Abstract
Palms (Arecaceae) contribute remarkable economic and environmental values to human life. However, many elite and commercial palm species are becoming critically endangered and demand immediate action to preserve their genetic resources. Cryopreservation has established itself as the definitive in vitro conservation method for recalcitrant-seeded species, such as those within the Arecaceae. Progress in this area has moved steadily forward over the last three decades with the development of various techniques for different explants and palm genotypes and for molecular testing methods to ensure genetic fidelity is maintained in the regenerants. There remains a key challenge to properly identify the components that will enable the long-term conservation of palms using cryopreservation. This review methodically analyzes the state-of-the-art cryopreservation techniques developed for palms and places them within a practical framework. This framework encompasses four underlying components, namely the tissue culture approaches required, the recalcitrant nature of the palm seed, the cryobiology and cryogenic techniques required, and fidelity assessment after cryopreservation. Through a critical analysis of this framework, further optimization of palm cryopreservation protocols and more fundamental studies on the physiological and molecular changes in cryopreserved palm tissues are recommended. The present review helps to showcase a multi-decade global attempt to preserve these mostly recalcitrant species through ex situ collections. From a conservationist’s perspective, this review hopes to stimulate awareness for further concerted efforts in the conservation of rare and endangered plant families. Meanwhile, from a managerial perspective, this work serves to inform decision-makers of the global research effort underway to improve key components of the cryopreservation program for palm species and to encourage funding bodies to appropriately allocate resources to these much-needed research areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The palms (Arecaceae) belong to a plant family that is predominantly found in tropical and sub-tropical regions of the world (Hunter and Bystriakova 2004; Simpson 2010). For many countries, palms provide food and industrial materials, as well as valuable sources of bioenergy. Several domesticated palm species are regarded as the key drivers for multiple economies. As an example, oil palm (Elaeis guineensis Jacq.) is the primary source of vegetable oil, the most consumed palm oil across many regions, especially in Southeast Asia, South America, and sub-Saharan Africa (Ritchie and Roser 2021). Also, coconut (Cocos nucifera L.) is cultivated on 12 million ha and is contributing to the livelihoods of millions of smallholder farmers around the globe (Nguyen et al. 2015). However, both oil palm and coconut production are facing significant threats from palm senility, pests, diseases, natural disasters, climate change, and genetic erosion (Nguyen et al. 2015; Abul-Soad et al. 2017). Therefore, a conscious effort needs to be made to conserve and enhance genetic diversity of the major economically important palms. Any conservation plan should not only preserve palm varieties with high economic values, but also maintain those with undiscovered values. Fortunately, palms so far are the targets of international conservation efforts, which also require cross-sectoral collaborations (Coates 2016; Griffith et al. 2021). This ambitious goal can be achieved through in situ farmers’ fields and through ex situ conservation collections.
In situ approaches often consist of storing germplasm in its natural habitat through on-farm conservation or using some other landscaping options. However, a major problem with many forms of in situ conservation is that they require significant financial support and labor for maintenance (Virchow 2005). Construction of more seed gardens is impossible due to the vast requirement for lands and the availability of so many varieties. Therefore, an alternative approach is to transport plant materials to designated facilities, such as field genebanks and in vitro laboratories. This method is called “ex situ conservation.” However, one major disadvantage of ex situ field genebanks, similar to in situ conservation, is that they are under constant threat of abiotic and biotic stresses and the appearance of devastating pests and diseases (Panis and Nagel 2020; Engels and Ebert 2021). In addition, the recalcitrant nature of palm seeds prevents their dehydration and storage in ex situ genebanks. Alternatively, in vitro collections are believed to provide a cost-effective, safe, and sustainable long-term ex situ conservation approach for plant genetic resources, including the recalcitrant palm species (Bourdeix et al. 2020). Furthermore, with the presently developed in vitro culture protocols, maintenance of selected in vitro-conserved lines can be undertaken and, when needed for breeding and research purposes, can be rapidly produced and sustained over a long period of time. From an economic point of view, an in vitro collection is regarded to be a more logistically efficient process for the reintroduction of superior plant types back into the environment. At present, there exist two main in vitro approaches to conservation, that is, short- to medium-term conservation (Table 1) and cryopreservation. The former is a process of utilizing a limited source of nutrients or using growth suppressive conditions (for example, using low incubation temperatures, or a medium that induces an osmotic stress) with the aim of keeping the cultured tissues viable for a longer period of time (Panis and Nagel 2020). However, this method still involves repetitive subculturing due to nutrient depletion and is prone to contamination risk and genetic shift. On the other hand, cryopreservation method uses extremely low temperatures (− 196°C) to bring all metabolic activities of living cells to a halt, thereby enabling long-term maintenance (Panis et al. 2001; Engelmann 2014). This process requires an initial reduction of intra-cellular water molecules by using either a physical or a chemical dehydration process and aims to turn the internal cell solutes into a vitreous state where ice cannot be formed and cause irreversible damage. Compared to the short- to medium-term method, cryopreservation provides a long-term option for plant genetic diversity conservation. In addition, cryopreservation can also serve as a powerful tool to single out cold-tolerant varieties from a mixed collection (cryo-selection) and to eradicate viruses from culture stocks (cryo-therapy) (Watanabe et al. 1985; Brison et al. 1997; Helliot et al. 2002; Engelmann 2004; Wang and Valkonen 2009; Wang et al. 2018).
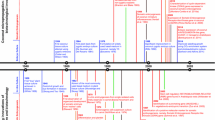
Over the past three decades, a range of cryopreservation techniques were developed and modified for various palm species. Most major palm cryopreservation studies focus on rapid cooling following either physical dehydration (N’Nan et al. 2008; Sisunandar et al. 2010a, 2010b) or chemical dehydration (Fki et al. 2013; Lédo et al. 2018; Wilms et al. 2019) techniques. For palm species, cryopreservation must be undertaken with careful consideration of the state-of-the-art techniques available. It should be mindful of the availability of explant types, species-specific biology, culture media, preculture conditions, cryoprotectants, and cooling and rewarming cycles and regrowth procedures. By using a proposed framework for palm cryopreservation, this paper aims to provide a more systematic approach to conducting cryopreservation techniques for palms and through which cryopreservation of palm tissues can be achieved efficiently and economically soon (Fig. 1).
In this review, the aim is to discuss four underlying elements to a framework for the cryopreservation of palm species, including (i) their response to tissue culture, (ii) the recalcitrant nature of their seed tissues, (iii) their cryobiology and responses to cryogenic techniques, and (iv) the fidelity assessment of regenerants. Together, these four elements form a basis to a practical framework for the long-term conservation of palm species using the core technology of cryopreservation.
Presently developed tissue culture pathways for the major palm species - Tissue culture for material preparation and post-cryostorage recovery
The first cornerstone of the framework is built upon a sound understanding of plant cell totipotency, which is the capability of an individual cell or cell clusters to develop into a whole plant (Su et al. 2021). To date, biotechnological interventions are used to help overcome the recalcitrant nature (low responsiveness to in vitro culture) of various palm species. These interventions, therefore, have enabled the regeneration of some economically important palm species via somatic embryogenesis (SE) or direct organogenesis (Al-Khayri and Naik 2017; Lédo et al. 2019; Yarra et al. 2019). Given the diminishing nature of sample quantities after going through different cryopreservation phases (Martinez-Montero and Harding 2015), it is necessary to multiply a surplus number of starting materials using the quickest method available, which is partially achieved by tissue culture. The process of SE involves a number of sequential steps, including the induction of callus, the formation, maturation and germination of somatic embryos, and the establishment of complete plantlets (Al-Khayri and Naik 2017). Meanwhile, direct organogenesis often starts with axillary shoot formation followed by shoot bud multiplication and plantlet establishment (Sidky 2017).
In oil palm, SE is reported to be a successful pathway for the multiple production of uniform plantlets, despite reported incidences of somaclonal variation (Weckx et al. 2019; Yarra et al. 2019). Also, SE is considered to be a fast and efficient pathway towards the large-scale propagation of date palm and coconut. Various explants (including zygotic embryos (Gomes et al. 2015; Monteiro et al. 2018), shoot tips (Al-Khayri 2010), immature leaves (Othmani et al. 2009; Gomes et al. 2017), adventitious bud and proximal leaf segments (Mazri et al. 2017), immature inflorescences (Teixeira et al. 1994; Jayanthi et al. 2015, anthers (Perera et al. 2008), plumules (Pérez-Núñez et al. 2006), and ovaries (Bandupriya et al. 2017)) were used as explants for the induction of callus in these palm species. On an industrial scale, it is speculated that advances in SE techniques may achieve a 10,000-fold multiplication (Pérez-Núñez et al. 2006; Sáenz et al. 2018).
On the other side, direct organogenesis was utilized in several regeneration protocols, especially for date palm (Bekheet 2013; Mazri and Meziani 2013; Meziani et al. 2015). In coconut, propagation of plant materials was mostly achieved through embryo culture (Assy-Bah et al. 1987; Rillo 1999; Sisunandar et al. 2015) and SE (Pérez-Núñez et al. 2006; Samosir and Adkins 2014) with a view towards conservation and germplasm exchange of coconut varieties (Nguyen et al. 2016; Oropeza et al. 2018). Very recently, coconut shoot tips have proven to be potential explants for both multiplication and cryopreservation, which completes the available repertoire of methodologies for regeneration of the most important palm species (Wilms et al. 2019, 2021). This new achievement has paved the way for in vitro establishment of regenerants in the post-cryopreservation stage.
