EMT and subsequent tumor metastasis at multiple levels [147]. However, studies have also delineated the antitumor activities of IFN-Is which negatively regulate premetastatic niche formation in the TME [148]. Further, the potent antiangiogenic activity of IFN-Is especially IFN-α has been reported [149]. IFN-α was approved for the treatment of hairy cell leukemia in 1986 [150]. A growing body of literature then investigated the efficacy of IFNs in both hematological malignancies and solid tumors. Thus, the role of IFN-Is in cancer may be highly dependent on cell type, timing, and various other factors.
Interleukin-1
Interleukin (IL)-1 is upregulated in multiple tumor types including breast, colon, head and neck, lung, pancreas cancer, and melanomas, the high expression of which is indicative of bad prognosis [151]. The endogenous IL-1 produced by cancer cells acts as a growth factor that promotes the synthesis of other cytokines such as IL-6 and TGF-β in a paracrine and autocrine manner [152, 153]. It was recently reported that the baseline IL-1 expression and the newly produced IL-1 in response to CD40 agonists are both correlated with the resistance of in melanomas to immunotherapy [154]. Positive correlations were identified between IL-1β expression and the infiltration of immunosuppressive MDSCs, as well as the expression of their chemoattractants in patients with K-ras-mutant lung adenocarcinoma (KM-LUAD), suggesting the therapeutic potential of IL-1β blockades. However, some studies presented different results that supported the antitumor role of IL-1. For example, IL-1 has been found to induce Th1-mediated immunity against cancer [155]. Such dual activities of IL-1 in cancer require more detailed assessment when developing therapeutic intervention strategies targeting IL-1 [156].
In the TME, immunosuppressive cells including MDSCs, TAMs, TANs, regulatory B (Breg) cells, and Th17 are a major source of IL-1, which also are in turn regulated by IL-1 [157]. IL-1 plays a pivotal role in the differentiation of Th17 cells from naïve T cells and facilitates the maintenance of Th17 cell phenotypes [158]. Tumor-released IL-1α promoted tumor development by recruiting MDSCs to inhibit T cell activation [159]. The elevated level of IL-1β in the serum of advanced melanoma patients was associated with higher frequency of MDSCs and Tregs [160]. In addition, MDSC-secreted IL-1β promotes the production of angiogenic factors such as VEGF by tissue-resident endothelial cells [161, 162]. The immunosuppressive TME provides rationale for the combinatorial use of checkpoint blockades and IL-1 inhibitors, which displayed a synergistic antitumor effect in a breast cancer mouse model [163]. Similar results were reported in pancreatic ductal adenocarcinoma (PDAC) model where IL-1β blockade sensitized tumors to the PD-1 blockade [164].
Interleukin-6
Interleukin (IL)-6 is a family of protumorigenic cytokines consisting of IL-11, IL-27, IL-31, leukemia inhibitory factor (LIF), oncostatin M (OSM), ciliary neurotrophic factor (CNTF), cardiotrophin-1 (CT-1), and cardiotrophin-like cytokine (CLC), the role of which has been well characterized in the regulation of tumor growth and metastasis. IL-6 can be produced by multiple cell types including T cells, B cells, monocytes, fibroblasts, keratinocytes, endothelial cells, mesangial cells, adipocytes, and tumor cells. By interacting with IL-6 receptor (IL-6R), IL-6 activates STAT3 by upregulating the expression of cyclin D1, D2, and B1, and c-Myc and downregulating the expression of the cyclin-dependent kinase (CDK) inhibitor p21, which collectively accelerates the entry of tumor cells into cell cycles [165]. Moreover, tumor cells partially rely on the IL-6/STAT3 axis to escape cell death induced by cytotoxic drugs. IL-6-activated STAT3 in turn promotes tumor cell survival by inducing the expression of Bcl-2, survivin, and X-linked inhibitor of apoptosis protein (XIAP), the overexpression of which is related to increased chemoresistance [166, 167]. IL-6 may also contribute to cell proliferation, survival, and chemoresistance of tumor cells by activating the Ras-ERK and PI3K-Akt pathways [168]. Other mechanisms for the protumorigenic effect of IL-6 include the suppression of tumor senescence [169, 170], the interaction with growth factor signaling [171], the induction of EMT [172, 173], and angiogenesis [174]. Notably, IL-6 has been found to be overexpressed in common metastatic organs such as lung, liver, brain, and bone marrow, which is conductive to the seeding of circulating tumor cells to establish metastatic lesions [175,176,177].
Interleukin-10
IL-10 was initially conceived as a secreted cytokine synthesis inhibitory factor, known to inhibit cytokine production of Th1 cells [178] and activate macrophages and DCs [179, 180]. As a key mediator of the anti-inflammatory response, IL-10 family cytokines are mostly produced by leukocytes, as well as human tumor cells. This cytokine family consists of IL-10 and IL-20 subfamily cytokines including IL-19, IL-20, IL-22, IL-24, and IL-26 [181]. IL-10 suppresses uncontrolled inflammatory responses, thereby maintaining homeostasis [182]. In tumors such as gastric cancer, TAM-produced IL-10 contributes to an immunosuppressive microenvironment that favors tumor growth [183]. A more recent study showed that the expression of IL-10 in tumor-infiltrating regulatory T cells may result in the exhaustion of intratumoral CD8 + T cells [184]. Some studies on the other hand suggested that IL-10 can be used as an immunotherapy in tumor models [185]. IL-10 could induce the expression of CD3 and CD8 molecules on thymocytes and thereby promotes the cytotoxic activity of CD8 + T cells [186]. Another mechanism for the antitumor action of IL-10 is the increased CD8 + T cell infiltration and IFN-γ level in tumor tissues induced by IL-10 [181]. The discrepancies may be attributed to the tumor types or different stages of T cells that respond to IL-10. It is thus critical to assess the context before determining the either protective or detrimental role of IL-10 in cancer therapy.
ROS
Reactive oxygen species (ROS) are a large family of reactive molecules, including hydrogen peroxide (H2O2), hydrogen radicals (·OH), hydroxyl ions (OH −), superoxide anions (·O2 −), singlet oxygen (1O2), nitric oxide (NO −), peroxynitrites (ONOO −), and hypochlorite (OCl −) [187]. ROS are capable of rapidly switching one specie to another through cascade reactions because they are equipped with. Due to their unpaired valence electrons and unstable bonds, ROS rapidly switch from one to another and are therefore short-lived. As an essential signal molecule, ROS is implicated in various physiological possess, whereas excessive generation of ROS is associated with oxidative stress overload, leading to cell dysfunction and inflammation [188, 189]. Mitochondria are the major source of ROS and are actively involved in oxidative phosphorylation chain [190]. During aberrant oxidative phosphorylation, electrons escape and react with O2 to produce superoxide anions, which are then converted to H2O2 in the mitochondrial matrix. It has to be addressed that not all mitochondria-produced ROS derive from oxidative phosphorylation, with approximately 30% of H2O2 generated from oxidation of cytochrome C [191], and recently reported to be generated from nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [192]. Glutathione peroxidase (GPx) represents another endogenous antioxidant mechanism which degrades hydroperoxides [193]. In addition, the external stimuli such as chemotherapy, radiotherapy, and ultraviolet may also trigger ROS production [194].
Cancer cells carry higher amount of ROS than their normal counterparts, due to aberrant oncogene activation and mitochondrial activity. The role of ROS in cancer development is intricate, making it a double-edged sword [195]. On one hand, the sustained ROS stress may damage cell structures, impede their biological functions, and cause mutagenesis, which collectively increase the risks for oncogenesis [196, 197]. On the contrary, ROS may accumulate upon exogenous stimuli such as chemotherapy and radiotherapy, leading to tumor cell death and thereby sensitizing tumor cells to treatments. Elucidating the complex roles of ROS in cancer will aid the design of ROS-targeting therapies for cancer. Recent studies suggest that hypoxic environment in tumors could activate ROS generation [198]. In response to hypoxia, the hypoxia-inducible factor-1 (HIF-1) is a well-characterized transcriptional activator that modulates oxygen homeostasis [199]. By interacting with hypoxia response elements of target genes, ROS promotes the activation of HIF-1α, leading to subsequent transactivation of genes that augment hypoxic adaptation [200, 201]. It was recently reported that hypoxia-induced ROS augment the hypoxic adaptation of glioblastoma by mediating the HIF-1α-SERPINE1 signaling pathway, making ROS a promising therapeutic target for glioblastoma [202].
Key inflammatory pathways in cancer
Despite the cellular components of cancer-related inflammation, the vast majority of regulatory molecules have been identified to facilitate the protumorigenic effect of inflammation. Such molecules range from inflammatory cytokines to their downstream target molecules and transcription factors, represented by the eicosanoid signaling, and the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling.
Eicosanoid signaling
Eicosanoids are highly bioactive oxidized derivatives of 20-carbon polyunsaturated fatty acids (PUFAs) that can be produced through the cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (cytP450) pathways. Whereas the COX pathway produces prostaglandins (PGs) and thromboxanes (TXs), the LOX pathway is known to generate leukotrienes (LTs) and lipoxins (LXs) [203]. The rapid catabolism of eicosanoids constrains their activities to the local sites of their production [110]. The eicosanoid signaling cascades play a pivotal role in both physiological processes and pathological processes such as tumorigenesis.
Cyclooxygenase (COX) signaling
The COX pathway is a well-studied mechanism through which eicosanoids are formed and link inflammation with cancer. COX-1 and COX-2 are two key isoforms of COX enzymes. Under physiologic conditions, the constitutive expression of COX-1 is important for maintaining tissue homeostasis. On the other hand, the expression of COX-2 is upregulated by proinflammatory stimuli. Another isomer COX-3 has recently been identified, the function of which remains to be further elucidated [204, 205]. Among them, COX-2 has been intensively studied for its regulation of cancer-associated inflammation and cancer progression. The upregulation of COX-2 was first identified in human colorectal adenomas and adenocarcinomas [206] and was found to correlate with inflammatory bowel disease and colorectal cancer [207]. The association between COX-2 overexpression and unfavorable prognosis has later extended to various cancer types including melanoma [208], breast [209], prostate [210, 211], laryngeal [212], esophageal [213], gastric [214], pancreatic [215], and ovarian cancer [216].
During the early stage of the inflammatory response, COX-2-derived PGs are assumed to display proinflammatory functions [217]. The prostaglandin D2, prostaglandin E2, prostaglandin F2α, prostaglandin I2, and thromboxane A2 are five key PGs derived via the COX pathway. Among them, PGE2 is the most common prostaglandin in cancer, the upregulation of which is associated with poor prognosis and more advanced tumor stage [218,219,220]. Accordingly, genetic deletion of microsomal PGE2 synthase 1 (mPGES-1) gene leads to decreased intestinal tumor growth by 66–95% [221]. Furthermore, PGE2 may also promote tumorigenesis by inducing immune suppression [222, 223]. PGE2 potently regulates IFN-γ synthesis of NK cells, which is an important proinflammatory event [224]. The MDSCs were found to express receptors for PGE2, the antagonists of which could block the differentiation of MDSCs [225]. PGE2 may enhance the immunosuppressive phenotype of mononuclear (M)-MDSCs and potentiate its inhibitory activities on T cell proliferation [226]. In response to IFN-γ, tumor-derived PGE2 also induces nuclear p50 NF-κB that epigenetically reprograms monocyte toward an immunosuppressive phenotype, providing another rationale for the tumorigenic effect of PGE2 [227].
In contrast to prostaglandin E2 the role has been established in cancer, prostaglandin D2, another COX-2 metabolite and may play dual roles in chronic inflammation and cancer. The interaction between PGD2 and its receptor PTGDR2 inhibits the self-renewal of gastric cancer cells and attenuates the growth and metastasis of gastric tumors [228]. In addition, PGD2 also inhibits colitis and colitis-associated colon cancer in mouse models [229]. It was recently reported that PGD2 could reduce the proliferation of lung cancer cells, but at the same time enhance their invasion and migration [230], leading to the hypothesis that the exact role of PGD2 in cancer may vary according to the tumor stage.
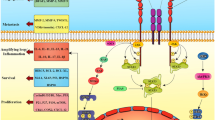
The contributing role of COX-2/PGE2 in immunosuppression has long been studied even before the advent of immunotherapy. The association between COX-2 expression and T cell exclusion was found in pancreatic cancer models [231]. The intrinsic TGF-β signaling of pancreatic tumor cells induced the overexpression of PTGS2, leading to decreased level of activated CD8 + T cells in the TME [231]. In addition, COX-2/PGE2 signaling is associated with the accumulation of MDSCs. Thus, blocking COX-2/PGE2 signaling could reshape TME by reversing the immunosuppressive activities of MDSCs [232]. Moreover, PGE2 also impacts the polarization status of macrophage by inducing monocyte differentiation into the M2-like macrophage [233]. Given that the COX-2/PGE2 pathway facilitates the maintenance of immunosuppressive TME by activating a wide range of immunosuppressive immune cells, inhibiting COX-2 signaling is potentially a good combination partner for immunotherapies, such as checkpoint inhibitors (Fig. 2).
Overview of the cyclooxygenase pathway and the action mechanisms of cyclooxygenase-targeting strategies in cancer. The COX-2/PGE2 pathway facilitates the maintenance of immunosuppressive TME by activating a wide range of immunosuppressive immune cells. Inhibitors of COX-2 signaling such as NSAIDs are potentially a good combination partner for immunotherapies. Figures created with BioRender. Abbreviations: PGH2, prostaglandin H2; PGG2, prostaglandin G2; PLA2, PLC, PLD, phospholipases A2, C, and D; PGE2, prostaglandin (PG) E2; PGI2, prostacyclin; PGD2, prostaglandin D2; PGF2α, prostaglandin F2α; TXA2, thromboxane A2; MDSC, myeloid-derived suppressor cells
Lipoxygenase (LOX) signaling
The LOX pathway mainly comprises 5-LOX, 12-LOX, and 15-LOX [110]. Whereas 5-LOX and 12-LOX have been identified with angiogenetic and protumorigenic activities, 15-LOX exerts both protumorigenic and antitumorigenic effects [234]. As a key enzyme in metabolizing arachidonic acid to leukotrienes, 5-LOX is highly expressed in epithelial cancers as well as lymphomas [235, 236]. Inhibiting approaches targeting 5-LOX were used to inhibit tumorigenesis [226, 237]. Given that both 5-LOX and COX-2 are upregulated in inflammation-related tumors, the concomitant inhibition of 5-LOX and COX-2 was designed to render more potent tumor suppression than inhibition of a single eicosanoid pathway [116, 238, 239].
The 12-LOX is a key enzyme that mediates the generation of 12-HETE which in recent years has been identified to facilitate tumor growth by activating the integrin-linked kinase/NF-κB pathway [240, 241]. 15-LOX-1, on the other hand, can be expressed in Hodgkin lymphoma cells, and its metabolites were found to enhance tumor-associated inflammation [242]. As discussed earlier, 15-LOX may have antitumorigenic role in cancer. A recent study suggested decreased levels of 15-LOX in doxorubicin (DOX)-resistant cells compared with their DOX-sensitive counterparts. The overexpression of 15-LOX could induce DOX accumulation in DOX-resistant breast cancer cells and promote their apoptosis [243]. Similar data were obtained from colorectal cancer (CRC) model where deficient 15-LOX-1 was correlated with the radioresistance of CRC cells, potentially by downregulating the histone H2A variant macroH2A2 [244].
The LOX pathways are responsible for metabolizing arachidonic acid to leukotrienes such as leukotriene A4 (LTA4) and leukotriene B4 (LTB4). Inflammatory cells including leukocytes, macrophages, and mast cells are the major source of leukotrienes [245]. LTB4 was found to promote inflammation-induced melanoma, and the inhibition of LTB4 receptors may suppress the progression of inflammation-associated tumors [246]. The leukotriene D4 (LTD4), derived from the 5-LOX-catalyzed oxygenation of arachidonic acid, is upregulated in the circulation of patients with hepatocellular carcinoma and chronic hepatitis B [247, 248]. Recent studies investigated the efficacy of leukotriene receptor antagonists as a novel combination partner for conventional multi-kinase inhibitors in the treatment of hepatic cancer [249].
On the contrary, another LOX-derived eicosanoids, lipoxins (LXs), are characterized as antitumorigenic [250]. Lipoxins stimulate monocytes without causing the inflammatory release of ROS [251]. Lipoxins may also promote the phagocytosis of apoptotic neutrophils by macrophages, thereby reducing inflammation[252]. Accumulating evidence suggests the anti-inflammatory effect of lipoxin A4 (LXA4) in inflammation-associated cancers such as colorectal cancer [253]. In prostate cancer, LXA4 promotes the M2 polarization of macrophages by inhibiting METTL3 [254]. Other mechanisms for the LXA4-induced polarization of M2 macrophages may be mediated via the FPR2/IRF4 pathway [255]. However, a recent study reported that lipid mediators such as lipoxins could induce the angiogenesis, proliferation, and treatment resistance of glioblastoma cells [256]. More studies are warranted to elucidate the potential of endogenous lipoxin administration in combating cancer.
JAK-STAT signaling
The JAK/STAT signaling is a highly conserved pathway with the ligand–receptor interaction machinery. The JAK family consists JAK1, JAK2, JAK3, and TYK2, and the STAT family members include STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6 [257]. In general, the receptors–ligand interaction induces the phosphorylation of JAKs which then form a docking site for STATs leading to STAT phosphorylation. As the core member of the STAT protein family, STAT3 plays a with versatile roles in the inflammatory response and tumor progression. Multiple growth factors and cytokines are implicated in the canonical STAT3 pathways, regulating the transcription of STAT3 target genes and downstream cellular processes such as cell differentiation, angiogenesis, and tumorigenesis [258]. The dysregulated STAT3 signaling has been implicated in a series of inflammatory diseases such as rheumatoid arthritis, multiple sclerosis, and inflammatory bowel disease [259]. Moreover, the persistent activation of the STAT3 signaling may result in the tumorigenesis of both solid and hematological malignancies [260].
Chronic inflammation is a key event of tumorigenesis [261]. Genome-wide association studies have identified a certain correlation between STAT3 and the susceptibility to inflammatory bowel disease (IBD) [262]. Cytokines that induce the activation of STAT3 are upregulated in IBD such as IL-1β, IL-6, IL-12, IL-15, IL-10, IFN, and TNF-α [263]. It has been well established that IL-6 and STAT3 are required for survival and proliferation of tumor-initiating intestinal epithelial cells [264]. As a critical regulator of the inflammatory process, the IL-6/STAT3 signaling is implicated in inflammation-associated tumors such as CRC and colitis-associated CRC (CAC) [265]. Furthermore, in CRC stroma, cancer-associated fibroblasts (CAFs) produce IL-6 which upregulates the expression of metastasis-associated markers such as Leucine Rich Alpha-2-Glycoprotein 1(LRG1) via the JAK2/STAT3 signaling [266].
The status of the gut microbiome which metabolizes bile acid in the intestine is another important determinant of intestinal inflammation, with certain microbes either promoting or suppressing tumorigenesis of CRC [267]. The loss of integrity of intestinal epithelial barriers and the recognition of PAMPs by PRRs leads to increased secretion of inflammatory factors that activate STAT3, thereby evoking inflammatory response in CRC. Similar results were observed in prostate cancer where gut dysbiosis increased gut permeability and intratumoral LPS which promotes tumor progression via NF-κB/IL6/STAT3 axis [268].
Metal metabolism
Iron is indispensable for multiple cellular events such as cell survival and biological processes such as oxygen transport and deoxyribonucleic acid (DNA) synthesis [269]. Dysregulated iron metabolism is a crucial hallmark of tumor cells where malignant cells need substantial amount of iron to survive and proliferate. In the Fenton reaction, the redox-active iron (Fe2 +) reacts with H2O2 which directly generates ferric iron (Fe3 +) and a large amount of hydroxyl radicals [270]. As aforementioned, the balance between ROS generation and detoxification is important to prevent the oxidative stress and ROS-mediated cell death [271]. Iron-dependent enzymes such as cytochrome P450 enzymes, nitric oxide synthases, NADPH oxidases, and lipoxygenases are involved in the generation of ROS [272]. Excessive iron is also associated with ferroptosis, a type of regulated cell death. GPX4 is the key regulating glutathione peroxidase of ferroptosis, which converts lipid hydroperoxides to lipid alcohols, and prevents the iron (Fe2 +)-dependent formation of ROS [273]. Thus, inhibiting GPX4 could enhance the antitumor response of therapies by inducing ferroptosis. Nevertheless, even with high oxidative stress, ferroptosis is not a frequent event in tumor cells. Several agents have been identified with ferroptosis-inducing capacity, including erastin, a voltage-dependent anion channels (VDAC)-2/3 inhibitor, and sorafenib, a multikinase inhibitor [274].
Zinc is the second most abundant fundamental nutritional element in human body, which was first documented in the 1960s regarding its role in human health [275]. Zinc is implicated in the production and signaling of numerous inflammatory cytokines, and upon acute response to stress stimuli, plasma concentrations of zinc rapidly drop. Zinc metabolism in humans is tightly associated with the activities of zinc transporters such as ZIP8. During inflammation, activated NF-κB increases the expression of ZIP8 which localizes to cell membrane and regulates zinc uptake. Following the entry of zinc into cytosol, zinc suppresses IKKβ activities and thereby attenuates the inflammatory response, all of which form a negative feedback loop [276]. These results highlight the regulating role of metal metabolism in inflammation and cancer and unveil the therapeutic potential of metabolic reprogramming in disease treatment.
