Abstract
Agrobacterium-mediated transformation of indica rice has been established in only a limited number of cultivars because the regeneration of plants from transformed embryogenic calli is highly cultivar-specific. Establishment of a highly efficient plant regeneration system from shoot apex explants applicable to many cultivars of indica rice will accelerate the application of transformation technology in breeding programs and functional genomics study. We established an efficient shoot multiplication and plant regeneration system from shoot apices of indica rice using thidiazuron (TDZ) as a plant growth regulator. Shoot apices cultured on MS basal medium devoid of plant growth regulators formed solitary shoots in 90% of cultures. Addition of TDZ or benzylaminopurine to regeneration medium significantly influenced formation of multiple shoots directly from shoot apex explants without an intervening callus stage. Best shoot proliferation response (10.3 shoots per explant) was recorded when shoot apices were cultured on media supplemented with 4 mg/l TDZ. No synergistic effect on shoot proliferation was observed when indole-3-acetic acid and indole-3-butyric acid were supplemented to media containing 4 mg/l TDZ. The regeneration system was efficient in evoking multiple shoot proliferation in eight different cultivars of indica rice. Shoots were rooted in MS basal medium and plantlets were acclimatized with 100% survival rate. The shoot apex explants of all the eight cultivars of indica rice were found competent to Agrobacterium-mediated transformation while explants from IR-64 showed highest transient GUS expression. This variety-independent transformation amenable regeneration system from shoot apices may widely be applicable for genetic transformation of indica varieties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is the main staple food for more than half of the world population. Around 80% of the world rice production is based on indica varieties, which are grown under subtropical and tropical conditions as long grain rice, and thus securing a unique position in agriculture (Khush 1997). It has also become a model monocot system for genetic and functional genomic studies (Jung et al. 2008). In recent years, considerable progress has been made in the improvement of important agronomic traits of rice through biotechnological approaches (Hao et al. 2009; Skamnioti and Gurr 2009). Genetic transformation has become an important tool in targeted improvement and gene function studies in rice (Xu et al. 2012). Most plant regeneration systems adapted to Agrobactrerium-mediated genetic transformation in indica rice varieties (Rashid et al. 1996; Aldemita and Hodges 1996; Nayak et al. 1997; Zhang et al. 1997; Khanna and Raina 1999, 2002; Mohanty et al. 2002; Supertana et al. 2005; Ignacimuthu and Arockiasamy 2006) involve regeneration of plants from transformed embryogenic calli, anther calli (Jiang et al. 2004), and protoplasts. Success in transformation of indica rice using such regeneration systems depends on the factors that favor the formation of friable and high quality callus in a shorter time competent for shoot regeneration. The potential for callus induction and regeneration have been reported to be variety-dependent, limiting efficient regeneration in large number of regional indica rice varieties for genetic manipulation (Ali et al. 2004). Moreover, identification of callus amenable for transformation is cumbersome and the regeneration process is time consuming. Furthermore, inflorescences and immature embryos are available only for a limited period in a year because of photoperiodic sensitivity of rice genotypes. Quick loss of regeneration potential in calli, several stages of subculture to select the transformed calli, and problems associated with isolation and sterilization of immature embryos are the serious limitations for use of these explants in transformation (Kishore et al. 2006).
Use of shoot apex for successful genetic transformation through both Agrobacterium and biolostic methods is reported in many cereals including rice (Sticklen and Oraby 2005). A major advantage with shoot apex for genetic transformation is its developmental plasticity which allows rapid and direct regeneration of transgenic plants from transformed shoot apices ensuring cultivar integrity and circumventing the appearance of cell culture induced mutations (Hirochika et al. 1996; Bao et al. 2001). Manipulation of transgenic meristematic cells in shoot apices by treatment with growth regulators for induction of multiple shoots is most attractive for generation of stable transformants. Thidiazuron (TDZ), a phenylurea-type cytokinin, has been reported to facilitate efficient multiplication of apical meristem cells and their reprogramming to appropriate developmental stage for shoot differentiation (Gairi and Rashid 2004; Goldman et al. 2003; Srivatanakul et al. 2000). However, to our knowledge, plant regeneration from shoot apex is available only for two varieties, White Ponni (WP) and Pusa Basmati 1 (PB1) of Indian origin (Arockiasamy and Ignacimuthu 2007) of indica rice.