Explant choice
Since recalcitrant species cannot be conserved by cryopreservation using whole seeds, different explants must be used from which whole plants can be recovered. Each type of explant has different requirements for cryopreservation (Supplementary Table 1). A broad range of explant types can be used for cryopreservation in major palm species, including pollen, zygotic embryos and isolated plumules, somatic embryos, friable embryogenic tissues (FET), embryogenic cell suspensions (ECS), nodular culture, polyembryonic masses (PEMs), and meristems (Table 2). Coconut cryopreservation was attempted on zygotic embryos, pollen, plumules, embryogenic callus, and recently vegetative shoot tips. However, cryopreservation of the zygotic embryo and pollen are considered the only complete protocols shown to be possible to date (Bourdeix et al. 2020). In date palm, attempts have been made on the palm’s pollen, meristems, shoot tips, FET, somatic embryos, and ECS. For oil palm, advances have been made in using a wide array of explants, including intact seeds, whole kernels containing embryos, pollen, zygotic embryos, somatic embryos, and friable embryogenic callus.
When embryos are used, their maturity stage is a significant factor that affects the success of cryopreservation. Previous studies on orthodox species showed that mature embryo is the best stage for cryopreservation (Kermode and Finch-Savage 2002; Wen and Song 2007; Vineesh et al. 2015). However, the embryo stage for cryopreservation varies depending on the species in recalcitrant plants. In coffee (Coffea arabica L.) and jackfruit (Artocarpus heterophyllus L.), zygotic embryos harvested at an intermediate stage of maturity were more successfully used in cryopreservation as compared to immature or fully mature embryos while in tea (Camellia sinensis L.) and coconut, the mature embryos gave the highest survival rate after cryopreservation (Abdelnour-Esquivel et al. 1992; Chandel et al. 1995; Kim et al. 2002; Sisunandar et al. 2014.
For small explants, such as plumular tissues, embryogenic callus, or meristems, it is also essential to determine the appropriate development stage at which cryopreservation will be most successful, and to determine if any preculture of the tissues (for example, preculture using high sucrose solutions or osmoprotectants) can aid the cryopreservation process. Researchers should also be mindful that careful excision of the explants and their appropriate recovery will help reduce the impact of unavoidable stress. According to Sisunandar et al. (2010a), the highest post-cryotreatment survival rate, germination, and normal seedling production belong to embryos harvested at 11 mo of age. In cryopreservation of date palm meristematic cell aggregates, explants with sizes below 3 mm and displaying the meristematic-aggregate-bearing structures were precultured on 18% sucrose and incubated at low temperatures for several days before being subjected to different vitrification schemes. After cryotreatment, they were able to produce proembryos and adventitious buds, which later multiplied using a temporary immersion system (Fki et al. 2014). In cryopreservation of oil palm polyembryoids, selected cell suspension-derived aggregates must be larger than 500 µm and contain developing embryoids. Observation of haustorium and torpedo structures was the prerequisite for high regeneration rates in cryopreserved oil palm polyembryoids (Gantait et al. 2015; Palanyandy et al. 2020).
Insight to be gained
Successful establishment of regenerative cultures should be achieved prior to a start of any cryopreservation work, so it is essential to establish a solid tissue culture skillset. This crucial milestone will provide sufficient in vitro materials for preliminary and long-term cryostorage trials, as well as a means for recovery of plantlets post-cryopreservation. In addition, the input materials (also known as explant types) also dictate the cryopreservation procedures and outcomes. By comprehending the most efficient regenerative pathways and mastering the factors needed for successful regeneration (including physical conditions, medium composition, type of explants, and genotype), researchers would be able to conduct cryopreservation tasks on a wide choice of palm materials.
Recalcitrant nature of palm species - Problem definition
Understanding the distinctive recalcitrant nature of a particular palm species is a crucial step towards developing an appropriate cryopreservation approach for that species. In the field of cryobiology, recalcitrance is mainly attributed to low tolerance of seed tissues to dehydration, and this has been extensively discussed in literature (Berjak and Pammenter 2013; Pammenter and Berjak 2014; Walters 2015; Ballesteros et al. 2021). A different response threshold to water removal and glassy state formation in the seed separates recalcitrant plant species from the rest of the plant kingdom (Walters 2015). It has been widely known that a 50 to 70% loss in total fresh weight due to dehydration is lethal to the seeds of most of recalcitrant plants (Engelmann 1997). This makes them highly vulnerable to extreme dehydration when compared with orthodox and intermediate plant species and must require specific dehydration approaches. Like other recalcitrant species, such as rubber (Hevea brasiliensis Müll.Arg.), avocado (Persea americana Mill.), and citrus (Citrus L.), the seeds of most palm species are unable to withstand excessive moisture loss and cannot tolerate the associated damage that is caused during inappropriate low-temperature storage (Umarani et al. 2015). Due to such limitations in drying, cryopreservation has been considered to provide a reliable method in place of traditional seed banks to conserve desiccation-intolerant seed crops, including most of palms (Engelmann 2004). Nevertheless, the in vitro culture and cryopreservation protocols of these recalcitrant-seeded species often take a great deal of time and effort to develop and yet are unable to provide a highly effective solution to the long-term conservation process.
Dehydration approaches for recalcitrant palms - Physical dehydration
In cryopreservation, physical dehydration (also known as desiccation) is a method by which a laminar air flow hood or silica gel is used to dry the samples (Uragami et al. 1990; Sherlock et al. 2005). In the encapsulation-dehydration technique, explants can be further protected by being encapsulated in calcium alginate beads before sucrose preculture and drastic moisture reduction by physical means (Dereuddre et al. 1990). Physical dehydration approaches, especially encapsulation-dehydration, were used to prepare the tissues of many essential crops for cryopreservation (Gupta and Reed 2006; Halmagyi and Deliu 2006; Barraco et al. 2014; Pinto et al. 2016; Nakkanong and Nualsri 2018; AlMousa and Hassan 2021). In addition, the speed of dehydration can be modified to accommodate each specific species. For example, rapid dehydration methods using silica gel are found to be the most useful methods to dehydrate zygotic embryos of recalcitrant species (Berjak and Pammenter 2001; Sisunandar et al. 2010b). As the traditional slow dehydration approach used for orthodox seeds is unable to be used with recalcitrant species, the drying process for these species needs to be undertaken rapidly but only down to certain tissue water contents, then immediately rapidly cooled, which enables the cytoplasm to ultimately achieve the vitrified state (Sisunandar et al. 2010b). A preculture period in high sucrose can be beneficial for palm tissue undergoing physical dehydration. Sucrose acts as a non-penetrating osmotic agent to prime the explants into a suitable physiological state that can withstand subsequent moisture loss. However, at high concentrations, this compound can become toxic and must be gradually adjusted to accommodate each explant type (Sakai et al. 2008; Engelmann 2009).
Chemical dehydration
Another cryopreservation approach is through chemical dehydration. This approach, which is shortened to “vitrification method” in many references and must be distinguished from physical dehydration methods that also aim at tissue vitrification, undertakes sample preparation using a mixture of cryoprotective agents, including dimethyl sulfoxide (DMSO), glycerol, and sucrose (Sakai et al. 1990; Panis and Lambardi 2005). Different plant vitrification solutions were developed by changing the amount and ratio of these components, but the major solutions used for palms are PVS1 (Uragami et al. 1989), PVS2 (Sakai et al. 1990), and PVS3 (non-DMSO containing) (Nishizawa et al. 1993). For horticultural and ornamental crops, which possess numerous vegetative shoot tips or buds, vitrification by chemical dehydration has become the technique of choice (Zamecnik et al. 2021) while physical approaches are still favored for palms with few vegetative shoot tips or buds (Table 2).
Over time, improved cryopreservation techniques that use vitrification solutions have been developed. Among those is encapsulation-vitrification, which, similar to encapsulation-dehydration, utilizes alginate beads to prevent the plant tissues from coming into direct contact with the vitrification solutions thereby reducing the chances of tissues becoming intoxicated (Sakai and Engelmann 2007). Another method called droplet-vitrification involves using aluminum foil strips to hold the samples to achieve a faster cooling rate by direct and rapid exposure to liquid nitrogen (Panis et al. 2009). Indeed, droplet-vitrification has become the method of choice to cryopreserve the highly organized tissues of several species (Wang et al. 2021) while for palms it is mainly used for the cryopreservation of meristems or proembryonic cultures (Table 2). In the chemical approach, it is important to prevent damage to the tissues by controlling the concentration of the cryoprotectants and vitrification solutions used. Although DMSO-containing cryoprotective agents are often associated with high anti-freeze properties, they could be toxic to some members of the Palm family, as has been shown in peach palm (Bactris gasipaes Kunth.) (Heringer et al. 2013b; Awan et al. 2020). As the effect of cryoprotective chemicals can be species-dependent, future empirical research is of importance to determine appropriate use of them.
Based on the aforementioned technologies, two cryoplate approaches have been developed, namely the V-cryoplate and D-cryoplate techniques. The V-cryoplate technique was first developed from droplet-vitrification (Yamamoto et al. 2011) while the D-cryoplate technique was developed from encapsulation-dehydration (Niino et al. 2014).