Inflammation-targeted therapies in cancer
As aforementioned, the inflammatory cells and mediators including cytokines, chemokines, and eicosanoids form an intricate network in the TME and regulate tumor-associated inflammatory responses. Emerging preclinical results have motivated the design of anti-inflammatory agents for the treatment of cancer, either as monotherapy or in combination with other therapeutic modalities (Table 3). We herein discuss the current application of inflammatory-targeted treatments and the potential for translating current knowledge on cancer-related inflammation into clinical practice. The molecular mechanisms that mediate the effects of inflammation-targeting strategies in cancer are presented in Fig. 3.
Molecular mechanisms that mediate the effects of inflammation-targeting strategies in cancer. These inflammation-targeting strategies inhibit the COX, JAK/STAT, and TGF-β signaling which support cancer cell survival, proliferation, and invasion. Figures created with BioRender. NSAIDs, non-steroidal anti-inflammatory drugs; COX, cyclooxygenase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa B; CXCR, CXC-chemokine receptor; CXCL, chemokine (C-X-C motif) ligand; TGF-β, transforming growth factor-β; TGF-βR, TGF-β receptor; IL, interleukin; IFN, interferon; STAT3, signal transducer and activator of transcription 3; SMAD, mothers against decapentaplegic
Non-steroidal anti-inflammatory drugs (NSAIDs)
With the advent of aspirin in the 1990s, the application of NSAIDs has been extended to the treatment of pain, fever, and other inflammatory processes. Multiple studies have addressed the preventative effect of NSAIDs on cancer, leading to reduced incidence of colorectal [277], breast [278], and esophageal cancer [279]. In a randomized clinical trial, daily administration of aspirin effectively prevented adenoma growth in patients with familial adenomatous polyposis [280, 281]. Another clinical trial demonstrated that aspirin decreased the recurrence rates of colorectal adenomas and the incidence of CRC in patients with hereditary Lynch syndrome [277]. A multicenter, randomized controlled clinical trial (AspECT) aimed to investigate the long-term chemoprevention effect of esomeprazole proton-pump inhibitor (PPI) and aspirin, suggesting that the combination treatment of aspirin and esomeprazole significantly improved the clinical outcome of patients with Barrett's esophagus, thereby reducing the risk of esophageal cancer [282].
A major mechanism through which NSAIDs suppress carcinogenesis is the eicosanoid signaling. NSAIDs inhibit the cyclooxygenases (COX-1 and COX-2), but not the lipoxygenases. As the levels of PGE2 and COX-2 are often elevated in cancers such as CRC [283, 284], COX-2 inhibitors especially COXIBs (selective COX-2 inhibitors) were developed, with potent anti-inflammatory activities without affecting the physiological functions of COX-1 [285]. Thus, COXIBs are believed to cause fewer gastrointestinal side effects compared with non-selective NSAIDs and at the same time derive the same benefits [238]. In 1999, the Food and Drug Administration (FDA) approved the use of celecoxib, a COXIB, in patients with familial adenomatous polyposis [286].
Multiple clinical trials have evaluated the potential of celecoxib for the prevention and treatment of cancer patients. For instance, the concomitant use of celecoxib and chemotherapy (FOLFIRI regimen consisting of 5-flourouracil, leucovorin, irinotecan) may represent an effective and safe synergetic protocol for patients with metastatic CRC (NCT03645187) [287]. Celecoxib also demonstrates excellent efficacy in the prevention of colorectal adenomas (NCT00005094) [288]. The administration of celecoxib significantly reduced the occurrence of colorectal adenomas in patients receiving polypectomy (NCT00141193) [289]. Celecoxib has also been tested in synergy with PD-1 blockade toripalimab, which induced a high pathological complete response rate and an acceptable safety profile in patients with mismatch repair (MMR) deficient or microsatellite instability (MSI)-high CRC (NCT03926338) [290]. A meta-analysis further confirmed the potential of celecoxib-combined cancer therapy in improving clinical outcomes in several cancer types [291]. In patients with positive COX-2-positive gastric cancer, combination therapy of celecoxib and chemotherapy significantly improved disease-free survival (DFS), progression-free survival (PFS), and short-term clinical efficacy, without increasing the incidence of adverse events (AEs) [292]. In lung cancer, celecoxib at a maximal tolerated dose of 800 mg/d can be safely administered concurrently with thoracic radiotherapy and resulted in PFS rates of 66.0% at 1 year and 42.2% at 2 years [293]. In other phase II trials however, celecoxib treatment (NCT00300729) or adding celecoxib to concurrent chemoradiation (NCT01503385) did not improve survival of NSCLC patients [294, 295]. In a phase II trial, celecoxib induced favorable changes in serum biomarkers and cytology in women with increased risk for breast cancer [296]. Notably, the improvement of prognosis by celecoxib-based combination treatment is more prominent in patients with tumors expressing higher levels of COX-2 [297]. No statistical difference in AEs was identified between treatment group and control group, such as dysphagia, anxiety, dry mouth, and hair loss. Celecoxib treatment induced a significantly higher pathological complete response (pCR) rate in breast cancer patients with COX2-overexpressing tumors [298].
However, a recent clinical trial suggested that the addition of celecoxib to the standard adjuvant chemotherapy regime failed to bring more benefits to patients with stage III colon cancer (NCT01150045) [299]. Another study evaluated the efficacy of celecoxib as a combination partner for conventional therapy in ERBB2-negative breast cancer, which demonstrated no significant benefits from celecoxib in terms of DFS following 2-year treatments (NCT02429427) [300]. Moreover, some studies suggested that the addition of celecoxib to chemotherapy might adversely impact the prognosis of breast cancer patients, especially those with prostaglandin-endoperoxide synthase 2 (PTGS2) low tumors (NCT01041781) [301]. Such conflicting results likely reflect the impact of different treatment regimens or administration doses of celecoxib, and the expression profile of biomarkers in tumors. Thus, all the above factors should be taken into account to investigate the therapeutic potential of celecoxib. In addition, long-term use of NSAIDs including COXIBs at high doses may lead to severe cardiovascular side effects in patients, especially in those with a history of atherosclerotic heart disease [302]. One way to prevent or reduce these side effects would be the alternative targeting of the downstream PGE2 pathway. Some researchers have introduced natural compounds with known inhibitory activities on COX-2, such as natural phenols, flavonoids, stilbenes, terpenoids, quinones, and alkaloids [303].
Antiviral therapies
Antihepatitis B virus (HBV) therapies
The majority of hepatocellular carcinoma (HCC) cases are associated with known risk factors, such as chronic hepatitis B virus infection. During chronic hepatitis B (CHB) infection, the immune response to persistent infection may cause chronic inflammation and hepatic fibrogenesis, leading to irreversible damage in the liver structure. The continuous replication of virus DNA and its integration into host genomes may cause genetic alterations, ultimately driving the carcinogenesis of hepatocytes [120]. On the other hand, viral proteins such as hepatitis B virus X protein may increase the sensitivity of the host to chemical carcinogens [304]. These preclinical studies have motivated the design of antiviral therapies in the treatment of HBV-related hepatocellular carcinoma.
The antiviral therapies aim to suppress HBV DNA replication, promote the serum conversion of hepatitis B e antigen (HBeAg), and attenuate the development of cirrhosis. Common antiviral drugs include the nucleoside and nucleotide analogs (NAs) and IFNs. Among them, the long-term administration of potent NAs with high barrier to resistance such as entecavir and tenofovir disoproxil, was recommended as first-line anti-HBV drugs in the clinical management consensus of CHB [305]. In a randomized controlled trial involving 299 centers in Asia, Europe, and North and South America with a 10 year of follow-up, patients treated with entecavir had a reduced risk of HBV-related events including HCC (NCT00388674) [306]. A nationwide population-based cohort study on CHB patients suggested that tenofovir treatment had lower incidence of HCC compared with entecavir treatment [307]. The superiority of tenofovir over entecavir in reducing HCC incidence in CHB patients was further confirmed in several other studies [303, 308]. However, some studies failed to identify clinically meaningful difference in the risk of liver-related events or deaths including HCC between entecavir- and tenofovir-treated cohorts, suggesting that the choice between tenofovir or entecavir should be based on patients’ tolerability (NCT019553458) [309, 310]. A recent study compared the long-term risk of tenofovir versus entecavir on HCC and intrahepatic cholangiocarcinoma (ICC) in CHB patients and suggested a comparable long-term risk between these two agents [311]. Recently, some antifibrotic Chinese herbs have been introduced to the antiviral therapy formulas for the treatment of CHB-related liver fibrosis. For instance the therapeutic potential of entecavir combined with Ruangan granule to reverse advanced liver fibrosis is currently being investigated in a number of clinical studies [312, 313].
Antihuman papillomavirus (HPV) therapies
Persistent HPV infection is a well-established risk factor for cervical cancer or precancerous cervical dysplasia [314, 315]. HPV proteins are implicated in the development of chronic inflammation [316]. The persistent HPV infection initiates a chain of reactions that regulate the secretion of inflammatory cytokines and immune cell infiltration [317]. For instance, the sustained elevation of systemic inflammatory cytokine levels was observed in older populations with chronic HPV infection [318], which potentially increased the risk for cervical cancer in this age group [319, 320].
The efficacy of HPV vaccines against cervical precancerous lesions has been confirmed by multiple large-scale reports. The population-based vaccination not only decreased the infection rates of HPV, but also the incidence of cervical intraepithelial neoplasia in women aged 20–24 years [321]. Recent results from a nationwide clinical study suggested that the cumulative incidence of cervical cancer was dramatically reduced by approximately 50% in women received the quadrivalent HPV vaccine at 10–30 years of age [322]. Given that antiviral drugs that specifically target HPV infections are still lacking, increasing HPV vaccination coverage in the population would potentially facilitate cervical cancer occurrence [323]. The first-in-human clinical trial of Vvax001, an alphavirus-based vaccine against HPV, was conducted in patients with HPV-induced cancers to assess its immunological activity, safety, and tolerability. The preliminary results supported the therapeutic application of Vvax001 in patients with HPV-related malignancies [324]. Similarly, the long-term follow-up results from a randomized, double-blind, controlled trial demonstrated that the bivalent HPV vaccine was highly effective in preventing HPV 16/18-associated precancer, further supporting the possibility to prevent invasive cervical cancer [325]. Another randomized trial investigated the combinational efficacy of anti-PD-1 antibody nivolumab with ISA 101, a synthetic HPV-16 vaccine, in patients with HPV-16-positive cancer. The combination therapy has increased both overall response rates and survival compared with PD-1 blockade monotherapy (NCT02426892) [326].
Cytokine- and chemokine-directed therapies
The intratumoral infiltration of leukocytes and their release of soluble factors are important parts of the cancer-associated inflammation. These secretory factors include inflammatory cytokines such as IL-6, TNF-α, and IL-1b which facilitate the proliferation and metastasis of tumor cells, and suppress antitumor immune responses. We herein describe the anticancer therapies targeting cytokines or chemokines involved in cancer-related inflammation.
IFN-α-directed therapies
During the past decades, the adjuvant IFN-α therapy was intensively studied for the treatment of pancreatic cancer, with markedly improved prognosis observed from several clinical trials [327,328,329,330]. IFN-α was initially used as adjuvant therapies for patients with high-risk melanoma, which improved both relapse-free survival (RFS) and OS in patients receiving surgical treatments [331]. Adjuvant treatment with IFN-α-2a could improve the DFS and potentially OS of melanoma, with no improvement in clinical outcomes by PEG-IFN over IFN (NCT00204529) [332]. Nevertheless, inconsistent data were reported by some clinical trials that IFN-α derived no apparent benefits on the OS of patients [333]. High-dose interferon (IFN) for 1 year (HDI) has been approved by the FDA as adjuvant therapy for melanoma. In Japanese populations, PEG IFN-α-2b was well tolerated and approved in 2015 as adjuvant therapy in patients with stage III malignant melanoma [334]. Though approved by FDA for the treatment of melanoma and RCC, recombinant IFN-α is currently not a mainstream option due to the high incidence of AEs [335, 336]. Long-term follow-up results from the randomized phase III trial EORTC 18991 suggested that adjuvant PEG-IFN-α-2b therapy was able to induce sustained improvement of RFS in stage III melanoma patients [337]. On the other hand, PEG-IFN-α-2b may also negatively impact the health-related quality of life (HRQOL) of patients [338]. A phase III trial S0008 compared the efficacy of HDI regimen with short-term biochemotherapy consisting of dacarbazine, cisplatin, vinblastine, IL-2, IFN-α-2b, and GCSF and reported significant improvement in RFS but no significant difference in OS [339]. The grade 3 and 4 adverse events occurred in 57% and 7% of HDI patients, compared with 36% and 40% in biochemotherapy patients. IFN-α is also frequently used as a combination partner for immunotherapies or target therapies. The combination of the BRAF inhibitor vemurafenib and PEG-IFN-α-2b was well tolerated in melanoma patients whose treatment response was correlated with IFNAR1 expression levels (NCT01959633) [340]. Previous data supported the prophylactic administration of PEG-IFN-α for leukemia patients during the treatment of peri-hematopoietic cell transplantation (HCT) to prevent leukemia relapse (NCT02328755) [341]. IFN-α treatment is an effective strategy for minimal residual disease (MRD)-positive leukemia patients receiving allogeneic hematopoietic stem cell transplantation (allo-HSCT) (NCT02185261) [342].
IFN-α is a promising combination therapy for target therapies and immune checkpoint inhibitors such as anti-PD-1 therapies [343]. The prolonged IFN-α treatment results in long-lasting complete responses and long-term outcome with acceptable toxicity in patients with metastatic RCC. Sorafenib, a kinase inhibitor drug approved for the treatment of primary kidney cancer, concurrently used with IFN-α has been proved safe and effective for metastatic RCC patients (UMIN000002466) [344]. Similarly, bevacizumab plus IFN led to superior benefits in terms of PFS and ORR in patients with metastatic RCC as compared with IFN monotherapy (CALGB 90206) [345]. Recent research has focused on the potential of IFN-α in combination with ICBs which may overcome the treatment resistance to ICBs [346]. In NSCLC patients treated with nivolumab, a significantly elevated level of peripheral IFN-α was observed in those with longer PFS, indicating the synergistic effect of regional IFN-α with anti-PD-1 therapy [347]. The combination of ipilimumab with high dose IFNα2b (HDI) demonstrated an acceptable toxicity profile and a promising tumor response in ICB naïve patients (no treatment history of ICB) [348, 349]. Another factor that limits the use of IFNs is the short half-life of IFNs which makes it difficult to deliver IFNs to tumor sites at sufficient concentrations. To solve this, IFNs conjugated to tumor-specific mAbs were developed. An early example is the anti-CD20-IFN-α2 conjugate which increased antibody-dependent cytotoxicity and overcame the resistance to anti-CD20 treatment alone in mouse models [350, 351]. In addition, the anti-VEGFR mAb-conjugated IFN-α could inhibit the angiogenesis and promote immune responses in CRC tumor models [352]. IL-4 fused to pseudomonas exotoxin represents another novel combination partner for IFNs, which was found to improve the OS of mice with ovarian cancer xenograft, potentially by activating the key mediators of apoptosis [353].
Given the potential antitumor activities of IFN-α described in previous literature, IFN-α is also used as an adjuvant in tumor vaccines such as DC vaccines, augmenting their efficacy in tumors [354, 355]. For instance, IFN-α-conditioned DCs significantly increased the number of tumor-specific CD8 + T cells with cytotoxic phenotypes than cytokine cocktail-mDCs in RCC patients [356]. In a phase I clinical study, IFN-DCs were well tolerated and included marked immunological responses in advanced melanoma patients [357]. More recently, IFN-DCs were used as a novel DC-based immunotherapy for non-Hodgkin lymphomas (NHL) [358].
TGF-β-directed therapies
Therapeutic approaches targeting TGF-β mainly include: (1) the small-molecule inhibitors of TGF-β receptor I (TGF-βRI) such as galunisertib; (2) anti-TGF-β mAbs such as fresolimumab; (3) antagonistic mAbs targeting TGF-βR and TGF-β ligand traps [359]. Fresolimumab (GC1008) is a TGF-β-blocking antibody that neutralizes all mammalian active isoforms of TGF-β and was reported to induce stable disease in 6 out of 29 melanoma patients [360]. In patients with advanced melanoma and RCC, fresolimumab displayed preliminary antitumor efficacy and acceptable safety profile at multiple doses [360]. For patients with advanced malignant melanoma and RCC, Fresolimumab was safe and displayed preliminary antitumor efficacy (NCT00356460) [360]. A recent study examined the efficacy and immune effects of fresolimumab in metastatic breast cancer patients during radiotherapy treatment, where a favorable systemic immune response was observed. Notably, fresolimumab improved the OS of patients in a dose-dependent manner, with longer median OS observed in those treated at higher dose [361].
Galunisertib is a TGF-β1 receptor type I inhibitor and was intensively studied for the treatment of HCC and pancreatic cancer. The combination of galunisertib and sorafenib demonstrated improved prognosis of HCC, with neutropenia, fatigue, anemia, increased bilirubin, hypoalbuminemia, and embolism being the most common treatment-related AEs. (NCT01246986) [362, 363]. The galunisertib–gemcitabine combination improved OS in patients with unresectable pancreatic cancer with minimal added toxicity [364]. Galunisertib co-administered with durvalumab was tolerable, but with limited clinical activity which required the selection of predictive biomarkers for TGF-β inhibition in pancreatic cancer patients (NCT02734160) [365]. In a phase Ib/II study, galunisertib combined with checkpoint inhibitor nivolumab was well tolerated in NSCLC (NCT02423343) [366]. In this phase of the trial, the most frequent AEs were pruritus, fatigue, and decreased appetite. In addition, the addition of galunisertib to neoadjuvant chemoradiotherapy was well tolerated and improved the complete response rate in patients with rectal cancer (NCT02688712) [367].
PF-03446962 is a monoclonal antibody (mAb) targeting activin receptor like kinase-1 (ALK1), a TGF-βR subtype, which showed limited activity in urothelial carcinoma and is thus not recommended as monotherapy [368]. A phase I study reported manageable safety and pharmacokinetic profiles with promising clinical activity, supporting further evaluation of PF-03446962 in patients with HCC and other solid malignancies (NCT00557856) [369]. However, several other clinical trials failed to identify improvement of objective responses in patients with HCC, RCC, NSCLC, and malignant pleural mesothelioma [369,370,371]. More recently, the combination of regorafenib and PF-03446962 was found to cause unacceptable toxicity with limited clinical activity in patients with refractory metastatic CRC [372]. Thus, PF-03446962 has not been developed further.
Based on the observation that TGF-β signaling was associated with treatment resistance to anti-PD-L1 therapies, a novel dual-targeting agent bintrafusp alfa was developed. Bintrafusp alfa is a bifunctional fusion protein consisting of the extracellular domain of the TGF-βRII receptor and a PD-L1-blocking immunoglobulin G1 (IgG1) mAb [373].
An expansion cohort of a phase trial suggested that bintrafusp alfa induced encouraging efficacy and manageable tolerability in patients with NSCLC previously treated with platinum (NCT02517398) [374]. Bintrafusp alfa has demonstrated potent clinical activity with manageable safety in patients with HPV-associated cancer (NCT02517398, NCT02517398, NCT04247282) and esophageal adenocarcinoma (NCT02517398, NCT02699515) [375,376,377,378,379]. Moreover, the simultaneous inhibition of TGF-β and PD-L1 by bintrafusp alfa could synergize with radiotherapy in radioresistant tumor models [380]. These results collectively support the clinical translation of this dual-targeting agent in treating therapy-resistant tumors, with minimal damage to normal tissues.
IL-1-directed therapies
In the clinical setting, many NSCLC tumors displayed low PD-L1 expression, which requires other treatment options to improve the efficacy of ICBs. As aforementioned, the elimination of MDSCs in the TME by inhibiting the IL-1 pathway is a potential strategy to overcame tumor resistance to immunotherapies such as immune checkpoint blockades [381],which has been evaluated in different models. Anti-IL-1β mAbs could enhance the efficacy of PD-1 blockades against breast cancer [163]. In a RCC mouse model, the combination of IL-1β blockade with either anti-PD-1 or tyrosine kinase inhibitors achieved greater antitumor efficacy than either monotherapy [382].
Canakinumab is an anti-IL1β mAb that has been approved for use in a variety of immune-related disorders. Clinical inhibition of IL-1β by canakinumab in lung cancer was first reported in a phase III study, the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) [383]. In this trial, canakinumab reduced both the occurrence and mortality of lung cancer, providing the first rationale for the assessment of canakinumab use in lung cancer patients [384]. Though with less lung cancer mortality, canakinumab 300 mg group had higher incidence of fatal infections or sepsis than the placebo group. CANOPY-N is a randomized phase II trial investigating the efficacy of combination therapy with canakinumab and pembrolizumab as neoadjuvant treatment in patients with non-small cell lung cancer (NSCLC) [385]. Later evidence suggested that blocking IL-1β with canakinumab may be a preventive approach for individuals with high risks for KM-LUAD [386].