We report establishment of an efficient plant regeneration system from shoot apices of indica rice applicable to eight cultivars using TDZ as a growth regulator and demonstrate their amenability to Agrobacterium-mediated transformation.
Materials and methods
Plant material and explant preparation
Seeds of eight indica rice cultivars IR-64, Anjali, Vandana, Chandan, Mahasuri, Nilagiri, Ranjit, and Luit having superior attributes including good grain quality and varying levels of disease resistance were obtained from Regional Rainfed Low Land Rice Research Station, Gerua, Assam, India. The cultivar IR-64 was used as a model for indica rice shoot apex regeneration and transformation experiments. Mature seeds were dehusked, surface sterilized with 70% ethanol for 30 s, rinsed with 1% bavistin, and 0.2% HgCl2 (w/v) for 5 min each. The seeds were then rinsed five times with sterile double distilled water and cultured on MS basal medium (Murashige and Skoog 1962) with 3% sucrose supplemented with or without 1 mg/l TDZ. Shoot apices (4–5 mm) were carefully excised from the four-day-old germinated seedlings and used for all experiments.
Multiple shoot induction and plant regeneration
In order to study the effect of most potential cytokinins, benzylaminopurine (BAP) and TDZ on multiple shoot induction, shoot apices of indica rice cultivar IR-64 were cultured on MS medium supplemented with different concentrations (1, 2, 3, 4, and 5 mg/l) of TDZ or BAP. The regenerating explants were subcultured twice onto fresh media at an interval of 15 days each. The efficiency of multiple shoot induction and plant regeneration were evaluated by scoring the mean number of shoots induced from responding explant, and measuring the mean shoot length. Regeneration frequency was calculated based on the number of shoot apices responding to regeneration by total number of shoot apices cultured.
The synergistic effect of auxin with 4 mg/l TDZ on shoot proliferation was examined by supplementing different concentrations (0.025, 0.1 and 0.25 mg/l) of indole-3-acetic acid (IAA) or indole-3-butyric acid (IBA) to MS medium containing 4 mg/l TDZ. The regenerating explants were subcultured following the methods described earlier. Percentage of regeneration, mean number of shoots, and average shoot length were recorded. All media were adjusted to pH 5.8 with 0.1 N NaOH or 0.1 N HCl prior to addition of 0.7% agar–agar (Hi-media, Mumbai), and autoclaving at 20 psi and 121 °C for 20 min.
Effect of genotype
The effect of genotype on multiple shoot induction and plant regeneration from shoot apices was studied by culturing shoot apex explants of indica rice cultivars, Anjali, Vandana, Chandan, Mahasuri, Nilagiri, Ranjit, and Luit in MS medium supplemented with 4 mg/l TDZ. The number of regeneration responsive explants, mean number of shoots, and average shoot length were recorded. The relative frequency of plant regeneration and efficiency of multiple shoot induction were compared among all cultivars.
Rooting and transplantation
The shoots (5–7 cm) were separated from multiple shoot clumps and transferred to MS medium devoid of growth regulator for 2 weeks for root formation. Subsequently plantlets with well-developed root system were washed in tap water and acclimatized in polybags containing soil and vermicompost (1:1), covered with transparent polybags at 27 °C and 16 h photoperiod for 14 days. Finally, the hardened plants were transferred to pots containing soil and established in a green house.
Culture conditions
All cultures were maintained under the same experimental conditions at 25 ± 2 °C under white fluorescent light at irradiance of 37.5 μmol/m2/s with 16-h photoperiod. Visual observations of the cultures were taken every week, and the percentage of cultures showing regeneration, number of shoots per explant, and shoot length were recorded after 30 days.