Potential approaches through molecular and oxidative responses
In temperate species, inducing the innate cold acclimation pathways before cryopreservation has long been applied so that the explants can better tolerate the extreme cooling process (Scottez et al. 1992; Wu et al. 2001; Senula et al. 2007; Kushnarenko et al. 2009; Bettoni et al. 2019), although efforts to improve cryopreservation on palms have not taken advantage of such molecular mechanisms. Empirical research showed that cold stress is likely to upregulate certain transcription factors (for example, the C-repeat binding factor (CBF)) that regulate the cold-related (COR) genes (Zhao and Zhu 2016; Mathivanan 2021). Despite the limited literature, some preliminary studies have shed some light on the cold-induced molecular pathways of palm species. For example, in oil palm, the CBF-binding capability is limited due to the lack of Dehydration Responsive Element (DRE) motifs in the COR genes, which leads to a lower tolerance to cold stress (Lei et al. 2014). One study on oil palm suggested that expression of certain CBF genes might be linked with tissue accumulation of sucrose or proline (Li et al. 2019). This prompts future investigators to conduct more basic research on similar pathways, which could be beneficial for understanding species-dependent responses to a particular cryogenic treatment (Volk 2010). However, even though desiccation-responsive and cold acclimation pathways can be universal among plant species, their expression levels substantially differ due to evolutionary adaptation (Lei et al. 2014; Subbiah et al. 2019). Therefore, practitioners should be considerate of any metabolic and proteomic differences that may be present between orthodox and recalcitrant plants.
Unlike physical damage, oxidative injury is induced only before and after the tissue is plunged into liquid nitrogen, and this is due to the stresses imposed by the dehydration and rewarming steps. Specifically, oxidative responses should be monitored when explants are recovered from cryostorage. In both orthodox and recalcitrant species, many studies have evidently shown a correlation between cryopreservation-induced oxidative stress and a direct uplift in antioxidant enzymes, lipid peroxidation, and an accumulation of reactive oxidative species (ROS) (Naidoo 2012; Ren et al. 2015; Pareek et al. 2017). Unfortunately, this is still a gap in cryopreservation research in palms. For instance, it is still elusive how ROS-induced oxidation correlates with palm explant viability after cryopreservation. In cryopreservation of Chinese fan palm, although plasma membrane destabilization and changes in antioxidant system were observed, induced oxidative stress was less likely to affect the viability of embryo explant (Wen et al. 2012). This implies that it is essential to identify stress tolerance threshold of explant types being used. In Arabidopsis, incorporation of dehydrins into vitrification solution can accelerate breakdown of ROS and promote expression of antioxidant enzymes, thereby enhancing survival of cryopreserved tissues (Yang et al. 2019). Hence, investigation of dehydrins, such as their expression and application, and similar antioxidative proteins (Uchendu et al. 2010) in palm cryopreservation should be given consideration.
Insight to be gained
Storage of recalcitrant species by cryopreservation is much more challenging due to low tolerance for extreme moisture loss, either by long storage time or by artificial dehydration, and the survival rates often widely oscillate between 0 and 100%. Thus, choosing the most appropriate explants and developing a species-specific dehydration approach are necessary for successful cryopreservation of palms (see comparison among explants and approaches in Supplementary Table 1 and Supplementary Table 2), since many protocols, while reportedly working on other species, were proven infeasible for these recalcitrant plants. In addition, although basic research has pointed out some potential areas to investigate in terms of molecular and oxidative changes in palm under cold stress, cryopreservation studies utilizing these pieces of information continue to be extensively underdeveloped in palms and should be given priorities in the future.
Cryobiology theory and responses of palm tissues to cryogenic techniques - The idea of achieving a vitreous state
The underlying principle of cryopreservation is to transform living tissues into a vitrified state without forming intra- or extra-cellular ice crystals, and then to store those tissues at ultra-low temperatures (Pegg 2007). This requires a clear understanding of several different fields of study, including plant physiology, cryobiology, and biochemical properties of water molecules (Martinez-Montero and Harding 2015). When water is frozen, ice crystals develop in a process known as “crystal seeding” (Huang et al. 2017). This can occur with water molecules in both intra-cellular and extra-cellular sites, and result in lethal damage to tissues when frozen (Pegg 2007). By coupling ultra-fast cooling with the addition of a physical or chemical dehydration approach, the cell cytoplasm can become vitrified without ice crystal formation (Wowk 2010). However, upon rewarming, further instability of the cryopreserved tissues can occur since unfrozen water molecules become remobilized within and out of individual cells and can reform ice crystals. The principle is to maintain a homogenous transition from normal to frozen state and vice versa to mitigate such instability and also cellular stress (Benson 2008). On the contrary, if the transition takes place heterogeneously, physical and biochemical states of the cells can be affected, leading to the so-called cryoinjury, or ruptures at intra- and extra-cellular sites (Gao and Critser 2000). The biology of recalcitrant species has in so far prevented them from being dehydrated and cryopreserved similarly to other plants and must follow specific procedural rules with regard to preculture, cryoprotectants, drying and cooling rate, and recovery (Pammenter and Berjak 2014; Roque-Borda et al. 2021).
Developments of cryopreservation techniques for palm species
In recent years, a wide range of cryopreservation techniques have been attempted in palm species with some notable achievements. These optimizations are summarized in this review section and listed in Table 2.
Oil palm
Among palm species, the cryopreservation of oil palm has been the most studied. Among physical dehydration techniques applied, silica gel-based desiccation is by far the most widely used (Norziha et al. 2017; Beulé et al. 2018; Prakash et al. 2019). In contrary to date palm, most of oil palm cryopreservation studies have been undertaken without a preculture treatment. There has been no clear explanation, except that the oil palm may not be a recalcitrant species as previously mentioned by Grout et al. (1983). Studies that actually used pretreatment reported that high-sucrose culture (17 to 26%) is important for cryopreservation of oil palm somatic embryos using both silica gel dehydration and vitrification techniques (Gantait et al. 2015; Beulé et al. 2018). There has been a variation in terms of physical dehydration duration, which could range from 4 to 6 h and towards a targeted water content, which varied between 10 and 63% (Table 2). In a recent study, other techniques have been tested, including encapsulation-dehydration on oil palm polyembryoids, which resulted in 73.3% survival rate (Palanyandy et al. 2020). A brief rehydration period following initial desiccation has also been successfully applied in oil palm, leading to improved survival and plantlet recovery (Norziha et al. 2017; Prakash et al. 2019; Araújo de Oliveira et al. 2021). Cryopreservation techniques that use vitrification solutions (PVS2) have been tested in oil palm with inconsistent results (Suranthran et al. 2012; Gantait et al. 2015; Prakash et al. 2019). Liquid nitrogen storage duration can also vary between 1 h and 20 yr time (Engelmann et al. 1995; Gantait et al. 2015; Norziha et al. 2017; Beulé et al. 2018). To the best of this review’s knowledge, cryopreservation of oil palm has endured the longest-ever storage time among the Palm family (20 yr), albeit at a relatively low survival rate (19.1% after rewarming, 33.2% after 20 yr), with fifty shoots regenerated from one specific clone (Beulé et al. 2018).
As for recovery, after removing the explants from liquid nitrogen storage, most rewarming methods involve a water bath rewarming at 40°C (Suranthran et al. 2012; Beulé et al. 2018; Palanyandy et al. 2020) or at room temperatures (Norziha et al. 2017). Oil palm tissues can be then transferred onto recovery media containing high sucrose concentrations (10 to 41% sucrose) for up to 3 wk before being subcultured back onto normal propagation medium (Beulé et al. 2018; Palanyandy et al. 2020). This high sucrose medium can sometimes be referred to as unloading solution (Gantait et al. 2015; Prakash et al. 2019).
Date palm
Both physical and chemical dehydration approaches have been applied for date palm cryopreservation (Metwali et al. 2020). PVS2 is a preferred solution for achieving chemical-based vitrification in date palm (Al-Bahrany and Al-Khayri 2012; Fki et al. 2013; Alansi et al. 2019). A high survival rate (up to 80%) was achieved with encapsulation-vitrification (chemical approach) (Bekheet et al. 2007; Alansi et al. 2019) and encapsulation-dehydration (physical approach) (Solliman et al. 2019). It is also important to note that droplet-vitrification in date palm requires a partial dehydration step to reduce the initial water content to 75% while the encapsulation-dehydration approach requires desiccation to 65% (Bekheet et al. 2007; Fki et al. 2011; Solliman et al. 2019). Physical dehydration using silica gel has also been demonstrated on date palm pollen; however, pollen moisture needs to be monitored and adjusted beforehand (Araújo de Oliveira et al. 2021). Data from other studies also indicated that cutoff moisture values can be a contributing factor for explant recovery, particularly for heterogenous explants (Gonzalez-Arnao et al. 2008; Engelmann 2014).