Anakinra is a human anti-IL-1R1 antibody and has been approved by the FDA for the treatment of rheumatoid arthritis. Anakinra has also been used for the treatment of several cancers [387,388,389,390]. Preclinical studies reported that gemcitabine and 5-fluorouracil (5-FU) could promote IL-1β production in a T-cell lymphoma-bearing mouse model, which restrained the efficacy of chemotherapeutic agents [391]. Thus, anakinra can be used as an adjunctive therapy to enhance the efficacy of chemotherapy of 5-FU. In the clinical context, the combination of chemotherapy with 5-FU, anakinra, and bevacizumab led to an increased median PFS and OS of patients with metastatic CRC with minimum AEs [392]. In patients with multiple myeloma at high risk of progression to active myeloma, treatment with anakinra decreased the proliferative rates of tumor, leading to a chronic disease state with improved PFS (NCT00635154) [393].
IL-2-directed therapies
IL-2 is a key growth factor for CD4 + T cells and NK cells and is involved in the regulation of T cell proliferation, survival, and differentiation [394,395,396]. IL-2 has been described as a immunostimulant, and its anticancer activities have been studied for more than 30 years [397]. The intravenous administration of recombinant IL-2 was approved by the FDA for the treatment of metastatic RCC in 1992 and melanoma in 1998. Though IL-2 treatment could induce durable response in melanoma and RCC patients [398], the short half-life of IL-2 requires a therapeutic schedule with an 8-h interval. Moreover, a high incidence of severe AEs including vascular leak syndrome and cardiac toxicities was frequently reported due to the high dose of IL-2 to reach its efficacy [399]. IL-2 was also shown to promote the activities of immunosuppressive Tregs, which casted doubt on the antitumor role of IL-2 [399]. The impact of IL-2 on Tregs might be attributed to the constitutive expression of IL-2 receptor on Tregs. This receptor consists 3 subunits (IL-2Rαβγ) and has higher affinity to IL-2 compared with those expressed on CD8 + T cells, memory T cells, and NK cells which lack the α subunit [400].
The differential expression of IL-2 receptors has motivated the design of IL-2R agonists that selectively activate the IL-2Rβγ complex on immunostimulatory immune cells. A PEGylated form of IL-2, bempegaldesleukin (NKTR-214/BEMPEG) preferentially interacts with the β subunit of IL-2R, specifically stimulating the antitumor activities of CD8 + T cells and NK cells [401]. Multiple clinical studies have identified bempegaldesleukin as a promising agent in reducing tumor volumes in pre-treated melanoma and RCC [402]. Bempegaldesleukin has also been investigated as a combination partner for nivolumab, which yielded objective response rates (ORRs) of approximately 33–75% in patients with melanoma, RCC, NSCLC, or triple-negative breast cancer (TNBC) [403]. A number of clinical trials are ongoing to assess the safety and clinical benefits of bempegaldesleukin when combined with pembrolizumab in patients with metastatic melanoma (NCT03635983) [404]. Bempegaldesleukin is also suggested to be used in combination with nivolumab as the first-line therapy for patients with metastatic urothelial carcinoma (NCT02983045) or metastatic melanoma (PIVOT-02), with manageable side effects [405, 406]. Nemvaleukin alfa (nemvaleukin, ALKS 4230) is a novel engineered forms of IL-2 that selectively binds to the IL-2R on antitumor CD8 + T cells and NK cells with minimal effect on immunosuppressive Tregs [325]. In a novel SCLC murine model, the mouse version of nemvaleukin (mNemvaleukin) significantly inhibited murine SCLC tumor growth and improved mouse survival, supporting the evaluation of nemvaleukin alone or in combination with chemotherapy in clinical trials [407]. Ongoing clinical trials such as ARTISTRY-7 trial compared efficacy and safety of nemvaleukin as monotherapy and combination therapy with pembrolizumab in patients with platinum-resistant ovarian cancer (NCT05092360) [408,409,410].
In addition to engineered IL-2 that activates the IL-2Rβγ complex, another therapeutic strategy is to target IL-2α (CD25) and thus deplete the immunosuppressive Tregs. Earlier studies reported that the intravenous infusion of daclizumab monotherapy induced a significant and persistent decrease in CD25 + FOXP3 + Tregs in peripheral blood of breast cancer patients [411]. This result was further confirmed in patients with glioblastoma [412] and metastatic melanoma [413]. More recently, preclinical evidence suggested that the antihuman CD25 mAb (RG6292) efficiently induced Treg depletion and held great potential for the anticancer treatments in combination with ICBs [414]. It was later identified that the combination of anti-CD25 antibodies and anti-PD1 antibodies markedly promoted the tumor rejection induced by CD25 antibodies [415]. Moreover, the inhibitory effect of anti-CD25 antibodies in combination with radiotherapy was assessed on the local tumor growth and hepatic metastasis rectal cancer, which suggested that the depletion of Tregs could improve the antitumor effect of radiotherapy plus and produce an abscopal effect [416]. These data collectively support the clinical evaluation of RG6292 incorporating non-IL-2 blocking anti-CD25 antibodies [414].
IL-6-directed therapies
The therapeutic targeting of IL-6 cytokine family members includes the direct blocking of cytokines or their receptors by monoclonal antibodies and small molecules that inhibit the receptor signaling of gp130 and JAK–STAT pathway. These therapeutic strategies are best represented by the monoclonal antibodies targeting IL-6.
IL-6 has long been identified as a key growth factor for myelomas. Between in 1988 and 1989, three laboratories independently reported the promoting effect of IL-6 on the proliferation of in human multiple myeloma (MM) [417]. In 1991, researchers found that the sequential injections of mouse anti-IL-6 antibodies led to reduced MM cell proliferation [418]. Since then, IL-6 has been intensively investigated as a therapeutic target for MM in a number of clinical trials [419]. However, results form later clinical trials were unsatisfactory, and anti-IL6 mAb has thus not been approved for MM to date [420, 421]. Siltuximab (CNTO 328) is an anti-interleukin-6 chimeric mAb, the addition of which to the bortezomib-melphalan-prednisone (VMP) regimen did not improve the complete response rate or long-term outcomes of MM patients (NCT00911859) [421]. A phase I/II study reported that siltuximab stabilized disease in > 50% of progressive metastatic RCC patients [422]. Results from SWOG S0354 trial suggested that siltuximab resulted in a prostate-specific antigen (PSA) response rate (defined as 50% reduction) of 3.8% and a stable disease rate of 23% in patients with castration-resistant prostate cancer (CRPC) [423]. For CRPC patients with prior chemotherapy treatment, siltuximab plus mitoxantrone/prednisone (M/P) was well tolerated and improved clinical outcomes [424].
Due to the elevation in systemic IL-6 levels caused by anti-IL-6 mAbs [425], some alternative IL-6-directed therapies have been developed such as functional blocking of IL-6 receptors (IL-6R). Administration of IL-6R inhibitor tocilizumab at 8 mg/kg combined with carboplatin/doxorubicin chemotherapy is feasible and safe for the treatment of ovarian cancer (NCT01637532) [426]. Unfortunately these modalities are not further investigated in the treatment of cancer patients. One possible explanation is that cytokine receptors such as IL-6Rα may interact with more than one cytokine. The therapeutic targeting of IL-6R may thus result in unexpected AEs compared with the inhibition of an individual cytokine.
IL-10-directed therapies
IL-10 was initially identified as an immunosuppressive cytokine [427], but recent researches have also identified the antitumor effect of IL-10 by stimulating CD8 + T cell in tumor models [428, 429]. As aforementioned, the dual role of IL-10 in tumor progression may vary according to tumor types, or the stage of T cells that respond to IL-10. Though tumor vaccines are known to upregulate tumor-specific CD8 + T cells, they often fail to increase the number of tumor reactive T cells in the TME. An earlier study suggested that the sustained treatment with IL-10 could induce the activation and expansion of tumor-resident CD8 + T cells in mouse tumor models [428]. IL-10-induced tumor rejection could not be impaired by the inhibition of T-cell trafficking from lymphoid organs, indicating its activation on tumor-resident CD8 + T cells. Moreover, the antitumor immune response is mediated directly through expansion of intratumoral CD8 + T cells, whereas the expression of IL-10 receptors on other cells was not necessary for such tumor rejection.
A series of trials have been conducted using the PEGylated recombinant human IL-10 (AM0010, pegilodecakin) in patients with advanced-stage solid tumors [430]. Pegilodecakin is a long-acting, PEGylated version of IL-10 which was found to induce the expression of IFN-γ and granzymes in tumor-infiltrating CD8+ T cells, thereby increasing the number and enhancing the activities of CD8 + T cells. In a multi-institution trial (NCT02009449), pegilodecakin was used as monotherapy and in combination with chemotherapies or anti-PD-1 blockade to treat tumors such as melanoma, NSCLC, CRC, and pancreatic cancer [431]. The safety profile of pegilodecakin significantly differs from other interleukin therapies with frequent occurrence of the cytokine release syndrome [432]. The most frequent treatment-related AEs of pegilodecakin are thrombocytopenia and anemia. The occurrence of anemia might be attributed to the increased phagocytosis of aging red blood cells by activated macrophages [433]. Given that pegilodecakin monotherapy could increase the number of activated infiltrating CD8 + T cells, pegilodecakin is particularly applicable for patients with low T cell-infiltrated tumors prior to therapy [434] and those with tumors refractory to standard therapies [431].
Pegilodecakin was further evaluated in combination with anti-PD-1 inhibitors nivolumab or pembrolizumab for patients with melanoma, NSCLC, or RCC [435]. In the phase II CYPRESS 1 and CYPRESS 2 trials, the concomitant use of pegilodecakin and PD-1 blockades was tested in patients with NSCLC. Unfortunately no significant synergistic effects were observed with the drug combinations relative to the respective PD-1 blockade alone [435,436,437]. More recently, results from a phase I/Ib multi-cohort IVY study reported that pegilodecakin and PD-1 blockades showed promising clinical activity and consistent safety profile as previously reported [438]. Pegilodecakin also enhanced the treatment response of patients with heavily pretreated RCC to anti-PD-1 therapies [438]. Though promising antitumor efficacy was reported in patients with metastatic PDAC [439], the addition of pegilodecakin to the second-line FOLFOX chemotherapy failed to improve either PFS or OS in a phase III trial [440].
CCL2/CCR2 axis-directed therapies
As a potent proinflammatory chemokine signaling, the CCL2/CCR2 axis is important for the recruitment and survival of myeloid cells including inflammatory monocytes, TAMs, and MDSCs [441]. The inhibition of the CCL2/CCR2 axis was thus investigated as a therapeutic strategy to modify the immunosuppressive TME and activate antitumor immunity. The first-in-human clinical trial of carlumab (CNTO 888), a human anti-CCL2 mAb, identified transient free CCL2 suppression and antitumor efficacy in patients with solid tumors [442]. In a phase II study, carlumab could be safely administered in patients with metastatic CRPC, but failed to demonstrate significant antitumor activities as a single agent [443]. Later in another phase I trial (NCT01204996), carlumab was tested in combination with four chemotherapy regimens in patients with solid tumors. Though carlumab was well tolerated in combination with standard chemotherapies, with the most common drug-related grade 3/4 AEs being neutropenia for docetaxel and gemcitabine, long-term tumor responses were not identified in tested patients [444].
Given the suboptimal clinical efficacy of CCR2 inhibitors as monotherapy, the therapeutic potential of CCR2 inhibitors to work in synergy with chemotherapies and immune checkpoint inhibitors was then evaluated. PF-04136309 is a small-molecule CCR2 inhibitor which was mainly studied in the context of pancreatic cancer. In a phase I trial, the targeting of TAMs with PF-04136309-FOLFIRINOX combination was safe and tolerable in patients with borderline resectable and locally advanced pancreatic cancer [445]. Unfortunately, PF-04136309 combined with nab-paclitaxel plus gemcitabine resulted in synergistic pulmonary toxicity, with no superiority over in terms efficacy in PDAC patients (NCT02732938) [446]. CCR2i is a competitive binding inhibitor with a selective and high affinity for the binding pocket of CCR2 and, when combined with an immune checkpoint inhibitor, could suppress tumor growth of cutaneous T-cell lymphomas [447]. BMS-687681, a dual inhibitor targeting CCR2 and CCR5, was used as a prolonged treatment following αPD-1 and radiotherapy in PDAC mouse models, which conferred better antitumor efficacy than other tested combination regimes [448, 449]. Notably, this combination treatment altered the TME by increasing intratumoral effector and memory T cell infiltration and reducing the infiltration of Tregs, M2 TAMs, and MDSCs. The simultaneous administration of CTLA-4 blockades and CCR2 inhibitors led to potent antitumor immunity, further supporting the clinical translation of CCR2/5i in combination with ICIs [450].
Natural anti-inflammatory therapies
Many natural compounds that derive form natural resources such as plants are currently used as therapeutic drugs in cancer. A well-known example is curcumin, also known as diferuloylmethane. Curcumin is the key component of turmeric and has long been used for multiple medical purposes since ancient times [451]. Curcumin is involved in a series of inflammatory pathways implicated in tumorigenesis and has been characterized as a potent antitumor agent. In a systematic review based on multiple databases, analyses on clinical trails between 1980 and 2019 showed that dietary curcumin could reduce the level of C-reactive protein, IL-6, TNF-α, and MCP-1, and increase the level of IL-10, providing evidence for the anti-inflammatory effect of curcumin in chronic inflammation [452]. Notably, the intended use of curcumin was approved by the FDA as “Generally Recognized As Safe” (GRAS) [453].
Curcumin not only reduces cancer risks, but also increases the sensitivity of tumors to chemotherapy and radiotherapy [454]. In light of the frequent AEs associated with 5FU-based or oxaliplatin-based chemotherapy in advanced CRC patients, natural compounds such as curcumin are used as adjuncts to currently available treatment options. In a phase I trial, curcumin administration for up to 4 months was well tolerated in CRC patients [455]. In a phase II randomized controlled trial, curcumin was a safe and tolerable adjunct to folinic acid/5-fluorouracil/oxaliplatin chemotherapy (FOLFOX) chemotherapy in patients with metastatic CRC [456]. In breast cancer, curcumin reduced the paclitaxel (PTX)-induced EGFR, ERK1/2, and AKT expression and could thus synergize with PTX in suppressed tumor growth [457]. Moreover, the increased apoptosis of breast cancer cells induced by PTX-curcumin combination may be mediated via the upregulation of activated caspase 3 and PARP cleavage [458]. Other natural compounds such as quercetin and resveratrol have demonstrated preclinical antitumor efficacy, but no clearly established results were reported from human trials (NCT01538316, NCT01879878, NCT00003365).
Resveratrol is another anti-inflammation agent that inhibits the release of proinflammatory cytokines of T cells [459]. Th17 is a predominant T cell subset targeted by resveratrol. By activating sirtuin-1, resveratrol reduces the acetylation of p65/relA, ultimately suppressing the activation of NF-kB pathway. Moreover, activated sirtuin-1 may also cause STAT3 deacetylation, impeding the activation of retinoid orphan receptor gamma t (RORγt) and the production of IL-17 [460]. RORγt suppresses Th1 differentiation and thus switches the Th1/Th2 balance toward anti-inflammatory (Th2) and immunoregulatory (Treg) responses. In addition, resveratrol also leads to an increased level of anti-inflammatory macrophages (M2). Resveratrol impedes LPS-induced macrophage activation by inhibiting NF-kB and COX-2 signaling and inflammasome activation [459]. In a clinical study, daily consumption of resveratrol induced substantial antitumor effect in 20 patients with colorectal cancer, suggesting the potential of resveratrol as a chemopreventive drug in cancer.
Conclusions and future perspectives
In this review, we described the key inflammatory mediators in cancer. Inflammation, particularly the chronic inflammation, may serve as tumor initiators and promote tumor survival, invasion, and metastasis. It is thus conceivable that targeting inflammation mediators may facilitate the treatment of cancer patients. On one hand, inflammation-directed therapies aim to increase the tumor-killing capability by activating the anticancer immune cells. On the other hand, they may also reshape the TME by altering the immunosuppressive phenotypes of immune cells.
To date, a wide array of inflammation-directed therapies has been developed and is under evaluation both preclinically and clinically in cancer models. With the advances outlined herein, some anti-inflammatory approaches have proven rather effective in cancer prevention and treatment, providing solid scientific rationale for further development of such strategies. Moreover, some inflammatory responses following cancer therapies would confer residual cancer cells with resistance to subsequent treatments. Immunotherapies induce durable responses in only a small subset of patients, with the majority of patients eventually experiencing primary or acquired therapy resistance. Treatment resistance to immunotherapies is often attributed to the presence of proinflammatory and immunosuppressive TME [461]. One such example is the use of anti-CTLA-4 therapies that are related to incidence of colitis and hypophysitis [462], and anti-PD-1 therapies are associated with thyroiditis [463]. Thus, the addition of anti-inflammatory therapies into cancer treatment regimes would yield better clinical responses in some clinical cases.
The initial aim of anti-inflammatory therapies is to suppress the protumoral inflammation and at the same time activate antitumor immune response. Unlike therapies that target specific tumor markers, biomarkers for the selection of anti-inflammatory therapies are lacking. Intrinsic differences of patients such as age, and tumor molecular profile would affect the therapeutic response to inflammation-directed treatments. Thus, high-resolution methods such as multiomics, single-cell, and spatial analyses are recommended to facilitate medical decision and to predict the therapeutic response to inflammation-directed therapies. In addition, it still remains challenging to maintain the balance of inflammation in immune system. The heterogeneity and plasticity of the TME also pose challenges to inflammation-directed therapies by targeting a single molecule or immune cell type. For example, the disrupted feedback loops by targeting one inflammatory cytokine may lead to the compensatory activation of its involved pathways. Future studies are warranted to investigate the combination of inflammation-directed therapies and other treatment options for cancer, facilitating the design of safe and personalized treatment.
Availability of data and materials
The materials supporting our conclusion of this review are included within the article.
Abbreviations
- TME:
-
Tumor microenvironment
- ROS:
-
Reactive oxygen species
- TNF-α:
-
Tumor necrosis factor-α
- MIF:
-
Migration inhibitory factor
- TAMs:
-
Tumor-associated macrophages
- TANs:
-
Tumor-associated neutrophils
- DCs:
-
Dendritic cells
- MDSCs:
-
Myeloid-derived suppressor cells
- MMP:
-
Matrix metallopeptidase
- IFN:
-
Interferon
- TGF-β:
-
Transforming growth factor-beta
- CXCL:
-
C-X-C motif chemokine ligand
- ANG1:
-
Angiopoietin-1
- NETs:
-
Neutrophil extracellular traps
- EMT:
-
Endothelial-to-mesenchymal transition
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- NK:
-
Natural killer
- ECM:
-
Extracellular matrix
- Tregs:
-
Regulatory T cells
- Th17:
-
T helper 17
- FLT3:
-
Fms-related tyrosine kinase receptor 3
- TAAs:
-
Tumor-associated antigens
- ICD:
-
Immunogenic cell death
- DAMPs:
-
Damage-associated molecular patterns
- ER:
-
Endoplasmic reticulum
- PRRs:
-
Pattern recognition receptors
- M-MDSCs:
-
Monocytic-myeloid-derived suppressor cells
- PBMC:
-
Peripheral blood mononuclear cells
- NO:
-
Nitric oxide
- TCR:
-
T cell receptor
- ICB:
-
Immune checkpoint blockade
- CSF-1R:
-
Colony-stimulating factor-1 receptor
- PrP:
-
Prion protein
- PAF:
-
Platelet-activating factor
- TIMPs:
-
Tissue inhibitors of MMPs
- HNSCC:
-
Head and neck squamous cell carcinoma
- IL:
-
Interleukin
- LDL-C:
-
Low-density lipoprotein cholesterol
- KM-LUAD:
-
K-ras-mutant lung adenocarcinoma
- Breg:
-
Regulatory B
- PDAC:
-
Pancreatic ductal adenocarcinoma
- NF-κB:
-
Nuclear factor kappa B
- LIF:
-
Leukemia inhibitory factor
- OSM:
-
Oncostatin M
- CNTF:
-
Ciliary neurotrophic factor
- CT-1:
-
Cardiotrophin-1
- CLC:
-
Cardiotrophin-like cytokine
- CDK:
-
Cyclin-dependent kinase
- XIAP:
-
X-linked inhibitor of apoptosis protein
- JAK:
-
Janus kinase
- STAT:
-
Signal transducer and activator of transcription
- PUFAs:
-
Polyunsaturated fatty acids
- COX:
-
Cyclooxygenase
- LOX:
-
Lipoxygenase
- PGs:
-
Prostaglandins
- LXs:
-
Lipoxins
- mPGES-1:
-
Microsomal PGE2 synthase 1
- LT:
-
Leukotriene
- CRC:
-
Colorectal cancer
- IBD:
-
Inflammatory bowel disease
- CAC:
-
Colitis-associated CRC
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- DFS:
-
Disease-free survival
- PFS:
-
Progression-free survival
- AEs:
-
Adverse events
- MMR:
-
Mismatch repair
- MSI:
-
Microsatellite instability
- HCC:
-
Hepatocellular carcinoma
- HBV:
-
Hepatitis B virus
- NAs:
-
Nucleotide analogs
- RFS:
-
Relapse-free survival
- NHL:
-
Non-Hodgkin lymphomas
- 5-FU:
-
5-Fluorouracil
- MM:
-
Multiple myeloma
References
Plytycz B, Seljelid R. From inflammation to sickness: historical perspective. Arch Immunol Ther Exp (Warsz). 2003;51(2):105–9.