Transformation procedure and GUS assay
Agrobacterium tumefactions strain EHA105 harboring a binary vector pCAMBIA2301pyl13 which contains a ABA receptor gene (pyl13), β-glucuronidase (gus) interrupted with an intron in the coding region and neomycin phosphotransferase (nptII) genes, all driven by CaMV35S promoter (Fig. 1), was used for transformation studies. The bacteria was grown on YEP (10 g/l yeast extract, 10 g/l peptone, 50 g/l NaCl, 15 g/l agar–agar and pH 7.0–7.2) solid medium containing 50 mg/l kanamycin and 10 mg/l rifampicin at 28 °C. A single bacterial colony was inoculated into 2 ml of liquid AB medium containing 5 mg/l rifampicin and 25 mg/l kanamycin and grown overnight on a rotary shaker at 200 rpm at 28 °C. Bacteria were pelleted at 5,000 rpm for 5 min and resuspended in liquid MS medium containing 100 μM acetosyringone at a density of OD600 = 1. Shoots apices excised from 4-day-old seedlings were gently stabbed four to five times using a sterile needle (24 G) at apex region before being immersed in bacterial suspension for 30 min with shaking at 80 rpm at 25 °C. Inoculated explants were blotted on sterile filter paper and co-cultivated on solid MS medium containing 1 mg/l TDZ and 100 μM acetosyringone for 3 days at 25 °C under dark condition. After co-cultivation, the explants were washed three to four times with sterile double distilled water by vigorous stirring, blotted dry on sterile filter paper and 20 explants were tested for transient gus expression by histochemical assay using 5-bromo-4-chloro-3-indolyl glucuronide (X-Gluc) as a substrate (Jefferson et al. 1987). The explants were then visually scored for transient gus activity.
Regeneration of transgenic plants
Following co-cultivation, the explants were washed three to four times with sterile double distilled water by vigorous stirring, blotted dry on sterile filter paper and were cultured on selection medium (MS medium containing 4 mg/l TDZ, 45 mg/l kanamycin, and 500 mg/l cefotaxime) for induction and selective regeneration of transformants. The cultures were transferred to fresh selection medium at an interval of 15 days. Same levels of antibiotics were maintained during subsequent subcultures. After 4 weeks of culture on selection, the proliferating kanamycin resistant shoots (>5 cm) were transferred to rooting medium (MS medium containing 500 mg/l cefotaxime). The putative transformed plants were established in soil:compost (1:1) and grown to maturity in transgenic greenhouse containment.
Molecular analysis of transformed plants by PCR
Genomic DNA was isolated from the leaves of transformed and untransformed (control) plants by modified CTAB method (Solleti et al. 2008). PCR analysis was carried out for gus gene to amplify 570 bp internal fragment using 18 mers (gus Fw: CTGTGGGCATTCAGTCTG; Rv: ACGCTGACATCACCATTG) primers. To rule out the possibility of Agrobacterium contamination, PCR was performed to check for amplification of 760 bp fragment of the bacterial virulence gene (virG) located outside the T-DNA using 21 mers (Fw: ATGGCTGGCCAGGATCCTAGA; Rv: TCAGGCCGCCATCACACC) primers. The amplification reaction was carried out under the following conditions: 94 °C for 5 min (1 cycle), 94 °C for 1 min (denaturation), 58 °C for 1 min (annealing), 72 °C for 1 min (extension) for 35 cycles followed by the final extension at 72 °C for 7 min (1 cycle). PCR was performed using ~100 ng of purified genomic DNA, 50 ng plasmid DNA (pCAMBIA2301Atpyl13 as positive control), and Taq DNA polymerase (Genei, Bangalore, India) according to manufacturer’s instruction. The negative and untransformed plant controls were set up with no DNA and untransformed rice plant DNA, respectively. The amplified products were resolved by electrophoresis on 1% agarose gel and visualized by ethidium bromide staining (Sambrook et al. 1989).