Survival rates can also be enhanced by incorporating pretreatments, including preculture with sucrose, cryoprotective agents, and a brief dehydration, before being plunged into liquid nitrogen (Table 2). Pretreatment with higher sucrose concentrations (between 17 and 34%) was found suitable for pro-embryogenic masses, embryogenic callus, and isolated shoot tip (Fki et al. 2011, 2013; Salma and Engelmann 2017). It is also worth noting that duration of cryogenic treatment may range from 60 min to 6 wk. There has been no longer duration for cryopreservation of this palm species since the first study conducted three decades ago (Towill et al. 1989). The most recent advancement involves the use of D-cryoplate method, which produced a high survival rate (95.8%) (Salma and Engelmann 2017). Similar to other Palm members, during recovery, a medium containing normal sucrose concentration or similar with one used for tissue culturing the respective explant was used to recover date palm embryogenic callus (Alansi et al. 2019; Solliman et al. 2019; Metwali et al. 2020). For D-cryoplate method and vitrification, an initial high sucrose concentration (41%) was used for unloading; then, a lowered concentration (10 to 17%) was used for recovery (Fki et al. 2014; Salma and Engelmann 2017; Alansi et al. 2019).
Coconut
Cryopreservation research in coconut has trailed behind that undertaken on other members of the Palm family. Due to a recent decline in coconut productivity around the globe and the rapid loss in diversity, there has been a concerted effort to develop such techniques (Karun et al. 1999, 2014; Sisunandar et al. 2010a; Malaurie et al. 2011; Sajini et al. 2011; Lédo et al. 2018; Kim et al. 2019). To date, most coconut germplasm is conserved in genebanks to be found in more than 30 countries around the world, including five International Coconut Genebanks located in Brazil, Côte d’Ivoire, India, Indonesia, and Papua New Guinea. The COGENT (The International Coconut Genetic Resources Network) is among the most active research organizations that facilitate global collaboration for coconut genetic resource conservation and utilization. It has released a 10-yr global strategy that includes a comprehensive analysis of the current coconut genetic diversity and the accompanying conservation methodologies (COGENT et al. 2017). Even though there are 39 country members that actively contribute to the network’s visions, work into the cryopreservation of coconut has only been undertaken by research teams in Australia, Belgium, Brazil, Côte d’Ivoire, France, India, Korea, Malaysia, Sri Lanka, and the UK (Welewanni and Bandupriya 2017) (Table 2).
For coconut cryopreservation, zygotic embryos are often physically dehydrated either using a laminar air flow hood or by silica gel in a desiccator (Assy-Bah and Engelmann 1992; N’Nan et al. 2008; Welewanni et al. 2020). One method using rapid dehydration of zygotic embryos with a silica gel chamber, followed by rapid cooling and rewarming, resulted in a high number of seedlings of uniform morphology, cytology, and molecular makeup growing in soil (Sisunandar et al. 2010a). In order to achieve such a high rate of recovery to soil, the physical dehydration approach needed to reduce embryo water content to 20% in just a few hours (Sisunandar et al. 2010a). More recently, work has been undertaken on coconut vegetative shoots (Normah et al. 2019; Wilms et al. 2019). In these studies, a PVS2- or PVS3-based droplet-vitrification approach was used to overcome ice crystal formation (Sajini et al. 2011; Lédo et al. 2018; Wilms et al. 2019), yet no plants have been recovered to soil using these methods.
Recovery medium for encapsulated-dehydrated coconut tissues does not strictly require high sucrose concentrations and may be identical in composition to normal culturing media for the respective tissues (Assy-Bah and Engelmann 1992; Sisunandar et al. 2010b; Sajini et al. 2011; Lédo et al. 2020). However, for techniques using vitrification solutions, it is reported that a high-to-low sucrose recovery medium was used for up to 2 wk post-cryostorage (Lédo et al. 2018, 2020; Wilms et al. 2019).
Insight to be gained
Due to the occurrences of lethal ice crystals during cooling and rewarming cycles, successful cryopreservation protocols for palms should be designed towards minimizing such events and achieving a homogenous vitreous state throughout the tissue. This state is called vitrification, which can be achieved with either physical or chemical dehydration approaches. Indeed, cryopreservation protocols have been refined for targeted palm species and varieties with a diverse array of techniques and explants used. The biggest gap in palm cryopreservation, however, is the lack of studies that adequately regenerate palm plantlets post-cryopreservation into field-grown plants. Taken together from past research, researchers should begin their studies by addressing a set of aspects: (i) explant choice and cryopreservation techniques; (ii) detailed procedures and chemicals; and (iii) clear outcomes and analytical measures (depicted in Supplementary Fig. 1).
Fidelity assessment approaches for cryopreservation in palms-Morphological and physiological assessments
Several ways have been used to assess the viability of recovered cryopreserved plant tissues. It is worth mentioning that the term “survival” and “recovery” are sometimes used ambiguously in reports, although they should be clearly distinguished. While survival rates consider post-cryostorage recovered tissues to contain viable cells, recovery demands that those tissues be able to grow back into a fully functional organ or a complete plantlet. Sometimes report may use the term “regrowth” to indicate the increase in size of recovered tissues with or without subsequent organ regeneration. In palms, the viability of zygotic embryos is determined by measuring certain phenotypic traits, such as shoot or root elongation or the increase in embryo biomass (Steinmacher et al. 2007; Sisunandar et al. 2010b). Cryopreserved callus tissues are considered to be viable when visible swelling or new callus formation occurs (Welewanni et al. 2020). Polyembryoids are considered to have survived if they show growth of meristematic regions or shoot apices (Gantait et al. 2015). However, since the final requirement for an effective cryopreservation protocol is the successful establishment of healthy plants in soil, using viability measurements alone cannot determine the efficiency of the protocol.
Morphological and physiological assessments of the recovering tissues should be monitored throughout the recovery process and to a point well after soil establishment. For coconut cryopreservation, only two studies have reported the successful soil establishment of seedlings coming from cryopreserved zygotic embryos (Sisunandar et al. 2010a; Sajini et al. 2011). The regenerated coconut seedlings produced after cryopreservation demonstrated normal morphological, cytological, and genetical character, and the only difference was in the rate of shoot growth, which was slower in plantlets coming from cryopreserved embryos (Sisunandar et al. 2010a). For oil palm, field observations carried out on plantlets coming from cryopreserved somatic embryos were reported to show normal morphology and floral conformity and identical to non-cryopreserved plantlets (Konan et al. 2006). In a similar study, long-term observations made on six oil palm clones coming from cryopreserved polyembryonic cultures showed an average recovery of 34% (Konan et al. 2007). The study revealed that the percentage of abnormal palms originating from cryopreserved polyembryonic cultures was considerably lower (5%) than those originating from non-cryopreserved culture (29%), suggesting the possibility of using cryopreservation as a tool to select highly proliferative and normally developing cultures of oil palms.
Histological, ultrastructural, and cytological assessments
In a histological analysis, chromosomal changes of cryopreserved palms can be clearly observed by microsectioning and using various staining techniques (Table 2). However, the extent of nuclear damage observed by these methods is unlikely to give a true understanding of what may occur in the fully regenerated plant. In palm cryopreservation, osmotic damage can be detected by histological observations made on cell plasmolysis, damaged plasma membranes, retracted nuclei, and increasing periplasmic space, while dehydration damage can be detected by broken plasma membrane with condensed and poorly stained cytoplasm, decreasing nucleus and cytoplasmic ratio, and leaking intra-cellular soluble proteins. Furthermore, pyknotic nuclei and disappearing nucleoli observed through histological methods can predict chromatin contraction, which can be helpful when coupled with epigenetic assessments (Welewanni et al. 2020). Histological staining is used to observe the epidermal, protodermal, and procambium layer of cryopreserved coconut zygotic embryos (var. Brazilian Green Dwarf) although not at a comparable resolution to ultrastructural analyses (Lédo et al. 2018). In addition, it was reported on peach palm and date palm that detecting viability through a histological method can help draw conclusions about the correlation between the remaining meristematic regions and cellular vacuolation during a later stage of recovery (Steinmacher et al. 2007; Fki et al. 2013).
Ultrastructurally, technologies, such as Scanning Electron Microscopy (SEM), can assist in observing epidermal layers and specific contraction of cell regions (Welewanni et al. 2020). For example, in peach palm somatic embryo and oil palm polyembryoid cryopreservation, SEM helped visualize cell shape and configuration, cell and nucleus disruption, cell wall breakdown, mitochondria and middle lamella damage, and cellular space appearance (Heringer et al. 2013b; Gantait et al. 2015; ). Detecting such morphological damages, especially in similar minuscule tissues, can help accelerate the evaluation process for a particular treatment. Also, there is currently a lack of studies looking closely into gas exchange or leaf chlorophyll formation, which are indicators of self-sustaining capabilities of non-damaged tissues. In palm species, using these methods is less destructive and allows for more frequent sampling of plant material (Equiza and Francko 2010).
Cytological tests on coconut showed that the cryopreserved regenerants maintain the same gross chromosome arrangement (numbers and size) as non-cryopreserved plantlets. There was also no significant difference in the global methylation rates between coconut seedlings coming from cryopreserved and non-cryopreserved embryos (Sisunandar et al. 2010a) although some minor differences were detected among genotypes. This study also reported a high frequency of black banding (N-banding) in the cryopreserved seedlings, suggesting a possible relationship between the desiccation pretreatment with chromosomal protein denaturation and inhibition of certain quintessential functional genes (Sisunandar et al. 2010a).