Granger DN, Senchenkova E. In: Inflammation and the Microcirculation. San Rafael (CA); 2010.
Virchow R. An address on the value of pathological experiments. Br Med J. 1881;2(1075):198–203.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Haddow A. Addendum to “molecular repair, wound healing, and carcinogenesis: tumor production a possible overhealing”? Adv Cancer Res. 1974;20:343–66.
Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–9.
Abramovitch R, Marikovsky M, Meir G, Neeman M. Stimulation of tumour angiogenesis by proximal wounds: spatial and temporal analysis by MRI. Br J Cancer. 1998;77(3):440–7.
Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125(Pt 23):5591–6.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70.
Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403.
Krugliak Cleveland N, Torres J, Rubin DT. What does disease progression look like in ulcerative colitis, and how might it be prevented? Gastroenterology. 2022;162(5):1396–408.
Shah SC, Itzkowitz SH. Colorectal cancer in inflammatory bowel disease: mechanisms and management. Gastroenterology. 2022;162(3):715–30.
Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, Graham DY. Association between helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology. 2016;150(5):1113–24.
Tian T, Song C, Jiang L, Dai J, Lin Y, Xu X, Yu C, Ge Z, Ding Y, Wen Y, et al. Hepatitis B virus infection and the risk of cancer among the Chinese population. Int J Cancer. 2020;147(11):3075–84.
Kawanishi S, Ohnishi S, Ma N, Hiraku Y, Murata M. Crosstalk between DNA damage and inflammation in the multiple steps of carcinogenesis. Int J Mol Sci. 2017;18(8).
Suresh V, Dash P, Suklabaidya S, Murmu KC, Sasmal PK, Jogdand GM, Parida D, Sethi M, Das B, Mohapatra D, et al. MIF confers survival advantage to pancreatic CAFs by suppressing interferon pathway-induced p53-dependent apoptosis. FASEB J. 2022;36(8): e22449.
Chen L, Zhou X, Fan LX, Yao Y, Swenson-Fields KI, Gadjeva M, Wallace DP, Peters DJ, Yu A, Grantham JJ, et al. Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease. J Clin Invest. 2015;125(6):2399–412.
Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol. 2022;19(4):237–53.
Li L, Yu R, Cai T, Chen Z, Lan M, Zou T, Wang B, Wang Q, Zhao Y, Cai Y. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int Immunopharmacol. 2020;88: 106939.
Li MO, Wolf N, Raulet DH, Akkari L, Pittet MJ, Rodriguez PC, Kaplan RN, Munitz A, Zhang Z, Cheng S, et al. Innate immune cells in the tumor microenvironment. Cancer Cell. 2021;39(6):725–9.
Aga E, Mukherjee A, Rane D, More V, Patil T, van Zandbergen G, Solbach W, Dandapat J, Tackenberg H, Ohms M, et al. Type-1 interferons prolong the lifespan of neutrophils by interfering with members of the apoptotic cascade. Cytokine. 2018;112:21–6.
Wu M, Ma M, Tan Z, Zheng H, Liu X. Neutrophil: a new player in metastatic cancers. Front Immunol. 2020;11: 565165.
Li S, Cong X, Gao H, Lan X, Li Z, Wang W, Song S, Wang Y, Li C, Zhang H, et al. Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J Exp Clin Cancer Res. 2019;38(1):6.
Albini A, Bruno A, Noonan DM, Mortara L. Contribution to tumor angiogenesis from innate immune cells within the tumor microenvironment: implications for immunotherapy. Front Immunol. 2018;9:527.
Singhal S, Bhojnagarwala PS, O’Brien S, Moon EK, Garfall AL, Rao AS, Quatromoni JG, Stephen TL, Litzky L, Deshpande C, et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell. 2016;30(1):120–35.
Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. 2016;150(7):1646–58.
Mishalian I, Bayuh R, Eruslanov E, Michaeli J, Levy L, Zolotarov L, Singhal S, Albelda SM, Granot Z, Fridlender ZG. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17–a new mechanism of impaired antitumor immunity. Int J Cancer. 2014;135(5):1178–86.
Sasaki S, Baba T, Muranaka H, Tanabe Y, Takahashi C, Matsugo S, Mukaida N. Involvement of prokineticin 2-expressing neutrophil infiltration in 5-fluorouracil-induced aggravation of breast cancer metastasis to lung. Mol Cancer Ther. 2018;17(7):1515–25.
Mutua V, Gershwin LJ. A review of neutrophil extracellular traps (NETs) in disease: potential anti-NETs therapeutics. Clin Rev Allergy Immunol. 2021;61(2):194–211.
Rayes RF, Mouhanna JG, Nicolau I, Bourdeau F, Giannias B, Rousseau S, Quail D, Walsh L, Sangwan V, Bertos N et al. Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight. 2019;5(16).
Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, Huang D, Li J, Li H, Chen F, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583(7814):133–8.
Lee W, Ko SY, Mohamed MS, Kenny HA, Lengyel E, Naora H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med. 2019;216(1):176–94.
Bates AM, Gomez Hernandez MP, Lanzel EA, Qian F, Brogden KA. Matrix metalloproteinase (MMP) and immunosuppressive biomarker profiles of seven head and neck squamous cell carcinoma (HNSCC) cell lines. Transl Cancer Res. 2018;7(3):533–42.
Nie M, Yang L, Bi X, Wang Y, Sun P, Yang H, Liu P, Li Z, Xia Y, Jiang W. Neutrophil extracellular traps induced by IL8 promote diffuse large B-cell lymphoma progression via the TLR9 signaling. Clin Cancer Res. 2019;25(6):1867–79.
Weiss E, Kretschmer D. Formyl-peptide receptors in infection, inflammation, and cancer. Trends Immunol. 2018;39(10):815–29.
Teijeira A, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, de Andrea C, Ochoa MC, Otano I, Etxeberria I, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity. 2020;52(5):856–71.
Azevedo PO, Paiva AE, Santos GSP, Lousado L, Andreotti JP, Sena IFG, Tagliati CA, Mintz A, Birbrair A. Cross-talk between lung cancer and bones results in neutrophils that promote tumor progression. Cancer Metastasis Rev. 2018;37(4):779–90.
Berger-Achituv S, Brinkmann V, Abed UA, Kuhn LI, Ben-Ezra J, Elhasid R, Zychlinsky A. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol. 2013;4:48.
Demkow U. Neutrophil extracellular traps (NETs) in cancer invasion, evasion and metastasis. Cancers (Basel). 2021;13(17).
Wang Y, Liu F, Chen L, Fang C, Li S, Yuan S, Qian X, Yin Y, Yu B, Fu B, et al. Neutrophil extracellular traps (NETs) promote non-small cell lung cancer metastasis by suppressing lncRNA MIR503HG to activate the NF-kappaB/NLRP3 inflammasome pathway. Front Immunol. 2022;13: 867516.
Deng J, Kang Y, Cheng CC, Li X, Dai B, Katz MH, Men T, Kim MP, Koay EA, Huang H et al. DDR1-induced neutrophil extracellular traps drive pancreatic cancer metastasis. JCI Insight. 2021;6(17).
Khan U, Chowdhury S, Billah MM, Islam KMD, Thorlacius H, Rahman M. Neutrophil extracellular traps in colorectal cancer progression and metastasis. Int J Mol Sci. 2021;22(14).
Xiao Y, Cong M, Li J, He D, Wu Q, Tian P, Wang Y, Yang S, Liang C, Liang Y, et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021;39(3):423–37.
Yang C, Wang Z, Li L, Zhang Z, Jin X, Wu P, Sun S, Pan J, Su K, Jia F et al. Aged neutrophils form mitochondria-dependent vital NETs to promote breast cancer lung metastasis. J Immunother Cancer. 2021;9(10).
Arelaki S, Arampatzioglou A, Kambas K, Papagoras C, Miltiades P, Angelidou I, Mitsios A, Kotsianidis I, Skendros P, Sivridis E, et al. Gradient infiltration of neutrophil extracellular traps in colon cancer and evidence for their involvement in tumour growth. PLoS ONE. 2016;11(5): e0154484.
Millrud CR, Kagedal A, Kumlien Georen S, Winqvist O, Uddman R, Razavi R, Munck-Wikland E, Cardell LO. NET-producing CD16(high) CD62L(dim) neutrophils migrate to tumor sites and predict improved survival in patients with HNSCC. Int J Cancer. 2017;140(11):2557–67.
Schedel F, Mayer-Hain S, Pappelbaum KI, Metze D, Stock M, Goerge T, Loser K, Sunderkotter C, Luger TA, Weishaupt C. Evidence and impact of neutrophil extracellular traps in malignant melanoma. Pigment Cell Melanoma Res. 2020;33(1):63–73.
Muqaku B, Pils D, Mader JC, Aust S, Mangold A, Muqaku L, Slany A, Del Favero G, Gerner C. Neutrophil extracellular trap formation correlates with favorable overall survival in high grade ovarian cancer. Cancers (Basel). 2020;12(2).
Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–55.
Mackaness GB. Cellular resistance to infection. J Exp Med. 1962;116(3):381–406.
Duan Z, Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther. 2021;6(1):127.
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20.
Eum HH, Kwon M, Ryu D, Jo A, Chung W, Kim N, Hong Y, Son DS, Kim ST, Lee J, et al. Tumor-promoting macrophages prevail in malignant ascites of advanced gastric cancer. Exp Mol Med. 2020;52(12):1976–88.
Bernsmeier C, van der Merwe S, Perianin A. Innate immune cells in cirrhosis. J Hepatol. 2020;73(1):186–201.
Bruns H, Buttner M, Fabri M, Mougiakakos D, Bittenbring JT, Hoffmann MH, Beier F, Pasemann S, Jitschin R, Hofmann AD, et al. Vitamin D-dependent induction of cathelicidin in human macrophages results in cytotoxicity against high-grade B cell lymphoma. Sci Transl Med. 2015;7(282):282–247.
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–55.
Ding P, Wang W, Wang J, Yang Z, Xue L. Expression of tumor-associated macrophage in progression of human glioma. Cell Biochem Biophys. 2014;70(3):1625–31.
Yuan X, Zhang J, Li D, Mao Y, Mo F, Du W, Ma X. Prognostic significance of tumor-associated macrophages in ovarian cancer: a meta-analysis. Gynecol Oncol. 2017;147(1):181–7.
Larionova I, Kazakova E, Gerashchenko T, Kzhyshkowska J. New angiogenic regulators produced by TAMs: perspective for targeting tumor angiogenesis. Cancers (Basel). 2021;13(13).
Gurevich DB, Severn CE, Twomey C, Greenhough A, Cash J, Toye AM, Mellor H, Martin P. Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J 2018;37(13).
Ramirez-Pedraza M, Fernandez M. Interplay between macrophages and angiogenesis: a double-edged sword in liver disease. Front Immunol. 2019;10:2882.
Zhou J, Li X, Wu X, Zhang T, Zhu Q, Wang X, Wang H, Wang K, Lin Y, Wang X. Exosomes released from tumor-associated macrophages transfer miRNAs that induce a Treg/Th17 cell imbalance in epithelial ovarian cancer. Cancer Immunol Res. 2018;6(12):1578–92.
Lan J, Sun L, Xu F, Liu L, Hu F, Song D, Hou Z, Wu W, Luo X, Wang J, et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. 2019;79(1):146–58.
Yin Z, Ma T, Huang B, Lin L, Zhou Y, Yan J, Zou Y, Chen S. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-beta signaling pathway. J Exp Clin Cancer Res. 2019;38(1):310.
Shima T, Shimoda M, Shigenobu T, Ohtsuka T, Nishimura T, Emoto K, Hayashi Y, Iwasaki T, Abe T, Asamura H, et al. Infiltration of tumor-associated macrophages is involved in tumor programmed death-ligand 1 expression in early lung adenocarcinoma. Cancer Sci. 2020;111(2):727–38.
Sumitomo R, Hirai T, Fujita M, Murakami H, Otake Y, Huang CL. PD-L1 expression on tumor-infiltrating immune cells is highly associated with M2 TAM and aggressive malignant potential in patients with resected non-small cell lung cancer. Lung Cancer. 2019;136:136–44.
Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52.
Ness S, Lin S, Gordon JR. Regulatory dendritic cells, t cell tolerance, and dendritic cell therapy for immunologic disease. Front Immunol. 2021;12: 633436.
Tsapogas P, Mooney CJ, Brown G, Rolink A. The cytokine Flt3-ligand in normal and malignant hematopoiesis. Int J Mol Sci. 2017;18(6).
Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265–77.
Fu C, Jiang A. Dendritic cells and CD8 T cell immunity in tumor microenvironment. Front Immunol. 2018;9:3059.
Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20(1):7–24.
Aras S, Zaidi MR. TAMeless traitors: macrophages in cancer progression and metastasis. Br J Cancer. 2017;117(11):1583–91.
Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173(2):945–54.
Rufo N, Garg AD, Agostinis P. The unfolded protein response in immunogenic cell death and cancer immunotherapy. Trends Cancer. 2017;3(9):643–58.
Wang Y, Xiang Y, Xin VW, Wang XW, Peng XC, Liu XQ, Wang D, Li N, Cheng JT, Lyv YN, et al. Dendritic cell biology and its role in tumor immunotherapy. J Hematol Oncol. 2020;13(1):107.
Hole CR, Wager CML, Castro-Lopez N, Campuzano A, Cai H, Wozniak KL, Wang Y, Wormley FL Jr. Induction of memory-like dendritic cell responses in vivo. Nat Commun. 2019;10(1):2955.
Alzeibak R, Mishchenko TA, Shilyagina NY, Balalaeva IV, Vedunova MV, Krysko DV. Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future. J Immunother Cancer. 2021;9(1).
Wiernicki B, Maschalidi S, Pinney J, Adjemian S, Vanden Berghe T, Ravichandran KS, Vandenabeele P. Cancer cells dying from ferroptosis impede dendritic cell-mediated anti-tumor immunity. Nat Commun. 2022;13(1):3676.
Mandruzzato S, Brandau S, Britten CM, Bronte V, Damuzzo V, Gouttefangeas C, Maurer D, Ottensmeier C, van der Burg SH, Welters MJ, et al. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: results from an interim study. Cancer Immunol Immunother. 2016;65(2):161–9.
Li BH, Garstka MA, Li ZF. Chemokines and their receptors promoting the recruitment of myeloid-derived suppressor cells into the tumor. Mol Immunol. 2020;117:201–15.
Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208–20.
Rodriguez PC, Zea AH, Culotta KS, Zabaleta J, Ochoa JB, Ochoa AC. Regulation of T cell receptor CD3zeta chain expression by L-arginine. J Biol Chem. 2002;277(24):21123–9.
Wang Y, Ding Y, Guo N, Wang S. MDSCs: key criminals of tumor pre-metastatic niche formation. Front Immunol. 2019;10:172.
Bruno A, Mortara L, Baci D, Noonan DM, Albini A. Myeloid derived suppressor cells interactions with natural killer cells and pro-angiogenic activities: roles in tumor progression. Front Immunol. 2019;10:771.
Johnson BW, Achyut BR, Fulzele S, Mondal AK, Kolhe R, Arbab AS. Delineating pro-angiogenic myeloid cells in cancer therapy. Int J Mol Sci 2018;19(9).
Zhou J, Nefedova Y, Lei A, Gabrilovich D. Neutrophils and PMN-MDSC: their biological role and interaction with stromal cells. Semin Immunol. 2018;35:19–28.
Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, Maio M, Sucker A, Schilling B, Schadendorf D, et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin Cancer Res. 2014;20(6):1601–9.
Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, Michielin O, Romano E, Speiser DE. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother. 2014;63(3):247–57.
Sade-Feldman M, Kanterman J, Klieger Y, Ish-Shalom E, Olga M, Saragovi A, Shtainberg H, Lotem M, Baniyash M. Clinical significance of circulating CD33+CD11b+HLA-DR-myeloid cells in patients with stage IV melanoma treated with ipilimumab. Clin Cancer Res. 2016;22(23):5661–72.
Martens A, Wistuba-Hamprecht K, Geukes Foppen M, Yuan J, Postow MA, Wong P, Romano E, Khammari A, Dreno B, Capone M, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. 2016;22(12):2908–18.
Holmgaard RB, Zamarin D, Lesokhin A, Merghoub T, Wolchok JD. Targeting myeloid-derived suppressor cells with colony stimulating factor-1 receptor blockade can reverse immune resistance to immunotherapy in indoleamine 2,3-dioxygenase-expressing tumors. EBioMedicine. 2016;6:50–8.
Holmgaard RB, Brachfeld A, Gasmi B, Jones DR, Mattar M, Doman T, Murphy M, Schaer D, Wolchok JD, Merghoub T. Timing of CSF-1/CSF-1R signaling blockade is critical to improving responses to CTLA-4 based immunotherapy. Oncoimmunology. 2016;5(7): e1151595.
Lamichhane P, Karyampudi L, Shreeder B, Krempski J, Bahr D, Daum J, Kalli KR, Goode EL, Block MS, Cannon MJ, et al. IL10 release upon PD-1 blockade sustains immunosuppression in ovarian cancer. Cancer Res. 2017;77(23):6667–78.
Gomes-Santos IL, Amoozgar Z, Kumar AS, Ho WW, Roh K, Talele NP, Curtis H, Kawaguchi K, Jain RK, Fukumura D. Exercise training improves tumor control by increasing CD8(+) T-cell infiltration via CXCR3 signaling and sensitizes breast cancer to immune checkpoint blockade. Cancer Immunol Res. 2021;9(7):765–78.
Holder KA, Grant MD. Human cytomegalovirus IL-10 augments NK cell cytotoxicity. J Leukoc Biol. 2019;106(2):447–54.
O’Carroll SJ, Kho DT, Wiltshire R, Nelson V, Rotimi O, Johnson R, Angel CE, Graham ES. Pro-inflammatory TNFalpha and IL-1beta differentially regulate the inflammatory phenotype of brain microvascular endothelial cells. J Neuroinflammation. 2015;12:131.
Hillyer P, Mordelet E, Flynn G, Male D. Chemokines, chemokine receptors and adhesion molecules on different human endothelia: discriminating the tissue-specific functions that affect leucocyte migration. Clin Exp Immunol. 2003;134(3):431–41.
Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167(2):223–9.
Tichet M, Prod’Homme V, Fenouille N, Ambrosetti D, Mallavialle A, Cerezo M, Ohanna M, Audebert S, Rocchi S, Giacchero D, et al. Tumour-derived SPARC drives vascular permeability and extravasation through endothelial VCAM1 signalling to promote metastasis. Nat Commun. 2015;6:6993.
Hiratsuka S, Goel S, Kamoun WS, Maru Y, Fukumura D, Duda DG, Jain RK. Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proc Natl Acad Sci U S A. 2011;108(9):3725–30.
Burdick MM, Henson KA, Delgadillo LF, Choi YE, Goetz DJ, Tees DF, Benencia F. Expression of E-selectin ligands on circulating tumor cells: cross-regulation with cancer stem cell regulatory pathways? Front Oncol. 2012;2:103.
Hauselmann I, Roblek M, Protsyuk D, Huck V, Knopfova L, Grassle S, Bauer AT, Schneider SW, Borsig L. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade. Cancer Res. 2016;76(18):5302–12.
Shea DJ, Li YW, Stebe KJ, Konstantopoulos K. E-selectin-mediated rolling facilitates pancreatic cancer cell adhesion to hyaluronic acid. FASEB J. 2017;31(11):5078–86.
Kang SA, Blache CA, Bajana S, Hasan N, Kamal M, Morita Y, Gupta V, Tsolmon B, Suh KS, Gorenstein DG, et al. The effect of soluble E-selectin on tumor progression and metastasis. BMC Cancer. 2016;16:331.
Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7(5):651–8.
Amin MN, Siddiqui SA, Ibrahim M, Hakim ML, Ahammed MS, Kabir A, Sultana F. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med. 2020;8:2050312120965752.
Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, Lee SR, Yang SH. The role of tumor necrosis factor alpha (TNF-alpha) in autoimmune disease and current TNF-alpha inhibitors in therapeutics. Int J Mol Sci. 2021;22(5).
Zhang GP, Yue X, Li SQ. Cathepsin C interacts with TNF-alpha/p38 MAPK signaling pathway to promote proliferation and metastasis in hepatocellular carcinoma. Cancer Res Treat. 2020;52(1):10–23.
Schroder SK, Asimakopoulou A, Tillmann S, Koschmieder S, Weiskirchen R. TNF-alpha controls lipocalin-2 expression in PC-3 prostate cancer cells. Cytokine. 2020;135: 155214.
Jo E, Jang HJ, Yang KE, Jang MS, Huh YH, Yoo HS, Park JS, Jang IS, Park SJ. Cordyceps militaris induces apoptosis in ovarian cancer cells through TNF-alpha/TNFR1-mediated inhibition of NF-kappaB phosphorylation. BMC Complement Med Ther. 2020;20(1):1.