Statistical analysis
Data were subjected to analysis of variance (ANOVA) and mean separation by Duncan’s multiple range test (DMRT) using single-factor completely randomized block design to study the effect of different treatments on shoot proliferation and frequencies of transient expression. All experiments were performed at least three times with a minimum of 30–40 explants per treatment.
Result and discussion
A prolific regeneration system based on multiple shoot induction from isolated shoot apices permits quicker plant regeneration owing to their extensive proliferative ability and presents amenability to germline transformation. Furthermore, use of shoot apices from in vitro germinated seedlings facilitates availability of explants round the year. We developed an efficient and reproducible plant regeneration system from isolated shoot apices of indica rice by use of a synthetic urea-cytokinin, thidiazuron (TDZ). The regeneration system was found applicable to eight indica rice cultivars investigated in this study and amenable to Agrobacterium-mediated transformation.
Effect of TDZ on shoot multiplication
The morphogenic potential of shoot apices of the eight indica rice cultivars was analyzed on MS medium augmented with various concentrations of TDZ and BAP. The efficiency of shoot induction from shoot apices on growth regulator free MS medium was considered as control. Shoot apices (Fig. 2a) cultured on MS medium formed a solitary shoot without callus formation at the base in 90% of responding explants (Table 1) within 2 weeks of culture. However, inclusion of various concentrations (1–5 mg/l) of TDZ and BAP in basal medium elicited a prime and distinct role in shoot buds differentiation within 2 weeks of culture. One of the main functions of cytokinin is known to confer morphogenic competence for initiation of shoot proliferation. However, the type and concentration of cytokinin influenced the regeneration frequency, average number of shoots produced per explant, and mean length of the shoots (Table 1). Although, addition of both TDZ and BAP into medium resulted in induction of multiple shoots, but the effect of TDZ was more pronounced than BAP at equimolar concentrations (Table 1). Of the different concentrations of TDZ and BAP tested, 4 mg/l TDZ was found to be most effective in inducing multiple shoot induction from the shoot apices by producing maximum of 10.3 shoots per explant in 89% of cultures (Table 1; Fig. 2b, c). A linear correlation between increases in TDZ concentration to an optimal dose (4 mg/l) and regeneration frequency as well as mean shoot number was recorded (Table 1). Thidiazuron, a phenylurea-based compound has shown to possess potent activity as a cytokinin in inducing efficient multiple shoot formation in several plant species (Huetteman and Preece 1993, Srivatanakul et al. 2000; Mithila et al.2001; Goldman et al. 2003; Gairi and Rashid 2004; Faisal et al. 2005; D’Onofrio and Morini 2005; Radhika et al. 2006; Siddique and Anis 2007). Higher concentration of TDZ (5 mg/l) resulted in a decrease in regeneration frequency, mean shoot number, and shoot length indicating suppression of shoot proliferation (Table 1). Furthermore, stunted shoot growth was recorded at 5 mg/l TDZ as the shoot buds appeared to be developmentally suppressed. Unlike BAP, an adenine and purine based cytokinin, TDZ is resistant to cytokinin degrading enzymes, and therefore at high dose remains persistent in the tissues inducing excessive suppression of shoot buds, consequently leading to reduced proliferation rates (Huetteman and Preece 1993). In comparison to TDZ, shoots induced on BAP containing medium were longer and slender.