Molecular change assessments
It is believed that plant cells possess an exceptional ability, known as plasticity, which allows them to adapt and survive through stress events. However, during cryopreservation, plant genetic makeup has to encounter many impactful factors, for example, injury during cooling and rewarming transition, osmotic disruption, toxicity of cryoprotective agents, oxidative damage, and accumulation of secondary products (Martinez-Montero and Harding 2015). Therefore, genetic fidelity assessments are necessary to ensure that cryo-related stress does not exceed the limit of cell plasticity as it would result in undesired mutations or malfunctioning gene expression. This kind of assessment is often carried out using molecular marker techniques (Khan et al. 2012), which were previously developed for gene mapping or studies of related species, and are now widely employed to ensure that targeted accessions are well-maintained in genebanks (Spooner et al. 2005). Still, the use of genetic markers in palm cryopreservation research has only been targeted at checking whether recovered progenies are of any significant difference from their source plants, while in fact, those assessments could have been serving a more integrative picture and applied consistently over an extended period.
In some other species, molecular marker methods detected a mixed result between some and no variation with some possible reasons. In Lamprocapnos spectabilis L., RAPD (Random amplified polymorphic DNA) and ISSR (Inter simple sequence repeat) methods detected 5% variation, mostly of non-encapsulated samples (Kulus 2020). Meanwhile, in Chrysanthemum × morifolium Ramat., RAPD and AFLP (Amplified fragment length polymorphism) methods helped conclude that other steps of the procedure, such as sucrose preculture, may have caused the variations (Martín et al. 2011). RAPD, ISSR, AFLP, and SSR (Simple sequence repeats) methods have also been used to detect instability in other species, although the results can be inconsistent between two different measurement periods, or between two methods used or two different studies on the same species (Kaity et al. 2008, 2013; Castillo et al. 2010; Martín et al. 2015; Bi et al. 2016; Ibáñez et al. 2019).
In contrast to aforementioned research, genetic variation has yet to be reported in different cryopreserved palm species, with different cryogenic techniques being used (Bekheet et al. 2007; Sisunandar et al. 2010a; Gantait et al. 2015; Alansi et al. 2017; Solliman et al. 2019; Welewanni et al. 2020). In coconut, all-round fidelity analyses were undertaken on cryopreserved regenerants and, thus far, no significant variation has been reported (Sisunandar et al. 2010a; Iroshini et al. 2017; Bandupriya et al. 2017). Among the methods used, RAPD and ISSR techniques are the most chosen for their low cost, high speed, and small amount of required DNA (Gantait et al. 2015; Alansi et al. 2017). Yet, one caveat to the use of RAPD and SSR markers to detect variations is that they can only perform screening on an extremely small portion of the DNA. Firm conclusions about the whole genome cannot be drawn if these methods detect no differences or detect differences that happen to be on non-coding DNA portions. Usage of fidelity assessment protocols on palms is further hindered by a lack of studies that aim to develop molecular markers based on recently completed genome sequences of palm species.
Epigenetic change assessments
Plant living materials are known for their great adaptability and this capacity is closely linked to changes at transcriptional level. These changes are due to a phenomenon called DNA methylation (Finnegan and Kovac 2000). Scientists believe that assessing its rate can help understand how plant cell and tissue cope with cold-storage condition. This phenomenon was hypothesized to protect DNA regions from the stress-induced damages during cryopreservation process (Heringer et al. 2013a). Evidence suggested that global methylation profiles of cryopreserved plantlets return to a normal level after a period of elevation (Harding 2004). Global DNA methylation rate (often expressed in percentage) is usually the generic measure for this particular analysis, which determines the frequency of 5′- methylated deoxycytosines over the total amount of cytosines (Xie et al. 2017). In peach palm, global methylation rates of PVS3-treated peach palm embryogenic clusters increased temporarily after they had been subjected to cryopreservation treatment and eventually returned almost to the control level after several weeks of recovery (Heringer et al. 2013a). Similarly, no significant differences were found in the percentages of global DNA methylation rate between plants recovered from cryopreservation and non-cryopreservation on coconut (Sisunandar et al. 2010a). This might suggest that elevation of methylation rates can be beneficial for the survival and regrowth of cryopreserved materials in palms as it enables cells to temporarily hold up normal gene regulation and functionality. It is also worth combining these epigenetic studies with DNA change assessment to produce more robust fidelity assessment.
Insight to be gained
Morpho-physiological assessments should involve consistent analysis of tissue viability and subsequent shoot and root growth upon rewarming until the plant is well established in the field. Major histological, ultrastructural, and cytological techniques should be applied to further observe gross changes which the cryogenic treatments can cause on the cryopreserved tissues of palms. These methods, however, demand destructive preparation of the tissues, and may not be suitable if there is low availability of materials. Finally, since the ultimate goal of palm conservation is to preserve true-to-type clones, an effective program should involve rigorous molecular and epigenetic evaluations of cryopreserved regenerants.
Conclusion and prospects
Palms are an example of economically important species that demand immediate conservation efforts and also offer decades of illustrative cryopreservation research trials. The current review differs from previous palm reviews in that it encourages researchers and conservationists to base their cryopreservation research on the proposed framework and a series of consideration checkpoints (tissue culture, recalcitrant nature, cryobiology, and fidelity assessments), and to progress to further topics created by the combination of two nearby foundations. Cryopreservation is a multifaceted process that requires careful construction of different research blocks and having a frail one can result in a futile research endeavor. In reality, even though considerable efforts have been made in cryopreservation of important palm crops to achieve a certain degree of regrowth rate, the goal of true regeneration of cryopreserved plantlets (also known as soil establishment) has not been met satisfactorily by the majority of reports. The long-term view of palm cryobank construction is yet to be fulfilled due to a number of factors, including the lack of a systematic effort and often times short duration of research projects. Indeed, most current palm collections are only serving the immediate consumer demands instead of creating broader and more collaborative collections to preserve the palm taxa. With a diversity of species-specific protocols and problems, it is easy to get lost in the minor details and forget about the big picture. Thus, this review provided the most important insights for each foundation of the framework. It is important to note that the approach used does depend on species recalcitrance and the availability of explant tissues. Looking ahead, technology transfer among research laboratories around the globe should be facilitated to ensure high reproducibility of cryopreservation protocols, and a fidelity assessment should be performed with robust and state-of-the-art methods.
Data Availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Abdelnour-Esquivel A, Villalobos V, Engelmann F (1992) Cryopreservation of zygotic embryos of Coffea spp. CryoLetters 13:297–302
Abul-Soad AA, Jain SM, Jatoi MA (2017) Biodiversity and conservation of date palm. In: Ahuja MR, Jain SM (eds) Biodiversity and conservation of woody plants. Springer International Publishing, Cham, pp 313–353
Alansi S, Al-Qurainy F, Khan S, Nadeem M, Tarroum M, Alshameri A, Gaafar A-RZ (2017) Genetic fidelity testing in regenerated plantlets of cryopreserved and non-cryopreserved cultivars of Phoenix dactylifera L. Pak J Bot 49:2313–2320