Cruceriu D, Baldasici O, Balacescu O, Berindan-Neagoe I. The dual role of tumor necrosis factor-alpha (TNF-alpha) in breast cancer: molecular insights and therapeutic approaches. Cell Oncol (Dordr). 2020;43(1):1–18.
Garcia-Tunon I, Ricote M, Ruiz A, Fraile B, Paniagua R, Royuela M. Role of tumor necrosis factor-alpha and its receptors in human benign breast lesions and tumors (in situ and infiltrative). Cancer Sci. 2006;97(10):1044–9.
Mercogliano MF, De Martino M, Venturutti L, Rivas MA, Proietti CJ, Inurrigarro G, Frahm I, Allemand DH, Deza EG, Ares S, et al. TNFalpha-induced mucin 4 expression elicits trastuzumab resistance in HER2-positive breast cancer. Clin Cancer Res. 2017;23(3):636–48.
Wu C, Fernandez SA, Criswell T, Chidiac TA, Guttridge D, Villalona-Calero M, Bekaii-Saab TS. Disrupting cytokine signaling in pancreatic cancer: a phase I/II study of etanercept in combination with gemcitabine in patients with advanced disease. Pancreas. 2013;42(5):813–8.
Yoshimatsu Y, Wakabayashi I, Kimuro S, Takahashi N, Takahashi K, Kobayashi M, Maishi N, Podyma-Inoue KA, Hida K, Miyazono K, et al. TNF-alpha enhances TGF-beta-induced endothelial-to-mesenchymal transition via TGF-beta signal augmentation. Cancer Sci. 2020;111(7):2385–99.
Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014: 149185.
Rossi S, Cordella M, Tabolacci C, Nassa G, D’Arcangelo D, Senatore C, Pagnotto P, Magliozzi R, Salvati A, Weisz A, et al. TNF-alpha and metalloproteases as key players in melanoma cells aggressiveness. J Exp Clin Cancer Res. 2018;37(1):326.
Bertrand F, Rochotte J, Colacios C, Montfort A, Tilkin-Mariame AF, Touriol C, Rochaix P, Lajoie-Mazenc I, Andrieu-Abadie N, Levade T, et al. Blocking tumor necrosis factor alpha enhances CD8 T-cell-dependent immunity in experimental melanoma. Cancer Res. 2015;75(13):2619–28.
Li H, Wang R, Yu Z, Shi R, Zhang J, Gao S, Shao M, Cui S, Gao Z, Xu J, et al. Tumor necrosis factor alpha reduces SNAP29 dependent autolysosome formation to increase prion protein level and promote tumor cell migration. Virol Sin. 2021;36(3):458–75.
Nagar M, Jacob-Hirsch J, Vernitsky H, Berkun Y, Ben-Horin S, Amariglio N, Bank I, Kloog Y, Rechavi G, Goldstein I. TNF activates a NF-kappaB-regulated cellular program in human CD45RA- regulatory T cells that modulates their suppressive function. J Immunol. 2010;184(7):3570–81.
Medler J, Wajant H. Tumor necrosis factor receptor-2 (TNFR2): an overview of an emerging drug target. Expert Opin Ther Targets. 2019;23(4):295–307.
Farrugia M, Baron B. The role of TNF-alpha in rheumatoid arthritis: a focus on regulatory T cells. J Clin Transl Res. 2016;2(3):84–90.
Salomon BL, Leclerc M, Tosello J, Ronin E, Piaggio E, Cohen JL. Tumor necrosis factor alpha and regulatory T cells in oncoimmunology. Front Immunol. 2018;9:444.
Chen X, Subleski JJ, Kopf H, Howard OM, Mannel DN, Oppenheim JJ. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol. 2008;180(10):6467–71.
Torrey H, Butterworth J, Mera T, Okubo Y, Wang L, Baum D, Defusco A, Plager S, Warden S, Huang D et al. Targeting TNFR2 with antagonistic antibodies inhibits proliferation of ovarian cancer cells and tumor-associated Tregs. Sci Signal. 2017;10(462).
Batlle E, Massague J. Transforming growth factor-beta signaling in immunity and cancer. Immunity. 2019;50(4):924–40.
Baba AB, Rah B, Bhat GR, Mushtaq I, Parveen S, Hassan R, Hameed Zargar M, Afroze D. Transforming growth factor-beta (TGF-beta) signaling in cancer-a betrayal within. Front Pharmacol. 2022;13: 791272.
Crane JL, Cao X. Bone marrow mesenchymal stem cells and TGF-beta signaling in bone remodeling. J Clin Invest. 2014;124(2):466–72.
Hu Q, Hisamatsu T, Haemmerle M, Cho MS, Pradeep S, Rupaimoole R, Rodriguez-Aguayo C, Lopez-Berestein G, Wong STC, Sood AK, et al. Role of platelet-derived Tgfbeta1 in the progression of ovarian cancer. Clin Cancer Res. 2017;23(18):5611–21.
Melzer C, Hass R, von der Ohe J, Lehnert H, Ungefroren H. The role of TGF-beta and its crosstalk with RAC1/RAC1b signaling in breast and pancreas carcinoma. Cell Commun Signal. 2017;15(1):19.
Melzer C, von der Ohe J, Otterbein H, Ungefroren H, Hass R. Changes in uPA, PAI-1, and TGF-beta Production during Breast Cancer Cell Interaction with Human Mesenchymal Stroma/Stem-Like Cells (MSC). Int J Mol Sci. 2019. 20(11).
Villalba M, Evans SR, Vidal-Vanaclocha F, Calvo A. Role of TGF-beta in metastatic colon cancer: it is finally time for targeted therapy. Cell Tissue Res. 2017;370(1):29–39.
Hao Y, Baker D, Ten Dijke P. TGF-beta-mediated epithelial-mesenchymal transition and cancer metastasis. Int J Mol Sci. 2019;20(11).
Tauriello DVF, Sancho E, Batlle E. Overcoming TGFbeta-mediated immune evasion in cancer. Nat Rev Cancer. 2022;22(1):25–44.
Tan X, Chen C, Zhu Y, Deng J, Qiu X, Huang S, Shang F, Cheng B, Liu Y. Proteotoxic stress desensitizes TGF-beta signaling through receptor downregulation in retinal pigment epithelial cells. Curr Mol Med. 2017;17(3):189–99.
Esquivel-Velazquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J. The role of cytokines in breast cancer development and progression. J Interferon Cytokine Res. 2015;35(1):1–16.
Laine A, Labiad O, Hernandez-Vargas H, This S, Sanlaville A, Leon S, Dalle S, Sheppard D, Travis MA, Paidassi H, et al. Regulatory T cells promote cancer immune-escape through integrin alphavbeta8-mediated TGF-beta activation. Nat Commun. 2021;12(1):6228.
Akkaya M, Akkaya B, Miozzo P, Rawat M, Pena M, Sheehan PW, Kim AS, Kamenyeva O, Kabat J, Bolland S, et al. B cells produce type 1 IFNs in response to the TLR9 agonist CpG-A conjugated to cationic lipids. J Immunol. 2017;199(3):931–40.
Ali S, Mann-Nuttel R, Schulze A, Richter L, Alferink J, Scheu S. Sources of type I interferons in infectious immunity: plasmacytoid dendritic cells not always in the driver’s seat. Front Immunol. 2019;10:778.
Gato-Canas M, Zuazo M, Arasanz H, Ibanez-Vea M, Lorenzo L, Fernandez-Hinojal G, Vera R, Smerdou C, Martisova E, Arozarena I, et al. PDL1 signals through conserved sequence motifs to overcome interferon-mediated cytotoxicity. Cell Rep. 2017;20(8):1818–29.
Chen J, Cao Y, Markelc B, Kaeppler J, Vermeer JA, Muschel RJ. Type I IFN protects cancer cells from CD8+ T cell-mediated cytotoxicity after radiation. J Clin Invest. 2019;129(10):4224–38.
Lee MS, Kim B, Oh GT, Kim YJ. OASL1 inhibits translation of the type I interferon-regulating transcription factor IRF7. Nat Immunol. 2013;14(4):346–55.
Cunningham CR, Champhekar A, Tullius MV, Dillon BJ, Zhen A, de la Fuente JR, Herskovitz J, Elsaesser H, Snell LM, Wilson EB, et al. Type I and type II interferon coordinately regulate suppressive dendritic cell fate and function during viral persistence. PLoS Pathog. 2016;12(1): e1005356.
Gong W, Donnelly CR, Heath BR, Bellile E, Donnelly LA, Taner HF, Broses L, Brenner JC, Chinn SB, Ji RR, et al. Cancer-specific type-I interferon receptor signaling promotes cancer stemness and effector CD8+ T-cell exhaustion. Oncoimmunology. 2021;10(1):1997385.
Musella M, Guarracino A, Manduca N, Galassi C, Ruggiero E, Potenza A, Maccafeo E, Manic G, Mattiello L, Soliman Abdel Rehim S, et al. Type I IFNs promote cancer cell stemness by triggering the epigenetic regulator KDM1B. Nat Immunol. 2022;23(9):1379–92.
Pidugu VK, Wu MM, Yen AH, Pidugu HB, Chang KW, Liu CJ, Lee TC. IFIT1 and IFIT3 promote oral squamous cell carcinoma metastasis and contribute to the anti-tumor effect of gefitinib via enhancing p-EGFR recycling. Oncogene. 2019;38(17):3232–47.
Boukhaled GM, Harding S, Brooks DG. Opposing roles of type I interferons in cancer immunity. Annu Rev Pathol. 2021;16:167–98.
Spaapen RM, Leung MY, Fuertes MB, Kline JP, Zhang L, Zheng Y, Fu YX, Luo X, Cohen KS, Gajewski TF. Therapeutic activity of high-dose intratumoral IFN-beta requires direct effect on the tumor vasculature. J Immunol. 2014;193(8):4254–60.
Golomb HM, Ratain MJ, Mick R, Daly K. Interferon treatment for hairy cell leukemia: an update on a cohort of 69 patients treated from 1983–1986. Leukemia. 1992;6(11):1177–80.
Bent R, Moll L, Grabbe S, Bros M. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int J Mol Sci. 2018;19(8).
Malik A, Kanneganti TD. Function and regulation of IL-1alpha in inflammatory diseases and cancer. Immunol Rev. 2018;281(1):124–37.
Fahey E, Doyle SL. IL-1 family cytokine regulation of vascular permeability and angiogenesis. Front Immunol. 2019;10:1426.
Xiao Z, Singh S, Singh M. Improving cancer immunotherapy by targeting IL-1. Oncoimmunology. 2021;10(1):2008111.
Haabeth OA, Lorvik KB, Yagita H, Bogen B, Corthay A. Interleukin-1 is required for cancer eradication mediated by tumor-specific Th1 cells. Oncoimmunology. 2016;5(1): e1039763.
Baker KJ, Houston A, Brint E. IL-1 family members in cancer; two sides to every story. Front Immunol. 2019;10:1197.
Zhang W, Borcherding N, Kolb R. IL-1 signaling in tumor microenvironment. Adv Exp Med Biol. 2020;1240:1–23.
Basu A, Ramamoorthi G, Albert G, Gallen C, Beyer A, Snyder C, Koski G, Disis ML, Czerniecki BJ, Kodumudi K. Differentiation and regulation of T(H) cells: a balancing act for cancer immunotherapy. Front Immunol. 2021;12: 669474.
Lin D, Mei Y, Lei L, Binte Hanafi Z, Jin Z, Liu Y, Song Y, Zhang Y, Hu B, Liu C, et al. Immune suppressive function of IL-1alpha release in the tumor microenvironment regulated by calpain 1. Oncoimmunology. 2022;11(1):2088467.
Jiang H, Gebhardt C, Umansky L, Beckhove P, Schulze TJ, Utikal J, Umansky V. Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int J Cancer. 2015;136(10):2352–60.
Voronov E, Carmi Y, Apte RN. The role IL-1 in tumor-mediated angiogenesis. Front Physiol. 2014;5:114.
Carmi Y, Dotan S, Rider P, Kaplanov I, White MR, Baron R, Abutbul S, Huszar M, Dinarello CA, Apte RN, et al. The role of IL-1beta in the early tumor cell-induced angiogenic response. J Immunol. 2013;190(7):3500–9.
Kaplanov I, Carmi Y, Kornetsky R, Shemesh A, Shurin GV, Shurin MR, Dinarello CA, Voronov E, Apte RN. Blocking IL-1beta reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc Natl Acad Sci U S A. 2019;116(4):1361–9.
Das S, Shapiro B, Vucic EA, Vogt S, Bar-Sagi D. Tumor cell-derived IL1beta promotes desmoplasia and immune suppression in pancreatic cancer. Cancer Res. 2020;80(5):1088–101.
Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10): a016295.
Qin B, Zhou Z, He J, Yan C, Ding S. IL-6 inhibits starvation-induced autophagy via the STAT3/Bcl-2 signaling pathway. Sci Rep. 2015;5:15701.
Shi R, Chen M, Litifu B. Serum interleukin-6 and survivin levels predict clinical response to etanercept treatment in patients with established rheumatoid arthritis. Mod Rheumatol. 2018;28(1):126–32.
Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M, Yao Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141(2):125–39.
Ortiz-Montero P, Londono-Vallejo A, Vernot JP. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun Signal. 2017;15(1):17.
Sapochnik M, Haedo MR, Fuertes M, Ajler P, Carrizo G, Cervio A, Sevlever G, Stalla GK, Arzt E. Autocrine IL-6 mediates pituitary tumor senescence. Oncotarget. 2017;8(3):4690–702.
Ray K, Ujvari B, Ramana V, Donald J. Cross-talk between EGFR and IL-6 drives oncogenic signaling and offers therapeutic opportunities in cancer. Cytokine Growth Factor Rev. 2018;41:18–27.
Gao S, Hu J, Wu X, Liang Z. PMA treated THP-1-derived-IL-6 promotes EMT of SW48 through STAT3/ERK-dependent activation of Wnt/beta-catenin signaling pathway. Biomed Pharmacother. 2018;108:618–24.
Liu W, Wang H, Bai F, Ding L, Huang Y, Lu C, Chen S, Li C, Yue X, Liang X, et al. IL-6 promotes metastasis of non-small-cell lung cancer by up-regulating TIM-4 via NF-kappaB. Cell Prolif. 2020;53(3): e12776.
Bharti R, Dey G, Das AK, Mandal M. Differential expression of IL-6/IL-6R and MAO-A regulates invasion/angiogenesis in breast cancer. Br J Cancer. 2018;118(11):1442–52.
Xu J, Lin H, Wu G, Zhu M, Li M. IL-6/STAT3 is a promising therapeutic target for hepatocellular carcinoma. Front Oncol. 2021;11: 760971.
Zhang B, Li Y, Wu Q, Xie L, Barwick B, Fu C, Li X, Wu D, Xia S, Chen J, et al. Acetylation of KLF5 maintains EMT and tumorigenicity to cause chemoresistant bone metastasis in prostate cancer. Nat Commun. 2021;12(1):1714.
Manore SG, Doheny DL, Wong GL, Lo HW. IL-6/JAK/STAT3 signaling in breast cancer metastasis: biology and treatment. Front Oncol. 2022;12: 866014.
Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170(6):2081–95.
Vieira P, de Waal-Malefyt R, Dang MN, Johnson KE, Kastelein R, Fiorentino DF, deVries JE, Roncarolo MG, Mosmann TR, Moore KW. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci U S A. 1991;88(4):1172–6.
Macatonia SE, Doherty TM, Knight SC, O’Garra A. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J Immunol. 1993;150(9):3755–65.
Ouyang W, O’Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity. 2019;50(4):871–91.
Wang X, Wong K, Ouyang W, Rutz S. Targeting IL-10 family cytokines for the treatment of human diseases. Cold Spring Harb Perspect Biol. 2019;11(2).
Zhang H, Li R, Cao Y, Gu Y, Lin C, Liu X, Lv K, He X, Fang H, Jin K, et al. Poor clinical outcomes and immunoevasive contexture in intratumoral IL-10-producing macrophages enriched gastric cancer patients. Ann Surg. 2022;275(4):e626–35.
Sawant DV, Yano H, Chikina M, Zhang Q, Liao M, Liu C, Callahan DJ, Sun Z, Sun T, Tabib T, et al. Adaptive plasticity of IL-10(+) and IL-35(+) T(reg) cells cooperatively promotes tumor T cell exhaustion. Nat Immunol. 2019;20(6):724–35.
Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, Blaisdell S, Basham B, Dai J, Grein J, et al. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell. 2011;20(6):781–96.
Chen WF, Zlotnik A. IL-10: a novel cytotoxic T cell differentiation factor. J Immunol. 1991;147(2):528–34.
Murphy MP, Holmgren A, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ, Partridge L, Gems D, et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011;13(4):361–6.
Ochoa CD, Wu RF, Terada LS. ROS signaling and ER stress in cardiovascular disease. Mol Aspects Med. 2018;63:18–29.
Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 2020;152:116–41.
Violi F, Carnevale R, Loffredo L, Pignatelli P, Gallin JI. NADPH oxidase-2 and atherothrombosis: insight from chronic granulomatous disease. Arterioscler Thromb Vasc Biol. 2017;37(2):218–25.
Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol. 2007;8(9):722–8.
Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–37.
Pei J, Pan X, Wei G, Hua Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front Pharmacol. 2023;14:1147414.
Zhang J, Li F, Yin Y, Liu N, Zhu M, Zhang H, Liu W, Yang M, Qin S, Fan X, et al. Alpha radionuclide-chelated radioimmunotherapy promoters enable local radiotherapy/chemodynamic therapy to discourage cancer progression. Biomater Res. 2022;26(1):44.
Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G, Castoria G, Migliaccio A. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52(2):192–203.
Fang J, Seki T, Maeda H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev. 2009;61(4):290–302.
Lee HC, Wei YH. Mitochondrial DNA instability and metabolic shift in human cancers. Int J Mol Sci. 2009;10(2):674–701.
Yu LM, Zhang WH, Han XX, Li YY, Lu Y, Pan J, Mao JQ, Zhu LY, Deng JJ, Huang W, et al. Hypoxia-induced ROS contribute to myoblast pyroptosis during obstructive sleep apnea via the NF-kappaB/HIF-1alpha signaling pathway. Oxid Med Cell Longev. 2019;2019:4596368.
Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71.
Willson JA, Arienti S, Sadiku P, Reyes L, Coelho P, Morrison T, Rinaldi G, Dockrell DH, Whyte MKB, Walmsley SR. Neutrophil HIF-1alpha stabilization is augmented by mitochondrial ROS produced via the glycerol 3-phosphate shuttle. Blood. 2022;139(2):281–6.
Sun B, Yu L, Xu C, Li YM, Zhao YR, Cao MM, Yang LY. NAD(P)HX epimerase downregulation promotes tumor progression through ROS/HIF-1alpha signaling in hepatocellular carcinoma. Cancer Sci. 2021;112(7):2753–69.
Zhang L, Cao Y, Guo X, Wang X, Han X, Kanwore K, Hong X, Zhou H, Gao D. Hypoxia-induced ROS aggravate tumor progression through HIF-1alpha-SERPINE1 signaling in glioblastoma. J Zhejiang Univ Sci B. 2023;24(1):32–49.
Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10(3):181–93.
Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002;99(21):13926–31.
Esh CJ, Chrismas BCR, Mauger AR, Taylor L. Pharmacological hypotheses: is acetaminophen selective in its cyclooxygenase inhibition? Pharmacol Res Perspect. 2021;9(4): e00835.
Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107(4):1183–8.
Hijos-Mallada G, Sostres C, Gomollon F. NSAIDs, gastrointestinal toxicity and inflammatory bowel disease. Gastroenterol Hepatol. 2022;45(3):215–22.
Tudor DV, Baldea I, Lupu M, Kacso T, Kutasi E, Hopartean A, Stretea R, Gabriela Filip A. COX-2 as a potential biomarker and therapeutic target in melanoma. Cancer Biol Med. 2020;17(1):20–31.
Solanki R, Agrawal N, Ansari M, Jain S, Jindal A. COX-2 expression in breast carcinoma with correlation to clinicopathological parameters. Asian Pac J Cancer Prev. 2018;19(7):1971–5.
Khor LY, Bae K, Pollack A, Hammond ME, Grignon DJ, Venkatesan VM, Rosenthal SA, Ritter MA, Sandler HM, Hanks GE, et al. COX-2 expression predicts prostate-cancer outcome: analysis of data from the RTOG 92–02 trial. Lancet Oncol. 2007;8(10):912–20.
Guo W, Zhang Z, Li G, Lai X, Gu R, Xu W, Chen H, Xing Z, Chen L, Qian J, et al. Pyruvate kinase M2 promotes prostate cancer metastasis through regulating ERK1/2-COX-2 signaling. Front Oncol. 2020;10: 544288.
Du J, Feng J, Luo D, Peng L. Prognostic and clinical significance of COX-2 overexpression in laryngeal cancer: a meta-analysis. Front Oncol. 2022;12: 854946.