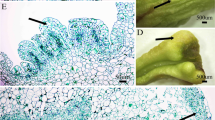
Multiple shoot induction and plant regeneration from apical meristems of Oryza sativa spp. indica cv. IR-64.a Shoot apex explant. b Induction of shoots from shoot apex in MS medium supplemented with 4 mg/l TDZ after 2 weeks in culture. c Shoot proliferation from shoot apex in MS medium supplemented with 4 mg/l TDZ after 4 weeks in culture. d Rooted shoot in MS medium. e Regenerated plants growing in greenhouse. Bar represents 2 mm (a); 1 cm (b); 2 cm (c); 2 cm (d)
Effective multiple shoot induction and shoot proliferation is often the manifestation of interactions among physiological state of the explants and a combination of plant growth regulators (Mallikarjuna and Rajendrudu 2007). In our study, we evaluated the effect of different concentrations of IAA and IBA in combination with optimal dose of TDZ (4 mg/l) on enhancement of multiple shoot induction from shoot apices. However, no incremental increase in regeneration frequency as well as mean shoot number was observed indicating the absence of synergistic effects of IAA and IBA with TDZ on cultured shoot apices of indica rice cultivars in our study (Table 2). Our results are contrary to the report on effective multiple shoot formation through the use of a combination of cytokinins, TDZ and BAP with auxins in immature embryo of another cereal, sorghum (Pola et al. 2007; Kishore et al. 2006). Hormonal metabolisms are known to be operated in an integrated manner (Gaspar et al. 2000) and that several, potential, mutual functional interacting points exist between different hormones (Coenen and Lomax 1997). Both synergic as well as antagonistic effects among plant growth regulators have been reported in effecting shoot proliferation in in vitro cultures.
After 4 weeks on multiple shoot induction medium, individual shoots were separated from each other and transferred to MS medium where they rooted within 2 weeks of culture (Fig. 2d). Over 100% of rooted plantlets survived when established in soil (Fig. 2e). The plants resumed growth in greenhouse reaching maturity and represented no phenotypic variation or sterility, irrespective of cultivars tested.
Genotype influences on tissue culture response
It is known that the potential for multiple shoot induction and plant regeneration in rice depends on a number of factors of which genotype of the donor plant and interaction between genotype and shoot proliferation medium are most important. Therefore, in the present study eight indica rice cultivars were screened to evaluate the genotype influence on shoot proliferation and plant regeneration. The study showed the sensitivities of different indica rice cultivars to the optimal shoot proliferation medium. The shoot apices of cultivars IR-64, Mahasuri, and Nilagiri showed significantly greater shoot proliferation response than the other five cultivars on MS medium containing 4 mg/l TDZ (Table 3). However, no significant variation in regeneration frequency as well as mean shoot number was detected among the five cultivars, Ranjit, Vandana, Luit, Anjali, and Chandana (Table 3). Although, shoot apices indica rice cultivars IR-64, Mahasuri, and Nilagiri showed significantly greater shoot proliferation response, other five cultivars also responded to multiple shoot proliferation. Regeneration of different indica rice cultivars on the medium showed the technique is genotype-independent. Although a large number of protocols are available for embryogenic calli-mediated plant regeneration in indica rice, they are mostly genotype-dependent and furthermore, no universal medium adaptable to a number of indica rice genotypes has been developed. Furthermore, multiple shoots developed directly from the meristem without an intervening callus stage in the present protocol is expected to maintain genotype fidelity that could be lost with shoots arising from callus.