Alansi S, Al-Qurainy F, Nadeem M, Khan S, Tarroum M, Alshameri A, Gaafar A-RZ (2019) Cryopreservation: A tool to conserve date palm in Saudi Arabia. Saudi J Biol Sci 26:1896–1902. https://doi.org/10.1016/j.sjbs.2019.02.004
Al-Bahrany AM, Al-Khayri JM (2012) Optimizing in vitro cryopreservation of date palm (Phoenix dactylifera L.). Biotechnology 11:59–66
Al-Khayri JM (2010) Somatic embryogenesis of date palm (Phoenix dactylifera L.) improved by coconut water. Biotechnology 9:477–484. https://doi.org/10.3923/biotech.2010.477.484
Al-Khayri JM, Naik PM (2017) Date palm micropropagation: Advances and applications. Ciênc Agrotec 41:347–358. https://doi.org/10.1590/1413-70542017414000217
AlMousa RN, Hassan NA-F (2021) Cryopreservation of grape (Vitis vinifera L.) using encapsulation-dehydration technique. J Genet Environ Resour Conserv 9:153–156
Araújo de Oliveira AC, da Silva LA, Polek M, Krueger R, Shepherd A, Volk GM (2021) Optimization of in vitro germination and cryopreservation conditions for preserving date palm pollen in the USDA National Plant Germplasm System. Plant Cell Tiss Org Cult 144:223–232. https://doi.org/10.1007/s11240-020-01907-1
Assy-Bah B, Durand-Gasselin T, Pannetier C (1987) Use of zygotic embryo culture to collect germplasm of coconut (Cocos nucifera L.). Plant Genet Resour Newsl 71:4–10
Assy-Bah B, Engelmann F (1992) Cryopreservation of mature embryos of coconut (Cocos nucifera L.) and subsequent regeneration of plantlets. CryoLetters 13:117–126
Assy-Bah B, Engelmann F (1993) Medium-term conservation of mature embryos of coconut. Plant Cell Tiss Org Cult 33:19–24
Ateyyeh AF (2012) Effect of storage method on date palm and pistachio pollen viability. Jor J Agric Sci 8:573–582
Awan M, Buriak I, Fleck R, Fuller B, Goltsev A, Kerby J, Lowdell M, Mericka P, Petrenko A, Petrenko Y (2020) Dimethyl sulfoxide: a central player since the dawn of cryobiology, is efficacy balanced by toxicity? Regen Med 15:1463–1491
Ballesteros D, Fanega-Sleziak N, Davies RM (2021) Cryopreservation of seeds and seed embryos in orthodox-, intermediate-, and recalcitrant-seeded species. In: Wolkers WF, Oldenhof H (eds) Cryopreservation and Freeze-Drying Protocols. Springer, US, New York, NY, pp 663–682
Bandupriya HDD, Iroshini WWMA, Perera SACN, Vidhanaarachchi VRM, Fernando SC, Santha ES, Gunathilake TR (2017) Genetic fidelity testing using SSR marker assay confirms trueness to type of micropropagated coconut (Cocos nucifera L.) plantlets derived from unfertilized ovaries. TOPSJ 10:46–54. https://doi.org/10.2174/1874294701710010046
Barraco G, Sylvestre I, Collin M, Escoute J, Lartaud M, Verdeil J-L, Engelmann F (2014) Histocytological analysis of yam (Dioscorea alata) shoot tips cryopreserved by encapsulation–dehydration. Protoplasma 251:177–189. https://doi.org/10.1007/s00709-013-0536-5
Bekheet S (2013) Direct organogenesis of date palm (Phoenix dactylifera L.) for propagation of true-to-type plants. Sci Agri 4:85–92
Bekheet S, Taha H, Saker M (2002) In vitro long-term storage of date palm. Biol Plant 45:121–124
Bekheet S, Taha H, Solliman M (2007) Cryopreservation of date palm (Phoenix dactylifera L) cultured in vitro. Acta Hortic 736:283–291. https://doi.org/10.17660/ActaHortic.2007.736.26
Benson EE (2008) Cryopreservation Theory. In: Reed BM (ed) Plant Cryopreservation: A Practical Guide. Springer, New York, NY, pp 15–32
Berjak P, Pammenter NW (2001) Seed recalcitrance — current perspectives. S Afr J Bot 67:79–89. https://doi.org/10.1016/S0254-6299(15)31111-X
Berjak P, Pammenter NW (2013) Implications of the lack of desiccation tolerance in recalcitrant seeds. Front Plant Sci 4:478. https://doi.org/10.3389/fpls.2013.00478
Bettoni JC, Kretzschmar AA, Bonnart R, Shepherd A, Volk GM (2019) Cryopreservation of 12 Vitis species using apical shoot tips derived from plants grown in vitro. HortScience 54:976–981
Beulé T, Ilbert P, Adeoti K, Durand-Gasselin T, Dumet D, Engelmann F, Morcillo F (2018) Recovery of oil palm (Elaeis guineensis Jacq.) somatic embryos cryostored for 20 years. CryoLetters 39:60–66
Bi W-L, Pan C, Liu J, Wang Q-C (2016) Greenhouse performance, genetic stability and biochemical compounds in Chrysanthemum morifolium ‘Hangju’ plants regenerated from cryopreserved shoot tips. Acta Physiol Plant 38:1–10
Bourdeix R, Adkins S, Johnson V, Perera L (2020) In situ and ex situ conservation of coconut genetic resources. In: Coconut biotechnology: Towards the sustainability of the ‘Tree of life’. Springer, pp 51–75
Brison M, de Boucaud M-T, Pierronnet A, Dosba F (1997) Effect of cryopreservation on the sanitary state of a cv Prunus rootstock experimentally contaminated with Plum Pox Potyvirus. Plant Sci 123:189–196
Castillo NRF, Bassil NV, Wada S, Reed BM (2010) Genetic stability of cryopreserved shoot tips of Rubus germplasm. In Vitro Cell Dev Biol - Plant 46:246–256. https://doi.org/10.1007/s11627-009-9265-z
Chandel K, Chaudhury R, Radhamani J, Malik S (1995) Desiccation and freezing sensitivity in recalcitrant seeds of tea, cocoa and jackfruit. Ann Bot 76:443–450
Coates D (2016) Strategic Plan for Biodiversity (2011–2020) and the Aichi Biodiversity Targets. In: Finlayson CM, Everard M, Irvine K, McInnes RJ, Middleton BA, van Dam AA, Davidson NC (eds) The Wetland Book I: Structure and function, management and methods. Springer, Netherlands, Dordrecht, pp 1–7
COGENT, Bourdeix R, Prades A (2017) A global strategy for the conservation and use of coconut genetic resources 2018–2028. Montpellier, France: Bioversity International 239 p. ISBN: 978–92–9043–984–4
Dereuddre J, Scottez C, Arnaud Y, Duron M (1990) Effets d’un endurcissement au froid des vitroplants de Poirier (Pyrus communis L. cv Beurré Hardy) sur la résistance des apex axillaires à une congélation dans l’azote liquide. Comptes rendus de l’Académie des sciences Série 3. Sciences De La Vie 310:265–272
Dumet D, Engelmann F, Chabrillange N, Duval Y (1993) Cryopreservation of oil palm (Elaeis guineensis Jacq.) somatic embryos involving a desiccation step. Plant Cell Rep 12:352–355
Eeuwens CJ (1976) Mineral requirements for growth and callus initiation of tissue explants excised from mature coconut palms (Cocos nucifera L.) and cultured in vitro. Physiol Plant 36:23–28. https://doi.org/10.1111/j.1399-3054.1976.tb05022.x
Ekaratne S, Senathirajah S (1983) Viability and storage of pollen of the oil palm, Elaeis guineensis Jacq. Ann Bot 51:661–668
Engelmann F (1997) Importance of desiccation for the cryopreservation of recalcitrant seed and vegetatively propagated species. Plant Genet Resour Newsl 112:9–18
Engelmann F (2004) Plant cryopreservation: progress and prospects. In Vitro Cell Dev Biol - Plant 40:427–433
Engelmann F (2009) Encapsulation-dehydration for cryopreservation: past, present and future. Acta Hortic 908:165–171
Engelmann F (2014) Cryopreservation of clonal crops: a review of key parameters. Acta Hortic 1039:31–39. https://doi.org/10.17660/ActaHortic.2014.1039.2
Engelmann F, Chabrillange N, Dussert S, Duval Y (1995) Cryopreservation of zygotic embryos and kernels of oil palm (Elaeis guineensis Jacq.). Seed Sci Res 5:81–86. https://doi.org/10.1017/S0960258500002658
Engels JM, Ebert AW (2021) A critical review of the current global ex situ conservation system for plant agrobiodiversity. I. History of the development of the global system in the context of the political/legal framework and its major conservation components. Plants 10:1557
Equiza MA, Francko DA (2010) Assessment of freezing injury in palm species by chlorophyll fluorescence. HortScience 45:845–848
Finnegan EJ, Kovac KA (2000) Plant DNA methyltransferases. In: Matzke MA, Matzke AJM (eds) Plant Gene Silencing. Springer, Netherlands, Dordrecht, pp 69–81
Fki L, Bouaziz N, Chkir O, Benjemaa-Masmoudi R, Rival A, Swennen R, Drira N, Panis B (2013) Cold hardening and sucrose treatment improve cryopreservation of date palm meristems. Biol Plant 57:375–379
Fki L, Bouaziz N, Sahnoun N, Swennen R, Drira N, Panis B (2011) Palm cryobanking. CryoLetters 32:451–462
Fki L, Chkir O, Nasri A, Bouaziz N, Sahnoun N, Drira N (2014) Cryopreservation of date palm meristematic cells. In: Abdelouahhab Zaid, Ghaleb Ali Alhadrami (eds) Proceedings of the Fifth International Date Palm Conference. Khalifa International Date Palm Award, Abu Dhabi, UAE, pp 155–159
Gantait S, Sinniah UR, Suranthran P, Palanyandy SR, Subramaniam S (2015) Improved cryopreservation of oil palm (Elaeis guineensis Jacq.) polyembryoids using droplet vitrification approach and assessment of genetic fidelity. Protoplasma 252:89–101
Gao D, Critser J (2000) Mechanisms of cryoinjury in living cells. ILAR J 41:187–196
Gomes HT, Bartos PMC, Scherwinski-Pereira JE (2015) Optimizing rooting and survival of oil palm (Elaeis guineensis Jacq.) plantlets derived from somatic embryos. In Vitro Cell Dev Biol - Plant 51:111–117