Hu Z, Yang Y, Zhao Y, Huang Y. The prognostic value of cyclooxygenase-2 expression in patients with esophageal cancer: evidence from a meta-analysis. Onco Targets Ther. 2017;10:2893–901.
Ren J, Liu J, Sui X. Correlation of COX-2 and MMP-13 expressions with gastric cancer and their effects on prognosis. J BUON. 2019;24(1):187–93.
Pomianowska E, Schjolberg AR, Clausen OP, Gladhaug IP. COX-2 overexpression in resected pancreatic head adenocarcinomas correlates with favourable prognosis. BMC Cancer. 2014;14:458.
Sun H, Zhang X, Sun D, Jia X, Xu L, Qiao Y, Jin Y. COX-2 expression in ovarian cancer: an updated meta-analysis. Oncotarget. 2017;8(50):88152–62.
Wang D, Cabalag CS, Clemons NJ, DuBois RN. Cyclooxygenases and prostaglandins in tumor immunology and microenvironment of gastrointestinal cancer. Gastroenterology. 2021;161(6):1813–29.
Li YF, Han CC, Wang Y, Cui DQ, Luo TT, Zhang YW, Ma Y, Wei W. Combined PGE2 with TNF-alpha promotes laryngeal carcinoma progression by enhancing GRK2 and TRAF2 interaction. Neoplasma. 2020;67(2):354–63.
Walker OL, Dahn ML, Power Coombs MR, Marcato P. The prostaglandin e2 pathway and breast cancer stem cells: evidence of increased signaling and potential targeting. Front Oncol. 2021;11: 791696.
Frejborg E, Salo T, Salem A. Role of cyclooxygenase-2 in head and neck tumorigenesis. Int J Mol Sci. 2020;21(23).
Nakanishi M, Montrose DC, Clark P, Nambiar PR, Belinsky GS, Claffey KP, Xu D, Rosenberg DW. Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res. 2008;68(9):3251–9.
Dean PT, Hooks SB. Pleiotropic effects of the COX-2/PGE2 axis in the glioblastoma tumor microenvironment. Front Oncol. 2022;12:1116014.
Knudsen NH, Manguso RT. Tumor-derived PGE2 gives NK cells a headache. Immunity. 2020;53(6):1131–2.
Walker W, Rotondo D. Prostaglandin E2 is a potent regulator of interleukin-12- and interleukin-18-induced natural killer cell interferon-gamma synthesis. Immunology. 2004;111(3):298–305.
Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67(9):4507–13.
Tomic S, Joksimovic B, Bekic M, Vasiljevic M, Milanovic M, Colic M, Vucevic D. Prostaglanin-E2 potentiates the suppressive functions of human mononuclear myeloid-derived suppressor cells and increases their capacity to expand IL-10-producing regulatory T cell subsets. Front Immunol. 2019;10:475.
Porta C, Consonni FM, Morlacchi S, Sangaletti S, Bleve A, Totaro MG, Larghi P, Rimoldi M, Tripodo C, Strauss L, et al. Tumor-derived prostaglandin E2 promotes p50 NF-kappaB-dependent differentiation of monocytic MDSCs. Cancer Res. 2020;80(13):2874–88.
Zhang B, Bie Q, Wu P, Zhang J, You B, Shi H, Qian H, Xu W. PGD2/PTGDR2 signaling restricts the self-renewal and tumorigenesis of gastric cancer. Stem Cells. 2018;36(7):990–1003.
Iwanaga K, Nakamura T, Maeda S, Aritake K, Hori M, Urade Y, Ozaki H, Murata T. Mast cell-derived prostaglandin D2 inhibits colitis and colitis-associated colon cancer in mice. Cancer Res. 2014;74(11):3011–9.
Vafaeinik F, Kum HJ, Jin SY, Min DS, Song SH, Ha HK, Kim CD, Bae SS. Regulation of epithelial-mesenchymal transition of A549 cells by prostaglandin D(2). Cell Physiol Biochem. 2022;56(2):89–104.
Conejo-Garcia JR. Breaking barriers for T cells by targeting the EPHA2/TGF-beta/COX-2 axis in pancreatic cancer. J Clin Invest. 2019;129(9):3521–3.
Yan G, Zhao H, Zhang Q, Zhou Y, Wu L, Lei J, Wang X, Zhang J, Zhang X, Zheng L, et al. A RIPK3-PGE(2) circuit mediates myeloid-derived suppressor cell-potentiated colorectal carcinogenesis. Cancer Res. 2018;78(19):5586–99.
Take Y, Koizumi S, Nagahisa A. Prostaglandin E receptor 4 antagonist in cancer immunotherapy: mechanisms of action. Front Immunol. 2020;11:324.
Clemente SM, Martinez-Costa OH, Monsalve M, Samhan-Arias AK. Targeting lipid peroxidation for cancer treatment. Molecules. 2020;25(21).
Lin H, Weng J, Mei H, Zhuang M, Xiao X, Du F, Lin L, Wu J, Chen Z, Huang Y, et al. 5-Lipoxygenase promotes epithelial-mesenchymal transition through the ERK signaling pathway in gastric cancer. J Gastroenterol Hepatol. 2021;36(2):455–66.
Xia C, Sadeghi L, Straat K, Merrien M, Wright AP, Sander B, Xu D, Osterborg A, Bjorkholm M, Claesson HE. Intrinsic 5-lipoxygenase activity regulates migration and adherence of mantle cell lymphoma cells. Prostaglandins Other Lipid Mediat. 2021;156: 106575.
Muthuraman S, Sinha S, Vasavi CS, Waidha KM, Basu B, Munussami P, Balamurali MM, Doble M, Saravana Kumar R. Design, synthesis and identification of novel coumaperine derivatives for inhibition of human 5-LOX: Antioxidant, pseudoperoxidase and docking studies. Bioorg Med Chem. 2019;27(4):604–19.
Doiphode S, Lokhande KB, Ghosh P, Swamy KV, Nagar S. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) by resveratrol derivatives in cancer therapy: in silico approach. J Biomol Struct Dyn. 2022:1–16.
Shi HY, Lv FJ, Zhu ST, Wang QG, Zhang ST. Dual inhibition of 5-LOX and COX-2 suppresses esophageal squamous cell carcinoma. Cancer Lett. 2011;309(1):19–26.
Chen S, Zou H. Key role of 12-lipoxygenase and its metabolite 12-hydroxyeicosatetraenoic acid (12-HETE) in diabetic retinopathy. Curr Eye Res. 2022;47(3):329–35.
Liu Q, Tan W, Che J, Yuan D, Zhang L, Sun Y, Yue X, Xiao L, Jin Y. 12-HETE facilitates cell survival by activating the integrin-linked kinase/NF-kappaB pathway in ovarian cancer. Cancer Manag Res. 2018;10:5825–38.
Mao F, Wang M, Wang J, Xu WR. The role of 15-LOX-1 in colitis and colitis-associated colorectal cancer. Inflamm Res. 2015;64(9):661–9.
Kazan HH, Urfali-Mamatoglu C, Yalcin GD, Bulut O, Sezer A, Banerjee S, Gunduz U. 15-LOX-1 has diverse roles in the resensitization of resistant cancer cell lines to doxorubicin. J Cell Physiol. 2020;235(5):4965–78.
Na YJ, Kim BR, Kim JL, Kang S, Jeong YA, Park SH, Jo MJ, Kim JY, Kim HJ, Oh SC et al. Deficiency of 15-LOX-1 induces radioresistance through downregulation of MacroH2A2 in colorectal cancer. Cancers (Basel). 2019;11(11).
Berger A. What are leukotrienes and how do they work in asthma? BMJ. 1999;319(7202):90.
Bachi AL, Kim FJ, Nonogaki S, Carneiro CR, Lopes JD, Jasiulionis MG, Correa M. Leukotriene B4 creates a favorable microenvironment for murine melanoma growth. Mol Cancer Res. 2009;7(9):1417–24.
Wendel A, Tiegs G. Leukotriene D4 mediates galactosamine/endotoxin-induced hepatitis in mice. Biochem Pharmacol. 1987;36(12):1867.
Zhou Y, Guo D, Li H, Jie S. Circulating LTD4 in patients with hepatocellular carcinoma. Tumour Biol. 2011;32(1):139–44.
Arai J, Goto K, Otoyama Y, Nakajima Y, Sugiura I, Kajiwara A, Tojo M, Ichikawa Y, Uozumi S, Shimozuma Y, et al. Leukotriene receptor antagonists enhance HCC treatment efficacy by inhibiting ADAMs and suppressing MICA shedding. Cancer Immunol Immunother. 2021;70(1):203–13.
Wang Z, Cheng Q, Tang K, Sun Y, Zhang K, Zhang Y, Luo S, Zhang H, Ye D, Huang B. Lipid mediator lipoxin A4 inhibits tumor growth by targeting IL-10-producing regulatory B (Breg) cells. Cancer Lett. 2015;364(2):118–24.
Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB J. 2011;25(5):1441–8.
Gleeson MJ, Felix H, Johnsson LG. Ultrastructural aspects of the human peripheral vestibular system. Acta Otolaryngol Suppl. 1990;470:80–7.
Liu H, Zeng J, Huang W, Xu Q, Ye D, Sun R, Zhang D. Colorectal cancer is associated with a deficiency of lipoxin A(4), an endogenous anti-inflammatory mediator. J Cancer. 2019;10(19):4719–30.
Jia G, Wang X, Wu W, Zhang Y, Chen S, Zhao J, Zhao W, Li W, Sun X, Han B. LXA4 enhances prostate cancer progression by facilitating M2 macrophage polarization via inhibition of METTL3. Int Immunopharmacol. 2022;107: 108586.
Yuan J, Lin F, Chen L, Chen W, Pan X, Bai Y, Cai Y, Lu H. Lipoxin A4 regulates M1/M2 macrophage polarization via FPR2-IRF pathway. Inflammopharmacology. 2022;30(2):487–98.
Korbecki J, Rebacz-Maron E, Kupnicka P, Chlubek D, Baranowska-Bosiacka I. Synthesis and significance of arachidonic acid, a substrate for cyclooxygenases, lipoxygenases, and cytochrome p450 pathways in the tumorigenesis of glioblastoma multiforme, including a pan-cancer comparative analysis. Cancers (Basel). 2023;15(3).
Lakshmanan K, Byran G, Bandlamudi S, Krishnamurthy PT. The role of STAT3 signaling in different types of cancers: a comprehensive review. Curr Enzym Inhib. 2020;16(3):189–98.
Bharadwaj U, Kasembeli MM, Robinson P, Tweardy DJ. Targeting janus kinases and signal transducer and activator of transcription 3 to treat inflammation, fibrosis, and cancer: rationale, progress, and caution. Pharmacol Rev. 2020;72(2):486–526.
Xin P, Xu X, Deng C, Liu S, Wang Y, Zhou X, Ma H, Wei D, Sun S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int Immunopharmacol. 2020;80: 106210.
Wong ALA, Hirpara JL, Pervaiz S, Eu JQ, Sethi G, Goh BC. Do STAT3 inhibitors have potential in the future for cancer therapy? Expert Opin Investig Drugs. 2017;26(8):883–7.
Hirano T, Hirayama D, Wagatsuma K, Yamakawa T, Yokoyama Y, Nakase H: Immunological Mechanisms in Inflammation-Associated Colon Carcinogenesis. Int J Mol Sci. 2020;21(9).
Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24.
Kasembeli MM, Bharadwaj U, Robinson P, Tweardy DJ. Contribution of STAT3 to inflammatory and fibrotic diseases and prospects for its targeting for treatment. Int J Mol Sci. 2018; 19(8).
Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–13.
Gargalionis AN, Papavassiliou KA, Papavassiliou AG. Targeting STAT3 signaling pathway in colorectal cancer. Biomedicines. 2021; 9(8).
Zhong B, Cheng B, Huang X, Xiao Q, Niu Z, Chen YF, Yu Q, Wang W, Wu XJ. Colorectal cancer-associated fibroblasts promote metastasis by up-regulating LRG1 through stromal IL-6/STAT3 signaling. Cell Death Dis. 2021;13(1):16.
Wang S, Dong W, Liu L, Xu M, Wang Y, Liu T, Zhang Y, Wang B, Cao H. Interplay between bile acids and the gut microbiota promotes intestinal carcinogenesis. Mol Carcinog. 2019;58(7):1155–67.
Roche B, Vanden-Bossche A, Normand M, Malaval L, Vico L, Lafage-Proust MH. Validated laser doppler protocol for measurement of mouse bone blood perfusion—response to age or ovariectomy differs with genetic background. Bone. 2013;55(2):418–26.
Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19(2):164–74.
D’Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8(10):813–24.
Florean C, Song S, Dicato M, Diederich M. Redox biology of regulated cell death in cancer: a focus on necroptosis and ferroptosis. Free Radic Biol Med. 2019;134:177–89.
Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313.
Forcina GC, Dixon SJ. GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics. 2019;19(18): e1800311.
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, Li B. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12(1):34.
Prasad AS, Miale A Jr, Farid Z, Sandstead HH, Schulert AR. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypognadism. J Lab Clin Med. 1963;61:537–49.
Liu MJ, Bao S, Galvez-Peralta M, Pyle CJ, Rudawsky AC, Pavlovicz RE, Killilea DW, Li C, Nebert DW, Wewers MD, et al. ZIP8 regulates host defense through zinc-mediated inhibition of NF-kappaB. Cell Rep. 2013;3(2):386–400.
Burn J, Sheth H, Elliott F, Reed L, Macrae F, Mecklin JP, Moslein G, McRonald FE, Bertario L, Evans DG, et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet. 2020;395(10240):1855–63.
Bens A, Cronin-Fenton D, Dehlendorff C, Jensen MB, Ejlertsen B, Kroman N, Friis S, Mellemkjaer L. Nonaspirin NSAIDs and contralateral breast cancer risk. Int J Cancer. 2019;144(6):1243–50.
Hao W, Shen Y, Feng M, Wang H, Lin M, Fang Y, Tan L. Aspirin acts in esophageal cancer: a brief review. J Thorac Dis. 2018;10(4):2490–7.
Burn J, Bishop DT, Chapman PD, Elliott F, Bertario L, Dunlop MG, Eccles D, Ellis A, Evans DG, Fodde R, et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev Res (Phila). 2011;4(5):655–65.
Ishikawa H, Wakabayashi K, Suzuki S, Mutoh M, Hirata K, Nakamura T, Takeyama I, Kawano A, Gondo N, Abe T, et al. Preventive effects of low-dose aspirin on colorectal adenoma growth in patients with familial adenomatous polyposis: double-blind, randomized clinical trial. Cancer Med. 2013;2(1):50–6.
Jankowski JAZ, de Caestecker J, Love SB, Reilly G, Watson P, Sanders S, Ang Y, Morris D, Bhandari P, Brooks C, et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet. 2018;392(10145):400–8.
Wu QB, Sun GP. Expression of COX-2 and HER-2 in colorectal cancer and their correlation. World J Gastroenterol. 2015;21(20):6206–14.
Sheng J, Sun H, Yu FB, Li B, Zhang Y, Zhu YT. The role of cyclooxygenase-2 in colorectal cancer. Int J Med Sci. 2020;17(8):1095–101.
Todoric J, Antonucci L, Karin M. Targeting inflammation in cancer prevention and therapy. Cancer Prev Res (Phila). 2016;9(12):895–905.
North GL. Celecoxib as adjunctive therapy for treatment of colorectal cancer. Ann Pharmacother. 2001;35(12):1638–43.
Mostafa TM, Alm El-Din MA, Rashdan AR. Celecoxib as an adjuvant to chemotherapy for patients with metastatic colorectal cancer: a randomized controlled clinical study. Saudi Med J. 2022;43(1):37–44.
Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, Tang J, Rosenstein RB, Wittes J, Corle D, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–84.
Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355(9):885–95.
Hu H, Kang L, Zhang J, Wu Z, Wang H, Huang M, Lan P, Wu X, Wang C, Cao W, et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7(1):38–48.
Ye SY, Li JY, Li TH, Song YX, Sun JX, Chen XW, Zhao JH, Li Y, Wu ZH, Gao P, et al. The efficacy and safety of celecoxib in addition to standard cancer therapy: a systematic review and meta-analysis of randomized controlled trials. Curr Oncol. 2022;29(9):6137–53.
Guo Q, Liu X, Lu L, Yuan H, Wang Y, Chen Z, Ji R, Zhou Y. Comprehensive evaluation of clinical efficacy and safety of celecoxib combined with chemotherapy in management of gastric cancer. Medicine (Baltimore). 2017;96(51): e8857.
Liao Z, Komaki R, Milas L, Yuan C, Kies M, Chang JY, Jeter M, Guerrero T, Blumenschien G, Smith CM, et al. A phase I clinical trial of thoracic radiotherapy and concurrent celecoxib for patients with unfavorable performance status inoperable/unresectable non-small cell lung cancer. Clin Cancer Res. 2005;11(9):3342–8.
Koch A, Bergman B, Holmberg E, Sederholm C, Ek L, Kosieradzki J, Lamberg K, Thaning L, Ydreborg SO, Sorenson S, et al. Effect of celecoxib on survival in patients with advanced non-small cell lung cancer: a double blind randomised clinical phase III trial (CYCLUS study) by the Swedish Lung Cancer Study Group. Eur J Cancer. 2011;47(10):1546–55.
Bi N, Liang J, Zhou Z, Chen D, Fu Z, Yang X, Feng Q, Hui Z, Xiao Z, Lv J, et al. Effect of concurrent chemoradiation with celecoxib vs concurrent chemoradiation alone on survival among patients with non-small cell lung cancer with and without cyclooxygenase 2 genetic variants: a phase 2 randomized clinical trial. JAMA Netw Open. 2019;2(12): e1918070.
Bayraktar S, Baghaki S, Wu J, Liu DD, Gutierrez-Barrera AM, Bevers TB, Valero V, Sneige N, Arun BK. Biomarker modulation study of celecoxib for chemoprevention in women at increased risk for breast cancer: a phase II pilot study. Cancer Prev Res (Phila). 2020;13(9):795–802.
Guo Q, Li Q, Wang J, Liu M, Wang Y, Chen Z, Ye Y, Guan Q, Zhou Y. A comprehensive evaluation of clinical efficacy and safety of celecoxib in combination with chemotherapy in metastatic or postoperative recurrent gastric cancer patients: a preliminary, three-center, clinical trial study. Medicine (Baltimore). 2019;98(27): e16234.
De Cremoux P, Hamy AS, Lehmann-Che J, Scott V, Sigal B, Mathieu MC, Bertheau P, Guinebretiere JM, Pierga JY, Giacchetti S, et al. COX2/PTGS2 expression is predictive of response to neoadjuvant celecoxib in HER2-negative breast cancer patients. Anticancer Res. 2018;38(3):1485–90.
Meyerhardt JA, Shi Q, Fuchs CS, Meyer J, Niedzwiecki D, Zemla T, Kumthekar P, Guthrie KA, Couture F, Kuebler P, et al. Effect of celecoxib vs placebo added to standard adjuvant therapy on disease-free survival among patients with stage III colon cancer: the CALGB/SWOG 80702 (alliance) randomized clinical trial. JAMA. 2021;325(13):1277–86.
Coombes RC, Tovey H, Kilburn L, Mansi J, Palmieri C, Bartlett J, Hicks J, Makris A, Evans A, Loibl S, et al. Effect of celecoxib vs placebo as adjuvant therapy on disease-free survival among patients with breast cancer: the REACT randomized clinical trial. JAMA Oncol. 2021;7(9):1291–301.
Hamy AS, Tury S, Wang X, Gao J, Pierga JY, Giacchetti S, Brain E, Pistilli B, Marty M, Espie M, et al. Celecoxib with neoadjuvant chemotherapy for breast cancer might worsen outcomes differentially by COX-2 expression and ER status: exploratory analysis of the REMAGUS02 trial. J Clin Oncol. 2019;37(8):624–35.
Schjerning AM, McGettigan P, Gislason G. Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat Rev Cardiol. 2020;17(9):574–84.
Cui J, Jia J. Natural COX-2 inhibitors as promising anti-inflammatory agents: an update. Curr Med Chem. 2021;28(18):3622–46.
Yang L, Zou T, Chen Y, Zhao Y, Wu X, Li M, Du F, Chen Y, Xiao Z, Shen J. Hepatitis B virus X protein mediated epigenetic alterations in the pathogenesis of hepatocellular carcinoma. Hepatol Int. 2022;16(4):741–54.
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L: EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–98.
Hou JL, Zhao W, Lee C, Hann HW, Peng CY, Tanwandee T, Morozov V, Klinker H, Sollano JD, Streinu-Cercel A, et al. Outcomes of long-term treatment of chronic HBV infection with entecavir or other agents from a randomized trial in 24 countries. Clin Gastroenterol Hepatol. 2020;18(2):457–67.
Choi J, Kim HJ, Lee J, Cho S, Ko MJ, Lim YS. Risk of hepatocellular carcinoma in patients treated with entecavir vs tenofovir for chronic hepatitis B: a Korean nationwide cohort study. JAMA Oncol. 2019;5(1):30–6.
Zhang Z, Zhou Y, Yang J, Hu K, Huang Y. The effectiveness of TDF versus ETV on incidence of HCC in CHB patients: a meta analysis. BMC Cancer. 2019;19(1):511.