Shoot apex transformation and gus expression
It is generally known that genotype remains the major limiting factor restricting successful transformation in indica rice (Ge et al. 2006). The shoot meristem based plant regeneration system is mostly genotype-independent and provides a potential target for T-DNA delivery by Agrobacterium and direct gene transfer method (Sticklen and Oraby 2005). Another major advantage using the shoot apex explants is that rapid regeneration of shoots can be achieved from transformed shoot apices unlike shoot regeneration from transformed calli and protoplasts which involve several rounds of subculture involving risk of generating mutations (Arockiasamy and Ignacimuthu 2007). To determine the competency of the shoot apex explants of eight indica rice cultivars to Agrobacterium-mediated genetic transformation, experiments were carried out to inoculate explants with A. tumefaciens. 3 days after coculture, explants were incubated with substrate for β-glucuronidase enzyme and assayed for gus expression. Strong transient gus expression was detected in the region of the shoot apices from where the shoots developed, i.e., apex region (Fig. 3a). The endogenous GUS activity (color) was not detected in non-transformed (control) explants (Fig. 3b). GUS activity at the regenerating sites indicated the amenability of explants to Agrobacterium-mediated transformation. Although, shoot apices of all the indica rice cultivars showed strong gus expression at the regenerating site, however, transient gus expression efficiency differed from cultivar to cultivar (Fig. 4). Highest transient gus expression efficiency was recorded in cultivar IR-64 in which 100% of shoot apex explants showed gus expression (Fig. 4). Our results demonstrated that shoot apex explants of eight indica rice cultivars are amenable to Agrobacterium-mediated transformation and combined with their shoot proliferation ability could lead to regeneration of stable transgenic plants in a genotype-independent fashion. Transformation of the shoot apex is advantageous as a region of the shoot apex differentiates into the germline, which gives rise to the seed enabling transfer of the trait to the progeny. Shoot apices in conjunction with Agrobacterium-mediated transformation have been used to develop transgenic plants in two indica rice cultivars of Indian origin, WP and PB1 (Arockiasamy and Ignacimuthu 2007). Shoot apex-based regeneration systems have also been employed successfully to recover stably transformed maize, wheat, oat, barley, sorghum, and millet (Sticklen and Oraby 2005) and finger millet (Antony Ceasar and Ignacimuthu 2011).
Molecular analysis of transgenic plants
The PCR analysis detected the presence of the expected 570 bp amplified product corresponding to gus (Fig. 5) in transformed shoots. No amplification was detected in the control untransformed shoots. Furthermore, the absence of signals in PCR by using virG primer in transformed shoots ruled out artefacts caused by A. tumefaciens contamination (Fig. 5).
PCR screening for virG and gus genes on transformed plants developed from shoot apex of Oryza sativa spp. indica cv. IR-64. Lane M λDNA/EcoRI + HindIII marker, lane P pCAMBIA2301Atpyl13 (positive control), lane C DNA from untransformed plant (negative control), lane At EHA105pCAMBIA2301Atpyl13 (positive control for virG gene), lanes 1–6 DNA from independently transformed plants
Conclusion
In conclusion, we have established rapid multiple shoot induction and efficient plant regeneration method from shoot apices of eight indica rice cultivars in a genotype-independent manner. The system was found amenable to Agrobacterium-mediated transformation in all the eight indica rice cultivars included in the study as evident from gus expression in transformed shoot apices and presence of gus gene in transformed plants by PCR analysis. This simple, rapid, and efficient plant regeneration system amenable to Agrobacterium-mediated transformation of eight indica rice cultivars may accelerate varietal development program through transgenic approach by incorporation of key candidate genes and for study of gene function.