Gomes HT, Bartos PMC, Scherwinski-Pereira JE (2017) Dynamics of morphological and anatomical changes in leaf tissues of an interspecific hybrid of oil palm during acquisition and development of somatic embryogenesis. Plant Cell Tiss Org Cult 131:269–282
Gonzalez-Arnao MT, Panta A, Roca WM, Escobar RH, Engelmann F (2008) Development and large scale application of cryopreservation techniques for shoot and somatic embryo cultures of tropical crops. Plant Cell Tiss Org Cult 92:1–13. https://doi.org/10.1007/s11240-007-9303-7
Griffith MP, Meyer A, Grinage A (2021) Global ex situ conservation of palms: Living treasures for research and education. Front Glob Change 4:711414
Grout B, Shelton K, Pritchard H (1983) Orthodox behaviour of oil palm seed and cryopreservation of the excised embryo for genetic conservation. Ann Bot 52:381–384
Gupta S, Reed BM (2006) Cryopreservation of shoot tips of blackberry and raspberry by encapsulation-dehydration and vitrification. CryoLetters 27:29–42
Halmagyi A, Deliu C (2006) Cryopreservation of strawberry shoot tips by encapsulation-dehydration. Not Bot Horti Agrobot Cluj Napoca 34:28–33
Harding K (2004) Genetic integrity of cryopreserved plant cells: a review. CryoLetters 25:3–22
Helliot B, Panis B, Poumay Y, Swennen R, Lepoivre P, Frison E (2002) Cryopreservation for the elimination of cucumber mosaic and banana streak viruses from banana (Musa spp.). Plant Cell Rep 20:1117–1122
Heringer AS, Steinmacher DA, Fraga HPF, Vieira LN, Ree JF, Guerra MP (2013a) Global DNA methylation profiles of somatic embryos of peach palm (Bactris gasipaes Kunth) are influenced by cryoprotectants and droplet-vitrification cryopreservation. Plant Cell Tiss Org Cult 114:365–372. https://doi.org/10.1007/s11240-013-0331-1
Heringer AS, Steinmacher DA, Schmidt ÉC, Bouzon ZL, Guerra MP (2013b) Survival and ultrastructural features of peach palm (Bactris gasipaes Kunth) somatic embryos submitted to cryopreservation through vitrification. Protoplasma 250:1185–1193
Huang H, Zhao G, Zhang Y, Xu J, Toth TL, He X (2017) Predehydration and ice seeding in the presence of trehalose enable cell cryopreservation. ACS Biomater Sci Eng 3:1758–1768
Hunter IR, Bystriakova N (2004) Tropical ecosystems: bamboos, palms and rattans. In: Evans J, Youngquist JA (eds) Encyclopedia of Forest Sciences. Academic Press, pp 1675–1681
Ibáñez MA, Alvarez-Mari A, Rodríguez-Sanz H, Kremer C, González-Benito ME, Martín C (2019) Genetic and epigenetic stability of recovered mint apices after several steps of a cryopreservation protocol by encapsulation-dehydration. A new approach for epigenetic analysis. Plant Physiol Biochem 143:299–307. https://doi.org/10.1016/j.plaphy.2019.08.026
Iroshini W, Jayasekera G, Perera S, Vidhanaarachchi V, Bandupriya H (2017) Genetic stability of coconut embryogenic calli after cryopreservation by encapsulation-dehydration technique. In: Proceedings of the 6th YSF symposium, Peradeniya, Sri Lanka
Jayanthi M, Susanthi B, Murali Mohan N, Mandal PK (2015) In vitro somatic embryogenesis and plantlet regeneration from immature male inflorescence of adult dura and tenera palms of Elaeis guineensis (Jacq.). SpringerPlus 4:256. https://doi.org/10.1186/s40064-015-1025-4
Kaity A, Ashmore S, Drew R, Dulloo M (2008) Assessment of genetic and epigenetic changes following cryopreservation in papaya. Plant Cell Rep 27:1529–1539
Kaity A, Drew R, Ashmore S (2013) Genetic and epigenetic integrity assessment of acclimatised papaya plants regenerated directly from shoot-tips following short-and long-term cryopreservation. Plant Cell Tiss Org Cult 112:75–86
Karun A, Sajini K (1994) Short-term storage of coconut zygotic embryos in sterile water. Curr Sci 67:118–120
Karun A, Sajini K, Niral V, Amarnath C, Remya P, Rajesh M, Samsudeen K, Jerard B, Engelmann F (2014) Coconut (Cocos nucifera L.) pollen cryopreservation. CryoLetters 35:407–417
Karun A, Sajini K, Shivashankar S (1999) Embryo culture of coconut: The CPCRI protocol. Indian J Hortic 56:348–353
Karunaratne S (1988) Short term in vitro preservation of coconut seed material: a method to facilitate field collection and transport of coconut germplasm. CORD 4:34–34
Kermode AR, Finch-Savage BE (2002) Desiccation sensitivity in orthodox and recalcitrant seeds in relation to development. In: Black M, Pritchard HW (eds) Desiccation and survival in plants: Drying without dying. CABI Publishing Wallingford, UK, pp 149–184
Khan S, Al-Qurainy F, Nadeem M (2012) Biotechnological approaches for conservation and improvement of rare and endangered plants of Saudi Arabia. Saudi J Biol Sci 19:1–11
Kim H-H, Cha Y-S, Baek H-J, Cho E-G, Chae Y-A, Engelmann F (2002) Cryopreservation of tea (Camellia sinensis L.) seeds and embryonic axes. CryoLetters 23:209–216
Kim HH, Lee HE, Cueto C, Rivera RL, Han BH, Kwon SW, Baek HJ, Park HJ, Rhee JH (2019) Bacterial contamination in cryopreservation of coconut zygotic embryos. Acta Hortic 1234:65–72. https://doi.org/10.17660/ActaHortic.2019.1234.8
Konan EK, Durand-Gasselin T, Kouadio Y, Niamké A, Dumet D, Duval Y, Rival A, Engelmann F (2007) Field development of oil palms (Elaeis guineensis Jacq.) originating from cryopreserved stabilized polyembryonic cultures. CryoLetters 28:377–386
Konan K, Rival A, Kouadio Y, Duval Y, Flori A, Adon B, Pene C, Gasselin T (2006) Assessment of various strategies for the preservation of clonal genetic resources in oil-palm (Elaeis guineensis Jacq.). Agron Afr 18:187–200
Kulus D (2020) Shoot tip cryopreservation of Lamprocapnos spectabilis (L.) Fukuhara using different approaches and evaluation of stability on the molecular, biochemical, and plant architecture levels. Int J Mol Sci 21:3901. https://doi.org/10.3390/ijms21113901
Kushnarenko SV, Romadanova NV, Reed BM (2009) Cold acclimation improves regrowth of cryopreserved apple shoot tips. CryoLetters 30:47–54
Lédo A da S, Jenderek M, Skogerboe D, Staats E, Machado C de A, Oliveira LAR de (2018) Cryopreservation of zygotic embryos of the Brazilian Green Dwarf coconut. Pesq agropec bras 53:514–517. https://doi.org/10.1590/S0100-204X2018000400013
Lédo A da S, Passos EEM, Fontes HR, Ferreira JMS, Talamini V, Vendrame WA (2019) Advances in Coconut palm propagation. Rev Bras Frutic 41:e–159. https://doi.org/10.1590/0100-29452019159
Lédo A da S, Santana FV, Oliveira ACA de, Oliveira LAR de, Silva AVC da (2020) Cryopreservation of Brazilian green dwarf coconut plumules by droplet-vitrification. Cienc Rural 50:e20190020. https://doi.org/10.1590/0103-8478cr20190020
Lei X, Xiao Y, Xia W, Mason AS, Yang Y, Ma Z, Peng M (2014) RNA-seq analysis of oil palm under cold stress reveals a different C-repeat binding factor (CBF) mediated gene expression pattern in Elaeis guineensis compared to other species. PLoS ONE 9:e114482
Li J, Yang Y, Iqbal A, Qadri R, Shi P, Wang Y, Wu Y, Fan H, Wu G (2019) Correlation analysis of cold-related gene expression with physiological and biochemical indicators under cold stress in oil palm. PLoS ONE 14:e0225768
Malaurie B, Tregear J, N’nan O, Bandupriya HDD, Borges M, Verdeil J-L (2011) Cryopreservation as a tool for the management of coconut germplasm. Acta Hortic 908:461–466. https://doi.org/10.17660/ActaHortic.2011.908.59
Martín C, Cervera MT, González-Benito ME (2011) Genetic stability analysis of chrysanthemum (Chrysanthemum x morifolium Ramat) after different stages of an encapsulation–dehydration cryopreservation protocol. J Plant Physiol 168:158–166. https://doi.org/10.1016/j.jplph.2010.06.025
Martín C, Kremer C, González I, González-Benito ME (2015) Influence of the cryopreservation technique, recovery medium and genotype on genetic stability of mint cryopreserved shoot tips. Plant Cell Tiss Org Cult 122:185–195
Martinez-Montero ME, Harding K (2015) Cryobionomics: Evaluating the concept in plant cryopreservation. In: Barh D, Khan MS, Davies E (eds) PlantOmics: The omics of plant science. Springer India, New Delhi, pp 655–682
Mathivanan S (2021) Abiotic stress-induced molecular and physiological changes and adaptive mechanisms in plants. In: Fahad S, Saud S, Chen Y, Wu C, Wang D (eds) Abiotic stress in plants. IntechOpen. https://doi.org/10.5772/intechopen.93367
Mazri MA, Belkoura I, Meziani R, Mokhless B, Nour S (2017) Somatic embryogenesis from bud and leaf explants of date palm (Phoenix dactylifera L.) cv. Najda. 3Biotech 7:58
Mazri MA, Meziani R (2013) An improved method for micropropagation and regeneration of date palm (Phoenix dactylifera L.). J Plant Biochem Biotechnol 22:176–184. https://doi.org/10.1007/s13562-012-0147-9