Tan DJH, Ng CH, Tay PWL, Syn N, Muthiah MD, Lim WH, Tang ASP, Lim KE, Lim GEH, Tamaki N, et al. Risk of hepatocellular carcinoma with tenofovir vs entecavir treatment for chronic hepatitis B virus: a reconstructed individual patient data meta-analysis. JAMA Netw Open. 2022;5(6): e2219407.
Pol S. group AAs: Similar 5-year HCC occurrence in Tenofovir- and Entecavir-treated HBV chronic infection in the French AFEF/ANRS CO22 Hepather cohort. Aliment Pharmacol Ther. 2021;53(5):616–29.
Chang TS, Yang YH, Chen WM, Shen CH, Tung SY, Yen CW, Hsieh YY, Lee CP, Tsai ML, Hung CH, et al. Long-term risk of primary liver cancers in entecavir versus tenofovir treatment for chronic hepatitis B. Sci Rep. 2021;11(1):1365.
Xing Y, Zhong W, Peng D, Han Z, Zeng H, Wang Y, Feng L, Huang J, Xu L, Chen M, et al. Chinese herbal formula ruangan granule enhances the efficacy of entecavir to reverse advanced liver fibrosis/early cirrhosis in patients with chronic HBV infection: a multicenter, randomized clinical trial. Pharmacol Res. 2023;190: 106737.
Ji D, Chen Y, Bi J, Shang Q, Liu H, Wang JB, Tan L, Wang J, Chen Y, Li Q, et al. Entecavir plus Biejia-Ruangan compound reduces the risk of hepatocellular carcinoma in Chinese patients with chronic hepatitis B. J Hepatol. 2022;77(6):1515–24.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Nahand JS, Taghizadeh-Boroujeni S, Karimzadeh M, Borran S, Pourhanifeh MH, Moghoofei M, Bokharaei-Salim F, Karampoor S, Jafari A, Asemi Z, et al. microRNAs: new prognostic, diagnostic, and therapeutic biomarkers in cervical cancer. J Cell Physiol. 2019;234(10):17064–99.
Sadri Nahand J, Moghoofei M, Salmaninejad A, Bahmanpour Z, Karimzadeh M, Nasiri M, Mirzaei HR, Pourhanifeh MH, Bokharaei-Salim F, Mirzaei H, et al. Pathogenic role of exosomes and microRNAs in HPV-mediated inflammation and cervical cancer: a review. Int J Cancer. 2020;146(2):305–20.
Laniewski P, Barnes D, Goulder A, Cui H, Roe DJ, Chase DM, Herbst-Kralovetz MM. Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women. Sci Rep. 2018;8(1):7593.
Kemp TJ, Hildesheim A, Garcia-Pineres A, Williams MC, Shearer GM, Rodriguez AC, Schiffman M, Burk R, Freer E, Bonilla J, et al. Elevated systemic levels of inflammatory cytokines in older women with persistent cervical human papillomavirus infection. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1954–9.
Yost S, Hoekstra A. Cervical cancer in women over 65: an analysis of screening. Gynecol Oncol Rep. 2018;25:48–51.
Sales KJ, Katz AA. Inflammatory pathways in cervical cancer—the UCT contribution. S Afr Med J. 2012;102(6):493–6.
Drolet M, Benard E, Perez N, Brisson M. Group HPVVIS: Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394(10197):497–509.
Lei J, Ploner A, Elfstrom KM, Wang J, Roth A, Fang F, Sundstrom K, Dillner J, Sparen P. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383(14):1340–8.
Brisson M, Kim JJ, Canfell K, Drolet M, Gingras G, Burger EA, Martin D, Simms KT, Benard E, Boily MC, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395(10224):575–90.
Komdeur FL, Singh A, van de Wall S, Meulenberg JJM, Boerma A, Hoogeboom BN, Paijens ST, Oyarce C, de Bruyn M, Schuuring E, et al. First-in-human phase I clinical trial of an SFV-based RNA replicon cancer vaccine against HPV-induced cancers. Mol Ther. 2021;29(2):611–25.
Porras C, Tsang SH, Herrero R, Guillen D, Darragh TM, Stoler MH, Hildesheim A, Wagner S, Boland J, Lowy DR, et al. Efficacy of the bivalent HPV vaccine against HPV 16/18-associated precancer: long-term follow-up results from the Costa Rica Vaccine Trial. Lancet Oncol. 2020;21(12):1643–52.
Massarelli E, William W, Johnson F, Kies M, Ferrarotto R, Guo M, Feng L, Lee JJ, Tran H, Kim YU, et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(1):67–73.
Bernhard H, Jager-Arand E, Bernhard G, Heike M, Klein O, Riemann JF, Meyer zum Buschenfelde KH, Dippold W, Knuth A. Treatment of advanced pancreatic cancer with 5-fluorouracil, folinic acid and interferon alpha-2A: results of a phase II trial. Br J Cancer. 1995;71(1):102–5.
David AK, Vaughn DJ, Holroyde CP, Armstead B, Haller DG. A phase II trial of 5-fluorouracil, leucovorin, and interferon alpha 2A (IFN-alpha 2a) in metastatic pancreatic carcinoma: a Penn Cancer Clinical Trials Group (PCCTG) trial. Am J Clin Oncol. 2000;23(1):37–9.
Ohman KA, Liu J, Linehan DC, Tan MC, Tan BR, Fields RC, Strasberg SM, Hawkins WG. Interferon-based chemoradiation followed by gemcitabine for resected pancreatic adenocarcinoma: long-term follow-up. HPB (Oxford). 2017;19(5):449–57.
Rocha FG, Hashimoto Y, Traverso LW, Dorer R, Kozarek R, Helton WS, Picozzi VJ. Interferon-based adjuvant chemoradiation for resected pancreatic head cancer: long-term follow-up of the Virginia mason protocol. Ann Surg. 2016;263(2):376–84.
Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14(1):7–17.
Eigentler TK, Gutzmer R, Hauschild A, Heinzerling L, Schadendorf D, Nashan D, Holzle E, Kiecker F, Becker J, Sunderkotter C, et al. Adjuvant treatment with pegylated interferon alpha-2a versus low-dose interferon alpha-2a in patients with high-risk melanoma: a randomized phase III DeCOG trial. Ann Oncol. 2016;27(8):1625–32.
Ives NJ, Suciu S, Eggermont AMM, Kirkwood J, Lorigan P, Markovic SN, Garbe C, Wheatley K. International Melanoma Meta-Analysis Collaborative G: Adjuvant interferon-alpha for the treatment of high-risk melanoma: An individual patient data meta-analysis. Eur J Cancer. 2017;82:171–83.
Yamazaki N, Uhara H, Wada H, Matsuda K, Yamamoto K, Shimamoto T, Kiyohara Y. Phase I study of pegylated interferon-alpha-2b as an adjuvant therapy in Japanese patients with malignant melanoma. J Dermatol. 2016;43(10):1146–53.
Dummer R, Mangana J. Long-term pegylated interferon-alpha and its potential in the treatment of melanoma. Biologics. 2009;3:169–82.
Najjar YG, Puligandla M, Lee SJ, Kirkwood JM. An updated analysis of 4 randomized ECOG trials of high-dose interferon in the adjuvant treatment of melanoma. Cancer. 2019;125(17):3013–24.
Eggermont AM, Suciu S, Testori A, Santinami M, Kruit WH, Marsden J, Punt CJ, Sales F, Dummer R, Robert C, et al. Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J Clin Oncol. 2012;30(31):3810–8.
Bottomley A, Coens C, Suciu S, Santinami M, Kruit W, Testori A, Marsden J, Punt C, Sales F, Gore M, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma: a phase III randomized controlled trial of health-related quality of life and symptoms by the European Organisation for Research and Treatment of Cancer Melanoma Group. J Clin Oncol. 2009;27(18):2916–23.
Flaherty LE, Othus M, Atkins MB, Tuthill RJ, Thompson JA, Vetto JT, Haluska FG, Pappo AS, Sosman JA, Redman BG, et al. Southwest Oncology Group S0008: a phase III trial of high-dose interferon Alfa-2b versus cisplatin, vinblastine, and dacarbazine, plus interleukin-2 and interferon in patients with high-risk melanoma–an intergroup study of cancer and leukemia Group B, Children’s Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. J Clin Oncol. 2014;32(33):3771–8.
Simeone E, Scognamiglio G, Capone M, Giannarelli D, Grimaldi AM, Mallardo D, Madonna G, Curvietto M, Esposito A, Sandomenico F, et al. A monocentric phase I study of vemurafenib plus cobimetinib plus PEG-interferon (VEMUPLINT) in advanced melanoma patients harboring the V600BRAF mutation. J Transl Med. 2021;19(1):17.
Magenau JM, Peltier D, Riwes M, Pawarode A, Parkin B, Braun T, Anand S, Ghosh M, Maciejewski J, Yanik G, et al. Type 1 interferon to prevent leukemia relapse after allogeneic transplantation. Blood Adv. 2021;5(23):5047–56.
Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, Chen YH, Han W, Wang FR, Wang JZ, et al. IFN-alpha is effective for treatment of minimal residual disease in patients with acute leukemia after allogeneic hematopoietic stem cell transplantation: results of a registry study. Biol Blood Marrow Transplant. 2017;23(8):1303–10.
Kankuri-Tammilehto M, Perasto L, Pyrhonen S, Salminen E. Long-term outcome with prolonged use of interferon-alpha administered intermittently for metastatic renal cell carcinoma: a phase II study. Anticancer Res. 2023;43(6):2645–57.
Eto M, Kawano Y, Hirao Y, Mita K, Arai Y, Tsukamoto T, Hashine K, Matsubara A, Fujioka T, Kimura G, et al. Phase II clinical trial of sorafenib plus interferon-alpha treatment for patients with metastatic renal cell carcinoma in Japan. BMC Cancer. 2015;15:667.
Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS, Archer L, Atkins JN, Picus J, Czaykowski P, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26(33):5422–8.
Rafique I, Kirkwood JM, Tarhini AA. Immune checkpoint blockade and interferon-alpha in melanoma. Semin Oncol. 2015;42(3):436–47.
Wang H, Xia L, Yao CC, Dong H, Yang Y, Li C, Ji WX, Sun RM, Duan HQ, Mengzhou W, et al. NLRP4 negatively regulates type I interferon response and influences the outcome in anti-programmed cell death protein (PD)-1/PD-ligand 1 therapy. Cancer Sci. 2022;113(3):838–51.
Brohl AS, Khushalani NI, Eroglu Z, Markowitz J, Thapa R, Chen YA, Kudchadkar R, Weber JS. A phase IB study of ipilimumab with peginterferon alfa-2b in patients with unresectable melanoma. J Immunother Cancer. 2016;4:85.
Tarhini A, Lin Y, Lin H, Rahman Z, Vallabhaneni P, Mendiratta P, Pingpank JF, Holtzman MP, Yusko EC, Rytlewski JA, et al. Neoadjuvant ipilimumab (3 mg/kg or 10 mg/kg) and high dose IFN-alpha2b in locally/regionally advanced melanoma: safety, efficacy and impact on T-cell repertoire. J Immunother Cancer. 2018;6(1):112.
Trinh KR, Vasuthasawat A, Steward KK, Yamada RE, Timmerman JM, Morrison SL. Anti-CD20-interferon-beta fusion protein therapy of murine B-cell lymphomas. J Immunother. 2013;36(5):305–18.
Xuan C, Steward KK, Timmerman JM, Morrison SL. Targeted delivery of interferon-alpha via fusion to anti-CD20 results in potent antitumor activity against B-cell lymphoma. Blood. 2010;115(14):2864–71.
Li Z, Zhu Y, Li C, Trinh R, Ren X, Sun F, Wang Y, Shang P, Wang T, Wang M, et al. Anti-VEGFR2-interferon-alpha2 regulates the tumor microenvironment and exhibits potent antitumor efficacy against colorectal cancer. Oncoimmunology. 2017;6(3): e1290038.
Green DS, Husain SR, Johnson CL, Sato Y, Han J, Joshi B, Hewitt SM, Puri RK, Zoon KC. Combination immunotherapy with IL-4 Pseudomonas exotoxin and IFN-alpha and IFN-gamma mediate antitumor effects in vitro and in a mouse model of human ovarian cancer. Immunotherapy. 2019;11(6):483–96.
Duggan MC, Jochems C, Donahue RN, Richards J, Karpa V, Foust E, Paul B, Brooks T, Tridandapani S, Olencki T, et al. A phase I study of recombinant (r) vaccinia-CEA(6D)-TRICOM and rFowlpox-CEA(6D)-TRICOM vaccines with GM-CSF and IFN-alpha-2b in patients with CEA-expressing carcinomas. Cancer Immunol Immunother. 2016;65(11):1353–64.
Sheng L, Chen X, Wang Q, Lyu S, Li P. Interferon-alpha2b enhances survival and modulates transcriptional profiles and the immune response in melanoma patients treated with dendritic cell vaccines. Biomed Pharmacother. 2020;125: 109966.
Gigante M, Mandic M, Wesa AK, Cavalcanti E, Dambrosio M, Mancini V, Battaglia M, Gesualdo L, Storkus WJ, Ranieri E. Interferon-alpha (IFN-alpha)-conditioned DC preferentially stimulate type-1 and limit Treg-type in vitro T-cell responses from RCC patients. J Immunother. 2008;31(3):254–62.
Rozera C, Cappellini GA, D’Agostino G, Santodonato L, Castiello L, Urbani F, Macchia I, Arico E, Casorelli I, Sestili P, et al. Intratumoral injection of IFN-alpha dendritic cells after dacarbazine activates anti-tumor immunity: results from a phase I trial in advanced melanoma. J Transl Med. 2015;13:139.
Cox MC, Lapenta C, Santini SM. Advances and perspectives of dendritic cell-based active immunotherapies in follicular lymphoma. Cancer Immunol Immunother. 2020;69(6):913–25.
Derynck R, Turley SJ, Akhurst RJ. TGFbeta biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 2021;18(1):9–34.
Morris JC, Tan AR, Olencki TE, Shapiro GI, Dezube BJ, Reiss M, Hsu FJ, Berzofsky JA, Lawrence DP. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFbeta) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS ONE. 2014;9(3): e90353.
Formenti SC, Lee P, Adams S, Goldberg JD, Li X, Xie MW, Ratikan JA, Felix C, Hwang L, Faull KF, et al. Focal irradiation and systemic TGFbeta blockade in metastatic breast cancer. Clin Cancer Res. 2018;24(11):2493–504.
Faivre S, Santoro A, Kelley RK, Gane E, Costentin CE, Gueorguieva I, Smith C, Cleverly A, Lahn MM, Raymond E, et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int. 2019;39(8):1468–77.
Kelley RK, Gane E, Assenat E, Siebler J, Galle PR, Merle P, Hourmand IO, Cleverly A, Zhao Y, Gueorguieva I, et al. A phase 2 study of galunisertib (TGF-beta1 receptor type I inhibitor) and sorafenib in patients with advanced hepatocellular carcinoma. Clin Transl Gastroenterol. 2019;10(7): e00056.
Melisi D, Garcia-Carbonero R, Macarulla T, Pezet D, Deplanque G, Fuchs M, Trojan J, Oettle H, Kozloff M, Cleverly A, et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br J Cancer. 2018;119(10):1208–14.
Melisi D, Oh DY, Hollebecque A, Calvo E, Varghese A, Borazanci E, Macarulla T, Merz V, Zecchetto C, Zhao Y et al. Safety and activity of the TGFbeta receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J Immunother Cancer. 2021;9(3).
Nadal E, Saleh M, Aix SP, Ochoa-de-Olza M, Patel SP, Antonia S, Zhao Y, Gueorguieva I, Man M, Estrem ST, et al. A phase Ib/II study of galunisertib in combination with nivolumab in solid tumors and non-small cell lung cancer. BMC Cancer. 2023;23(1):708.
Yamazaki T, Gunderson AJ, Gilchrist M, Whiteford M, Kiely MX, Hayman A, O’Brien D, Ahmad R, Manchio JV, Fox N, et al. Galunisertib plus neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: a single-arm, phase 2 trial. Lancet Oncol. 2022;23(9):1189–200.
Necchi A, Giannatempo P, Mariani L, Fare E, Raggi D, Pennati M, Zaffaroni N, Crippa F, Marchiano A, Nicolai N, et al. PF-03446962, a fully-human monoclonal antibody against transforming growth-factor beta (TGFbeta) receptor ALK1, in pre-treated patients with urothelial cancer: an open label, single-group, phase 2 trial. Invest New Drugs. 2014;32(3):555–60.
Simonelli M, Zucali P, Santoro A, Thomas MB, de Braud FG, Borghaei H, Berlin J, Denlinger CS, Noberasco C, Rimassa L, et al. Phase I study of PF-03446962, a fully human monoclonal antibody against activin receptor-like kinase-1, in patients with hepatocellular carcinoma. Ann Oncol. 2016;27(9):1782–7.
Wheatley-Price P, Chu Q, Bonomi M, Seely J, Gupta A, Goss G, Hilton J, Feld R, Lee CW, Goffin JR, et al. A phase II study of PF-03446962 in patients with advanced malignant pleural mesothelioma. CCTG trial IND207. J Thorac Oncol. 2016;11(11):218–21.
Goff LW, Cohen RB, Berlin JD, de Braud FG, Lyshchik A, Noberasco C, Bertolini F, Carpentieri M, Stampino CG, Abbattista A, et al. A phase I study of the anti-activin receptor-like kinase 1 (ALK-1) monoclonal antibody PF-03446962 in patients with advanced solid tumors. Clin Cancer Res. 2016;22(9):2146–54.
Clarke JM, Blobe GC, Strickler JH, Uronis HE, Zafar SY, Morse M, Dropkin E, Howard L, O’Neill M, Rushing CN, et al. A phase Ib study of the combination regorafenib with PF-03446962 in patients with refractory metastatic colorectal cancer (REGAL-1 trial). Cancer Chemother Pharmacol. 2019;84(4):909–17.
Gulley JL, Schlom J, Barcellos-Hoff MH, Wang XJ, Seoane J, Audhuy F, Lan Y, Dussault I, Moustakas A. Dual inhibition of TGF-beta and PD-L1: a novel approach to cancer treatment. Mol Oncol. 2022;16(11):2117–34.
Paz-Ares L, Kim TM, Vicente D, Felip E, Lee DH, Lee KH, Lin CC, Flor MJ, Di Nicola M, Alvarez RM, et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-beta and PD-L1, in second-line treatment of patients with NSCLC: results from an expansion cohort of a phase 1 trial. J Thorac Oncol. 2020;15(7):1210–22.
Lin CC, Doi T, Muro K, Hou MM, Esaki T, Hara H, Chung HC, Helwig C, Dussault I, Osada M, et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGFbeta and PD-L1, in patients with esophageal squamous cell carcinoma: results from a phase 1 cohort in Asia. Target Oncol. 2021;16(4):447–59.
Tan B, Khattak A, Felip E, Kelly K, Rich P, Wang D, Helwig C, Dussault I, Ojalvo LS, Isambert N. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-beta and PD-L1, in patients with esophageal adenocarcinoma: results from a phase 1 cohort. Target Oncol. 2021;16(4):435–46.
Cho BC, Daste A, Ravaud A, Salas S, Isambert N, McClay E, Awada A, Borel C, Ojalvo LS, Helwig C et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-beta and PD-L1, in advanced squamous cell carcinoma of the head and neck: results from a phase I cohort. J Immunother Cancer. 2020;8(2).
Strauss J, Gatti-Mays ME, Cho BC, Hill A, Salas S, McClay E, Redman JM, Sater HA, Donahue RN, Jochems C et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-beta and PD-L1, in patients with human papillomavirus-associated malignancies. J Immunother Cancer. 2020;8(2).
Redman JM, Friedman J, Robbins Y, Sievers C, Yang X, Lassoued W, Sinkoe A, Papanicolau-Sengos A, Lee CC, Marte JL et al. Enhanced neoepitope-specific immunity following neoadjuvant PD-L1 and TGF-beta blockade in HPV-unrelated head and neck cancer. J Clin Invest. 2022;132(18).
Lan Y, Moustafa M, Knoll M, Xu C, Furkel J, Lazorchak A, Yeung TL, Hasheminasab SM, Jenkins MH, Meister S, et al. Simultaneous targeting of TGF-beta/PD-L1 synergizes with radiotherapy by reprogramming the tumor microenvironment to overcome immune evasion. Cancer Cell. 2021;39(10):1388–403.
Singh S, Xiao Z, Bavisi K, Roszik J, Melendez BD, Wang Z, Cantwell MJ, Davis RE, Lizee G, Hwu P, et al. IL-1alpha mediates innate and acquired resistance to immunotherapy in melanoma. J Immunol. 2021;206(8):1966–75.
Aggen DH, Ager CR, Obradovic AZ, Chowdhury N, Ghasemzadeh A, Mao W, Chaimowitz MG, Lopez-Bujanda ZA, Spina CS, Hawley JE, et al. Blocking IL1 beta promotes tumor regression and remodeling of the myeloid compartment in a renal cell carcinoma model: multidimensional analyses. Clin Cancer Res. 2021;27(2):608–21.