References
Aldemita RR, Hodges TK (1996) Agrobacterium tumefaciens mediated transformation of japonica and indica rice varieties. Planta 199:612–617
Ali CA, Rashid M, Ashraf M, Qamar ZU (2004) Genetic divergence in rice collections. Pak J Bot 36(3):557–565
Antony Ceasar S, Ignacimuthu S (2011) Agrobacterium-mediated transformation of finger millet (Eleusine coracana (L.) Gaertn.) using shoot apex explants. Plant Cell Rep 30:1759–1770
Arockiasamy I, Ignacimuthu S (2007) Regeneration of transgenic plants from two indica rice (Oryza sativa) cultivars using shoot apex explants. Plant Cell Rep 26:1745–1753
Bao PH, Granata S, Castiglione S, Wang G, Giordani C, Cuzzoni E, Damiani G, Bandi C, Datta SK, Datta K, Potrykus I, Callegarin A, Sala F (2001) Genomic changes in transgenic rice (Oryza sativa L.) plants produced by infecting calli with Agrobacterium tumefaciens. Plant Cell Rep 20:325–330
Coenen C, Lomax TL (1997) Auxin–cytokinin interactions in higher plants: old problems and new tools. Trends Plant Sci 2:351–356
D’Onofrio C, Morini S (2005) Development of adventitious shoots from in vitro grown Cydonia oblonga leaves as influenced by different cytokinins and treatment duration. Biol Plant 49:17–21
Faisal M, Ahmad N, Anis M (2005) Shoot multiplication in Rauwolfia tetraphylla L. using thidiazuron. Plant Cell Tissue Organ Cult 80:187–190
Gairi A, Rashid A (2004) TDZ-induced somatic embryogenesis in non-responsive caryopses of rice using a short treatment with 2,4-D. Plant Cell Tissue Organ Cult 76:29–33
Gaspar T, Bisbis B, Kevers C, Franck T, Dommes J, Crevecouer M, Penel C, Greppin H (2000) Integrating photohormone metabolism and action with primary biochemical pathways.II. Interrelationships between disturbed nitrogen and carbon metabolism and changes in hormonal concentrations and sensitivities in tissue cultures. In: Greppin H, Penel C, Broughton W, Strasser R (eds) Integrated plant systems. University of Geneve, Geneva, pp 139–225
Ge XJ, Chu ZH, Lin YJ, Wang SP (2006) A tissue culture system for different germplasms of indica rice. Plant Cell Rep 25:392–402
Goldman JJ, Hanna WW, Fleming G, Ozias-Akins P (2003) Fertile transgenic pearl millet [Pennisetum glaucum (L.) R.Br.] plants recovered through micro-projectile bombardment and phosphinothricin selection of apical meristem inflorescence and immature embryo-derived embryogenic tissues. Plant Cell Rep 21:999–1009
Hao ZN, Wang J, Wang LP, Tao RX (2009) Influences of the disease resistance conferred by the individual transgenes, Pi-d2, Pi-d3 and Xa21, on the transgenic rice plants in yield and grain quality. African J Biotech 8:4845–4848
Hirochika H, Sugimoto K, Otsuki Y, Tsugawa H, Kanda M (1996) Retrotransposans of rice involved in mutations induced by tissue culture. Proc Natl Acad Sci USA 93:7783–7788
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult 33:105–119
Ignacimuthu S, Arockiasamy S (2006) Agrobacterium-mediated transformation of an elite indica rice for insect resistance. Curr Sci 90:829–835
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jiang S, Chen CY, Cheng ZK, Cai R, Zhai WX, Zhu LH (2004) Analysis of the transgenic rice plants derived from transformed anther calli. Acta Genetica Sincia 31:1381–1387
Jung KH, An G, Ronald PC (2008) Towards a better bowl of rice: assigning function to tens of thousands of rice genes. Nat Rev Genet 9:91–101
Khanna HK, Raina SK (1999) Agrobacterium-mediated transformation of indica rice cultivars using binary and superbinary vectors. Aust J Plant Physiol 26:311–324
Khanna HK, Raina SK (2002) Elite indica transgenic rice plants expressing modified Cry1Ac endotoxin of Bacillus thuringiensis show enhanced resistance to yellow stem borer (Scirpophaga incertulas). Transgenic Res 11:411–423
Khush GS (1997) Origin, dispersal, cultivation and variation of rice. Plant Mol Biol 35:25–34