Wong CC, Baum J, Silvestro A, Beste MT, Bharani-Dharan B, Xu S, Wang YA, Wang X, Prescott MF, Krajkovich L, et al. Inhibition of IL1beta by canakinumab may be effective against diverse molecular subtypes of lung cancer: an exploratory analysis of the CANTOS trial. Cancer Res. 2020;80(24):5597–605.
Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, Group CT. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10105):1833–42.
Garrido P, Pujol JL, Kim ES, Lee JM, Tsuboi M, Gomez-Rueda A, Benito A, Moreno N, Gorospe L, Dong T, et al. Canakinumab with and without pembrolizumab in patients with resectable non-small-cell lung cancer: CANOPY-N study design. Future Oncol. 2021;17(12):1459–72.
Yuan B, Clowers MJ, Velasco WV, Peng S, Peng Q, Shi Y, Ramos-Castaneda M, Zarghooni M, Yang S, Babcock RL et al. Targeting IL-1beta as an immunopreventive and therapeutic modality for K-ras-mutant lung cancer. JCI Insight. 2022;7(11).
Wu TC, Xu K, Martinek J, Young RR, Banchereau R, George J, Turner J, Kim KI, Zurawski S, Wang X, et al. IL1 Receptor antagonist controls transcriptional signature of inflammation in patients with metastatic breast cancer. Cancer Res. 2018;78(18):5243–58.
Lust JA, Lacy MQ, Zeldenrust SR, Witzig TE, Moon-Tasson LL, Dinarello CA, Donovan KA. Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. Am J Hematol. 2016;91(6):571–4.
Becerra C, Paulson AS, Cavaness KM, Celinski SA. Gemcitabine, nab-paclitaxel, cisplatin, and anakinra (AGAP) treatment in patients with localized pancreatic ductal adenocarcinoma (PDAC). J Clin Oncol. 2018;36(4_suppl):449–449.
Voigt C, May P, Gottschlich A, Markota A, Wenk D, Gerlach I, Voigt S, Stathopoulos GT, Arendt KAM, Heise C, et al. Cancer cells induce interleukin-22 production from memory CD4(+) T cells via interleukin-1 to promote tumor growth. Proc Natl Acad Sci USA. 2017;114(49):12994–9.
Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19(1):57–64.
Isambert N, Hervieu A, Rebe C, Hennequin A, Borg C, Zanetta S, Chevriaux A, Richard C, Derangere V, Limagne E, et al. Fluorouracil and bevacizumab plus anakinra for patients with metastatic colorectal cancer refractory to standard therapies (IRAFU): a single-arm phase 2 study. Oncoimmunology. 2018;7(9): e1474319.
Lust JA, Lacy MQ, Zeldenrust SR, Dispenzieri A, Gertz MA, Witzig TE, Kumar S, Hayman SR, Russell SJ, Buadi FK, et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1beta-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84(2):114–22.
Briukhovetska D, Dorr J, Endres S, Libby P, Dinarello CA, Kobold S. Interleukins in cancer: from biology to therapy. Nat Rev Cancer. 2021;21(8):481–99.
Waldmann TA. Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol. 2018;10(12).
Yang Y, Lundqvist A. Immunomodulatory Effects of IL-2 and IL-15; Implications for Cancer Immunotherapy. Cancers (Basel). 2020;12(12)
Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–8.
Payne R, Glenn L, Hoen H, Richards B, Smith JW 2nd, Lufkin R, Crocenzi TS, Urba WJ, Curti BD. Durable responses and reversible toxicity of high-dose interleukin-2 treatment of melanoma and renal cancer in a Community Hospital Biotherapy Program. J Immunother Cancer. 2014;2:13.
Baik AH, Oluwole OO, Johnson DB, Shah N, Salem JE, Tsai KK, Moslehi JJ. Mechanisms of cardiovascular toxicities associated with immunotherapies. Circ Res. 2021;128(11):1780–801.
Damoiseaux J. The IL-2—IL-2 receptor pathway in health and disease: the role of the soluble IL-2 receptor. Clin Immunol. 2020;218: 108515.
Doberstein SK. Bempegaldesleukin (NKTR-214): a CD-122-biased IL-2 receptor agonist for cancer immunotherapy. Expert Opin Biol Ther. 2019;19(12):1223–8.
Bentebibel SE, Hurwitz ME, Bernatchez C, Haymaker C, Hudgens CW, Kluger HM, Tetzlaff MT, Tagliaferri MA, Zalevsky J, Hoch U, et al. A first-in-human study and biomarker analysis of NKTR-214, a novel IL2Rbetagamma-biased cytokine, in patients with advanced or metastatic solid tumors. Cancer Discov. 2019;9(6):711–21.
Diab A, Tannir NM, Bentebibel SE, Hwu P, Papadimitrakopoulou V, Haymaker C, Kluger HM, Gettinger SN, Sznol M, Tykodi SS, et al. Bempegaldesleukin (NKTR-214) plus nivolumab in patients with advanced solid tumors: phase i dose-escalation study of safety, efficacy, and immune activation (PIVOT-02). Cancer Discov. 2020;10(8):1158–73.
Khushalani NI, Diab A, Ascierto PA, Larkin J, Sandhu S, Sznol M, Koon HB, Jarkowski A, Zhou M, Statkevich P, et al. Bempegaldesleukin plus nivolumab in untreated, unresectable or metastatic melanoma: phase III PIVOT IO 001 study design. Future Oncol. 2020;16(28):2165–75.
Siefker-Radtke AO, Cho DC, Diab A, Sznol M, Bilen MA, Balar AV, Grignani G, Puente E, Tang L, Chien D, et al. Bempegaldesleukin plus nivolumab in first-line metastatic urothelial carcinoma: results from PIVOT-02. Eur Urol. 2022;82(4):365–73.
Diab A, Tykodi SS, Daniels GA, Maio M, Curti BD, Lewis KD, Jang S, Kalinka E, Puzanov I, Spira AI, et al. Bempegaldesleukin plus nivolumab in first-line metastatic melanoma. J Clin Oncol. 2021;39(26):2914–25.
Pan Y, Hao Y, Han H, Chen T, Ding H, Labbe KE, Shum E, Guidry K, Hu H, Sherman F et al. Nemvaleukin alfa, a novel engineered IL-2 fusion protein, drives antitumor immunity and inhibits tumor growth in small cell lung cancer. J Immunother Cancer. 2022; 10(9).
Boni V, Winer IS, Gilbert L, Vaishampayan UN, Rosen SD, Muzaffar J, Spreafico A, McDermott DF, Chu QS, Dumas O, et al. ARTISTRY-1: Nemvaleukin alfa monotherapy and in combination with pembrolizumab in patients (pts) with advanced solid tumors. J Clin Oncol. 2021;39(15_suppl):2513–2513.
Hamid O, Liu SV, Boccia RV, Call JA, Wise-Draper TM, Alistar AT, Powderly JD, Carthon BC, Vaishampayan UN, Olszanski AJ, et al. Selection of the recommended phase 2 dose (RP2D) for subcutaneous nemvaleukin alfa: ARTISTRY-2. J Clin Oncol. 2021;39(15_suppl):2552–2552.
Vaishampayan UN, Tomczak P, Muzaffar J, Winer IS, Rosen SD, Hoimes CJ, Chauhan A, Spreafico A, Lewis KD, Bruno DS, et al. Nemvaleukin alfa monotherapy and in combination with pembrolizumab in patients (pts) with advanced solid tumors: ARTISTRY-1. J Clin Oncol. 2022;40(16_suppl):2500–2500.
Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci. 2009;1174:99–106.
Sampson JH, Schmittling RJ, Archer GE, Congdon KL, Nair SK, Reap EA, Desjardins A, Friedman AH, Friedman HS, Herndon JE 2nd, et al. A pilot study of IL-2Ralpha blockade during lymphopenia depletes regulatory T-cells and correlates with enhanced immunity in patients with glioblastoma. PLoS ONE. 2012;7(2): e31046.
Jacobs JF, Punt CJ, Lesterhuis WJ, Sutmuller RP, Brouwer HM, Scharenborg NM, Klasen IS, Hilbrands LB, Figdor CG, de Vries IJ, et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patients. Clin Cancer Res. 2010;16(20):5067–78.
Solomon I, Amann M, Goubier A, Arce Vargas F, Zervas D, Qing C, Henry JY, Ghorani E, Akarca AU, Marafioti T, et al. CD25-T(reg)-depleting antibodies preserving IL-2 signaling on effector T cells enhance effector activation and antitumor immunity. Nat Cancer. 2020;1(12):1153–66.
Arce Vargas F, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH, Miranda Rota E, Dahan R, Georgiou A, Sledzinska A, et al. Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory T cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity. 2017;46(4):577–86.
Ji D, Song C, Li Y, Xia J, Wu Y, Jia J, Cui X, Yu S, Gu J. Combination of radiotherapy and suppression of Tregs enhances abscopal antitumor effect and inhibits metastasis in rectal cancer. J Immunother Cancer. 2020;8(2).
Anderson KC, Jones RM, Morimoto C, Leavitt P, Barut BA. Response patterns of purified myeloma cells to hematopoietic growth factors. Blood. 1989;73(7):1915–24.
Klein B, Wijdenes J, Zhang XG, Jourdan M, Boiron JM, Brochier J, Liautard J, Merlin M, Clement C, Morel-Fournier B, et al. Murine anti-interleukin-6 monoclonal antibody therapy for a patient with plasma cell leukemia. Blood. 1991;78(5):1198–204.
Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16(5):448–57.
Voorhees PM, Manges RF, Sonneveld P, Jagannath S, Somlo G, Krishnan A, Lentzsch S, Frank RC, Zweegman S, Wijermans PW, et al. A phase 2 multicentre study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2013;161(3):357–66.
San-Miguel J, Blade J, Shpilberg O, Grosicki S, Maloisel F, Min CK, Polo Zarzuela M, Robak T, Prasad SV, Tee Goh Y, et al. Phase 2 randomized study of bortezomib-melphalan-prednisone with or without siltuximab (anti-IL-6) in multiple myeloma. Blood. 2014;123(26):4136–42.
Rossi JF, Negrier S, James ND, Kocak I, Hawkins R, Davis H, Prabhakar U, Qin X, Mulders P, Berns B. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br J Cancer. 2010;103(8):1154–62.
Dorff TB, Goldman B, Pinski JK, Mack PC, Lara PN Jr, Van Veldhuizen PJ Jr, Quinn DI, Vogelzang NJ, Thompson IM Jr, Hussain MH. Clinical and correlative results of SWOG S0354: a phase II trial of CNTO328 (siltuximab), a monoclonal antibody against interleukin-6, in chemotherapy-pretreated patients with castration-resistant prostate cancer. Clin Cancer Res. 2010;16(11):3028–34.
Fizazi K, De Bono JS, Flechon A, Heidenreich A, Voog E, Davis NB, Qi M, Bandekar R, Vermeulen JT, Cornfeld M, et al. Randomised phase II study of siltuximab (CNTO 328), an anti-IL-6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. Eur J Cancer. 2012;48(1):85–93.
Lu ZY, Brochier J, Wijdenes J, Brailly H, Bataille R, Klein B. High amounts of circulating interleukin (IL)-6 in the form of monomeric immune complexes during anti-IL-6 therapy. Towards a new methodology for measuring overall cytokine production in human in vivo. Eur J Immunol. 1992;22(11):2819–24.
Dijkgraaf EM, Santegoets SJ, Reyners AK, Goedemans R, Wouters MC, Kenter GG, van Erkel AR, van Poelgeest MI, Nijman HW, van der Hoeven JJ, et al. A phase I trial combining carboplatin/doxorubicin with tocilizumab, an anti-IL-6R monoclonal antibody, and interferon-alpha2b in patients with recurrent epithelial ovarian cancer. Ann Oncol. 2015;26(10):2141–9.
Saraiva M, Vieira P, O'Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med. 2020;217(1).
Emmerich J, Mumm JB, Chan IH, LaFace D, Truong H, McClanahan T, Gorman DM, Oft M. IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res. 2012;72(14):3570–81.
Oft M. Immune regulation and cytotoxic T cell activation of IL-10 agonists—preclinical and clinical experience. Semin Immunol. 2019;44: 101325.
Autio K, Oft M. Pegylated interleukin-10: clinical development of an immunoregulatory cytokine for use in cancer therapeutics. Curr Oncol Rep. 2019;21(2):19.
Naing A, Papadopoulos KP, Autio KA, Ott PA, Patel MR, Wong DJ, Falchook GS, Pant S, Whiteside M, Rasco DR, et al. Safety, antitumor activity, and immune activation of pegylated recombinant human interleukin-10 (AM0010) in patients with advanced solid tumors. J Clin Oncol. 2016;34(29):3562–9.
Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthelemy P, Porta C, George S, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90.
Tilg H, Ulmer H, Kaser A, Weiss G. Role of IL-10 for induction of anemia during inflammation. J Immunol. 2002;169(4):2204–9.
Naing A, Infante JR, Papadopoulos KP, Chan IH, Shen C, Ratti NP, Rojo B, Autio KA, Wong DJ, Patel MR, et al. PEGylated IL-10 (pegilodecakin) induces systemic immune activation, CD8(+) T cell invigoration and polyclonal T cell expansion in cancer patients. Cancer Cell. 2018;34(5):775–91.
Naing A, Wong DJ, Infante JR, Korn WM, Aljumaily R, Papadopoulos KP, Autio KA, Pant S, Bauer TM, Drakaki A, et al. Pegilodecakin combined with pembrolizumab or nivolumab for patients with advanced solid tumours (IVY): a multicentre, multicohort, open-label, phase 1b trial. Lancet Oncol. 2019;20(11):1544–55.
Pal S, Hu-Lieskovan S, Agarwal N. Can pegylated IL-10 add to a backbone of PD-1 inhibition for solid tumours? Lancet Oncol. 2019;20(11):1473–4.
Spigel D, Jotte R, Nemunaitis J, Shum M, Schneider J, Goldschmidt J, Eisenstein J, Berz D, Seneviratne L, Socoteanu M, et al. Randomized phase 2 studies of checkpoint inhibitors alone or in combination with pegilodecakin in patients with metastatic NSCLC (CYPRESS 1 and CYPRESS 2). J Thorac Oncol. 2021;16(2):327–33.
Tannir NM, Papadopoulos KP, Wong DJ, Aljumaily R, Hung A, Afable M, Kim JS, Ferry D, Drakaki A, Bendell J, et al. Pegilodecakin as monotherapy or in combination with anti-PD-1 or tyrosine kinase inhibitor in heavily pretreated patients with advanced renal cell carcinoma: Final results of cohorts A, G, H and I of IVY Phase I study. Int J Cancer. 2021;149(2):403–8.
Hecht JR, Papadopoulos KP, Falchook GS, Patel MR, Infante JR, Aljumaily R, Wong DJ, Autio KA, Wainberg ZA, Bauer TM, et al. Immunologic and tumor responses of pegilodecakin with 5-FU/LV and oxaliplatin (FOLFOX) in pancreatic ductal adenocarcinoma (PDAC). Invest New Drugs. 2021;39(1):182–92.
Hecht JR, Lonardi S, Bendell J, Sim HW, Macarulla T, Lopez CD, Van Cutsem E, Munoz Martin AJ, Park JO, Greil R, et al. Randomized phase III study of FOLFOX alone or with pegilodecakin as second-line therapy in patients with metastatic pancreatic cancer that progressed after gemcitabine (SEQUOIA). J Clin Oncol. 2021;39(10):1108–18.
Grossman JG, Nywening TM, Belt BA, Panni RZ, Krasnick BA, DeNardo DG, Hawkins WG, Goedegebuure SP, Linehan DC, Fields RC. Recruitment of CCR2(+) tumor associated macrophage to sites of liver metastasis confers a poor prognosis in human colorectal cancer. Oncoimmunology. 2018;7(9): e1470729.
Sandhu SK, Papadopoulos K, Fong PC, Patnaik A, Messiou C, Olmos D, Wang G, Tromp BJ, Puchalski TA, Balkwill F, et al. A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71(4):1041–50.
Pienta KJ, Machiels JP, Schrijvers D, Alekseev B, Shkolnik M, Crabb SJ, Li S, Seetharam S, Puchalski TA, Takimoto C, et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest New Drugs. 2013;31(3):760–8.
Brana I, Calles A, LoRusso PM, Yee LK, Puchalski TA, Seetharam S, Zhong B, de Boer CJ, Tabernero J, Calvo E. Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter phase 1b study. Target Oncol. 2015;10(1):111–23.
Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, Toriola AT, Nieman RK, Worley LA, Yano M, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016;17(5):651–62.
Noel M, O’Reilly EM, Wolpin BM, Ryan DP, Bullock AJ, Britten CD, Linehan DC, Belt BA, Gamelin EC, Ganguly B, et al. Phase 1b study of a small molecule antagonist of human chemokine (C-C motif) receptor 2 (PF-04136309) in combination with nab-paclitaxel/gemcitabine in first-line treatment of metastatic pancreatic ductal adenocarcinoma. Invest New Drugs. 2020;38(3):800–11.
Wu X, Singh R, Hsu DK, Zhou Y, Yu S, Han D, Shi Z, Huynh M, Campbell JJ, Hwang ST. A small molecule CCR2 antagonist depletes tumor macrophages and synergizes with anti-PD-1 in a murine model of cutaneous T-cell lymphoma (CTCL). J Invest Dermatol. 2020;140(7):1390–400.
Wang J, Saung MT, Li K, Fu J, Fujiwara K, Niu N, Muth S, Wang J, Xu Y, Rozich N et al. CCR2/CCR5 inhibitor permits the radiation-induced effector T cell infiltration in pancreatic adenocarcinoma. J Exp Med. 2022;219(5).
Tu MM, Abdel-Hafiz HA, Jones RT, Jean A, Hoff KJ, Duex JE, Chauca-Diaz A, Costello JC, Dancik GM, Tamburini BAJ, et al. Inhibition of the CCL2 receptor, CCR2, enhances tumor response to immune checkpoint therapy. Commun Biol. 2020;3(1):720.
Bartkowiak T, Jaiswal AR, Ager CR, Chin R, Chen CH, Budhani P, Ai M, Reilley MJ, Sebastian MM, Hong DS, et al. Activation of 4–1BB on liver myeloid cells triggers hepatitis via an interleukin-27-dependent pathway. Clin Cancer Res. 2018;24(5):1138–51.
Abd Wahab NA, Lajis NH, Abas F, Othman I, Naidu R. Mechanism of anti-cancer activity of curcumin on androgen-dependent and androgen-independent prostate cancer. Nutrients. 2020;12(3).
Ferguson JJA, Abbott KA, Garg ML. Anti-inflammatory effects of oral supplementation with curcumin: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2021;79(9):1043–66.
Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15(1):195–218.
Peng Y, Ao M, Dong B, Jiang Y, Yu L, Chen Z, Hu C, Xu R. Anti-inflammatory effects of curcumin in the inflammatory diseases: status, limitations and countermeasures. Drug Des Devel Ther. 2021;15:4503–25.
Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10(20):6847–54.
Howells LM, Iwuji COO, Irving GRB, Barber S, Walter H, Sidat Z, Griffin-Teall N, Singh R, Foreman N, Patel SR, et al. Curcumin combined with FOLFOX chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa trial. J Nutr. 2019;149(7):1133–9.
Zhan Y, Chen Y, Liu R, Zhang H, Zhang Y. Potentiation of paclitaxel activity by curcumin in human breast cancer cell by modulating apoptosis and inhibiting EGFR signaling. Arch Pharm Res. 2014;37(8):1086–95.
Calaf GM, Ponce-Cusi R, Carrion F. Curcumin and paclitaxel induce cell death in breast cancer cell lines. Oncol Rep. 2018;40(4):2381–8.
Malaguarnera L. Influence of resveratrol on the immune response. Nutrients. 2019;11(5).
Delmas D, Limagne E, Ghiringhelli F, Aires V. Immune Th17 lymphocytes play a critical role in the multiple beneficial properties of resveratrol. Food Chem Toxicol. 2020;137: 111091.
Hou J, Karin M, Sun B. Targeting cancer-promoting inflammation—have anti-inflammatory therapies come of age? Nat Rev Clin Oncol. 2021;18(5):261–79.
Barnabei A, Carpano S, Chiefari A, Bianchini M, Lauretta R, Mormando M, Puliani G, Paoletti G, Appetecchia M, Torino F. Case report: ipilimumab-induced panhypophysitis: an infrequent occurrence and literature review. Front Oncol. 2020;10: 582394.
Kotwal A, Kottschade L, Ryder M. PD-L1 inhibitor-induced thyroiditis is associated with better overall survival in cancer patients. Thyroid. 2020;30(2):177–84.
Acknowledgements
Not applicable.
Funding
This work is supported by “National Natural Science Foundation of China” (82203029), “China Postdoctoral Science Foundation” (2021M702347), and “Fundamental Research Funds for the Central Universities” (20826041F4164).
Author information
Authors and Affiliations
Contributions
XW brought up the topic of the review. MW wrote the manuscript and table. MW, XH, and SC prepared the figures. YY and XW reviewed the final revision of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, M., Chen, S., He, X. et al. Targeting inflammation as cancer therapy. J Hematol Oncol 17, 13 (2024). https://doi.org/10.1186/s13045-024-01528-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-024-01528-7