Kishore SN, Visarada KBRS, Aravinda Lakshmi Y, Pashupatinath E, Rao SV, Seetharama N (2006) In vitro culture methods in Sorghum with shoot tips the explants material. Plant Cell Rep 25:174–182
Mallikarjuna K, Rajendrudu G (2007) High frequency in vitro propagation of Holarrhena anti dysenterica from nodal buds of mature tree. Biol Plant 51:525–529
Mithila J, Murch SJ, Krishnaraj S, Saxena PK (2001) Recent advances in Pelargonium in in vitro regeneration systems. Plant Cell Tissue Organ Cult. 67:1–9
Mohanty A, Kathuria H, Ferjani A, Sakamoto A, Mohanty P, Murata N, Tyagi AK (2002) Transgenics of an elite indica rice variety Pusa Basmati1 harbouring the codA gene are highly tolerant to salt stress. Theor Appl Genet 106:51–57
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nayak P, Basu D, Das S, Basu A, Ghosh D, Ramakrishnan NA, Ghosh M, Sen SK (1997) Transgenic elite indica rice plants expressing CryIAc-endotoxin of Bacillus thuringiensis are resistant against yellow stem borer (Scirpophaga incertulas). Proc Natl Acad Sci USA 94:2111–2116
Pola S, Saradamani N, Ramana T (2007) Enhanced shoot regeneration in tissue culture studies of Sorghum bicolor. J Agric Technol 3(2):275–286
Radhika K, Sujatha M, Nageswar Rao T (2006) Thidiazuron stimulates adventitious shoot regeneration in different safflower explants. Biol Plant 50:174–179
Rashid H, Yokoi S, Toriyama K, Hinata K (1996) Transgenic plant production mediated by Agrobacterium in indica rice. Plant Cell Rep 15:727–730
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. vol 1, 2nd edn. Cold Spring Harbor Press, Cold Spring Harbor, New York, pp 33–39
Siddique I, Anis M (2007) Rapid micropropagation of Ocimum basilicum using shoot tip explants pre-cultured in thidiazuron supplemented liquid medium. Biol Plant 51(4):787–790
Skamnioti P, Gurr SJ (2009) Against the grain: safeguarding rice from rice blast disease. Trends Biotechnol 27(3):141–150
Solleti SK, Bakshi S, Sahoo L (2008) Additional virulence genes in conjunction with efficient selection scheme, and compatible culture regime enhance recovery of stable transgenic plants in cowpea via Agrobacterium tumefaciens-mediated transformation. J Biotech 135:97–104
Srivatanakul M, Park S, Sanders J, Salas M, Smith R (2000) Multiple shoot regeneration of kenaf (Hibiscus cannabinus L.) from a shoot apex culture system. Plant Cell Rep 19:1165–1170
Sticklen MB, Oraby HF (2005) Shoot apical meristem a sustainable explant for genetic transformation of cereal crops. In Vitro Cell Dev Biol Plant 41:187–200
Supertana P, Shimizu T, Shioiri H, Nogawa M, Nozue M, Kojima M (2005) Development of simple and efficient in planta transformation method for rice (Oryza sativa L.) using Agrobacterium tumefaciens. J Biosci Bioeng 100:391–397
Xu X, Liu X, Ge S, Jensen JD, Hu F, Li X, Dong Y, Gutenkunst RN, Fang L, Huang L, Li J, He W, Zhang G, Zheng X, Zhang F, Li Y, Yu C, Kristiansen K, Zhang X, Wang J, Wright M, McCouch S, Nielsen R, Wang J, Wang W (2012) Resequencing 50 accessions of cultivated and wild rice yields markers for identifying agronomically important genes. Nat Biotechnol 30:105–111
Zhang J, Xu RJ, Elliott MC, Chen DF (1997) Agrobacterium mediated transformation of elite indica and japonica rice cultivars. Mol Biotech 8:223–231
Acknowledgments
We thank Prof. K. Veluthambi, MKU, Madurai, India for Agrobacterium tumefaciens strain and Center for Application of Molecular Biology to International Agriculture (CAMBIA), Australia for pCAMBIA2301. The research was supported in part by grants from Department of Biotechnology (DBT), Government of India. MD is grateful to DBT for Junior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Dey, M., Bakshi, S., Galiba, G. et al. Development of a genotype independent and transformation amenable regeneration system from shoot apex in rice (Oryza sativa spp. indica) using TDZ. 3 Biotech 2, 233–240 (2012). https://doi.org/10.1007/s13205-012-0051-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13205-012-0051-y