Metwali EMR, Kadasa NMS, Soliman HIA, Almaghrabi OA, Fuller MP (2020) Cryopreservation of embryogenic callus of date palm (Phoenix dactylifera) cv Magdoul through encapsulation-dehydration technology. Int J Agric Biol 24:1754–1762. https://doi.org/10.17957/IJAB/15.1619
Meziani R, Jaiti F, Mazri MA, Anjarne M, Chitt MA, El Fadile J, Alem C (2015) Effects of plant growth regulators and light intensity on the micropropagation of date palm (Phoenix dactylifera L.) cv. Mejhoul. J Crop Sci Biotechnol 18:325–331
Monteiro T, Freitas E, Nogueira G, Scherwinski-Pereira J (2018) Assessing the influence of subcultures and liquid medium during somatic embryogenesis and plant regeneration in oil palm (Elaeis guineensis Jacq.). J Hortic Sci Biotechnol 93:196–203
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
N’Nan O, Hocher V, Verdeil J-L, Konan J-L, Ballo K, Mondeil F, Malaurie B (2008) Cryopreservation by encapsulation–dehydration of plumules of coconut (Cocos nucifera L.). CryoLetters 29:339–350
Naidoo CD (2012) Oxidative status and stress associated with cryopreservation of germplasm of recalcitrant-seeded species. Master’s thesis, University of KwaZulu-Natal, Durban, South Africa
Nakkanong K, Nualsri C (2018) Cryopreservation of Hevea brasiliensis zygotic embryos by vitrification and encapsulation-dehydration. J Plant Biotechnol 45:333–339. https://doi.org/10.5010/JPB.2018.45.4.333
Nguyen QT, Bandupriya HDD, Foale M, Adkins SW (2016) Biology, propagation and utilization of elite coconut varieties (makapuno and aromatics). Plant Physiol Biochem 109:579–589. https://doi.org/10.1016/j.plaphy.2016.11.003
Nguyen QT, Bandupriya HDD, López-Villalobos A, Sisunandar S, Foale M, Adkins SW (2015) Tissue culture and associated biotechnological interventions for the improvement of coconut (Cocos nucifera L.): a review. Planta 242:1059–1076. https://doi.org/10.1007/s00425-015-2362-9
Niino T, Watanabe K, Nohara N, Rafique T, Yamamoto S, Fukui K, Arizaga MV, Martinez CRC, Matsumoto T, Engelmann F (2014) Cryopreservation of mat rush lateral buds by air dehydration using aluminum cryo-plate. Plant Biotechnol 31:281–287
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1993) Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci 91:67–73
Normah MN, Sulong N, Reed BM (2019) Cryopreservation of shoot tips of recalcitrant and tropical species: Advances and strategies. Cryobiology 87:1–14. https://doi.org/10.1016/j.cryobiol.2019.01.008
Norziha A, Marhalil M, Fadila A, Zulkifli Y, Maizura I, Din MA, Rajanaidu N, Khusairi A (2017) Long-term storage of oil palm germplasm zygotic embryo using cryopreservation. J Oil Palm Res 29:541–547
Oropeza C, Sandoval-Cancino G, Sáenz L, Narváez M, Rodríguez G, Chan JL (2018) Coconut (Cocos nucifera L.) somatic embryogenesis using immature inflorescence explants. In: Jain SM, Gupta P (eds) Step wise protocols for somatic embryogenesis of important woody plants. Springer International Publishing, Cham, pp 103–111
Othmani A, Bayoudh C, Drira N, Marrakchi M, Trifi M (2009) Somatic embryogenesis and plant regeneration in date palm Phoenix dactylifera L., cv. Boufeggous is significantly improved by fine chopping and partial desiccation of embryogenic callus. Plant Cell Tiss Org Cult 97:71–79. https://doi.org/10.1007/s11240-009-9500-7
Palanyandy SR (2013) Establishment of short term and long term storage protocols for oil palm (Elaeis guineensis Jacq.) polyembryoids. Master’s thesis, Universiti Putra Malaysia, Malaysia
Palanyandy SR, Gantait S, Subramaniam S, Sinniah UR (2020) Cryopreservation of oil palm (Elaeis guineensis Jacq.) polyembryoids via encapsulation–desiccation. 3Biotech 10:1–10
Pammenter NW, Berjak P (2014) Physiology of desiccation-sensitive (recalcitrant) seeds and the implications for cryopreservation. Int J Plant Sci 175:21–28. https://doi.org/10.1086/673302
Panis B, Lambardi M (2005) Status of cryopreservation technologies in plants (crops and forest trees). In: International Workshop on “The role of biotechnology for the characterisation and conservation of crop, forestry, animal and fishery genetic resources”. Villia Gualino Turin, Italy, pp 43–54
Panis B, Nagel M (2020) Challenges and prospects for the conservation of crop genetic resources in field genebanks, in in vitro collections and/or in liquid nitrogen. Plants 9:1634
Panis B, Piette B, Andre E, Van den Houwe I, Swennen R (2009) Droplet vitrification: the first generic cryopreservation protocol for organized plant tissues? Acta Hortic 908:157–162. https://doi.org/10.17660/ActaHortic.2011.908.17
Panis B, Swennen R, Engelmann F (2001) Cryopreservation of plant germplasm. Acta Hortic 560:79–86. https://doi.org/10.17660/ActaHortic.2001.560.8
Pareek A, Khurana A, Sharma AK, Kumar R (2017) An overview of signaling regulons during cold stress tolerance in plants. Curr Genomics 18:498–511. https://doi.org/10.2174/1389202918666170228141345
Pegg DE (2007) Principles of Cryopreservation. In: Day JG, Stacey GN (eds) Cryopreservation and Freeze-Drying Protocols. Humana Press, Totowa, NJ, pp 39–57
Perera PI, Hocher V, Verdeil J-L, Bandupriya H, Yakandawala D, Weerakoon LK (2008) Androgenic potential in coconut (Cocos nucifera L.). Plant Cell Tiss Org Cult 92:293–302
Pérez-Núñez M, Chan J, Saenz L, González T, Verdeil J-L, Oropeza C (2006) Improved somatic embryogenesis from Cocos nucifera (L.) plumule explants. In Vitro Cell Dev Biol - Plant 42:37–43
Pinto M de S, Paiva R, Silva DPC da, Santos PAA, Freitas RT de, Silva LC (2016) Cryopreservation of coffee zygotic embryos: dehydration and osmotic rehydration. Ciênc Agrotec 40:380–389. https://doi.org/10.1590/1413-70542016404007616
Prakash K, Kumar K, Chaudhury R (2019) Cryopreservation of kernel and zygotic embryos of oil palm (Elaeis guineensis Jacq). J Plant Crops 47:16–23. https://doi.org/10.25081/jpc.2019.v47.i1.5530
Ree JF, Guerra MP (2021) Exogenous inorganic ions, partial dehydration, and high rewarming temperatures improve peach palm (Bactris gasipaes Kunth) embryogenic cluster post-vitrification regrowth. Plant Cell Tiss Org Cult 144:157–169. https://doi.org/10.1007/s11240-020-01852-z
Ren L, Zhang D, Chen G, Reed BM, Shen X, Chen H (2015) Transcriptomic profiling revealed the regulatory mechanism of Arabidopsis seedlings response to oxidative stress from cryopreservation. Plant Cell Rep 34:2161–2178. https://doi.org/10.1007/s00299-015-1859-9
Rillo EP (1999) Coconut embryo culture. In: Oropeza C, Verdeil JL, Ashburner GR, Cardeña R, Santamaría JM (eds) Current advances in coconut biotechnology. Springer, Netherlands, Dordrecht, pp 279–288
Ritchie H, Roser M (2021) Forests and Deforestation. In: Our World in Data. https://ourworldindata.org/palm-oil. Accessed: 18 Oct 2021
Roque-Borda CA, Kulus D, Vacaro de Souza A, Kaviani B, Vicente EF (2021) Cryopreservation of agronomic plant germplasm using vitrification-based methods: an overview of selected case studies. IJMS 22:6157. https://doi.org/10.3390/ijms22116157
Sáenz L, Chan JL, Narvaez M, Oropeza C (2018) Protocol for the micropropagation of coconut from plumule explants. In: Loyola-Vargas VM, Ochoa-Alejo N (eds) Plant Cell Culture Protocols. Springer, New York, New York, NY, pp 161–170
Sajini K, Karun A, Amarnath C, Engelmann F (2011) Cryopreservation of coconut (Cocos nucifera L.) zygotic embryos by vitrification. CryoLetters 32:317–328
Sakai A, Engelmann F (2007) Vitrification, encapsulation-vitrification and droplet-vitrification: a review. CryoLetters 28:151–172
Sakai A, Hirai D, Niino T (2008) Development of PVS-based vitrification and encapsulation–vitrification protocols. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer, New York, NY, pp 33–57
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Salma M, Engelmann F (2017) Cryopreservation of date palm pro-embryonic masses using the D cryo-plate technique. In: Al-Khayri JM, Jain SM, Johnson DV (eds) Date Palm Biotechnology Protocols, vol II. Springer. New York, NY, pp 25–37
Samosir YMS, Adkins S (2014) Improving acclimatization through the photoautotrophic culture of coconut (Cocos nucifera) seedlings: an in vitro system for the efficient exchange of germplasm. In Vitro Cell Dev Biol - Plant 50:493–501. https://doi.org/10.1007/s11627-014-9599-z
Scottez C, Chevreau E, Godard N, Arnaud Y, Duron M, Dereuddre J (1992) Cryopreservation of cold-acclimated shoot tips of pear in vitro cultures after encapsulation-dehydration. Cryobiology 29:691–700
Senula A, Keller E, Sanduijav T, Yohannes T (2007) Cryopreservation of cold-acclimated mint (Mentha spp.) shoot tips using a simple vitrification protocol. CryoLetters 28:1–12
Sherlock G, Block W, Benson EE (2005) Thermal analysis of the plant encapsulation-dehydration cryopreservation protocol using silica gel as the desiccant. CryoLetters 26:45–54
Sidky R (2017) Optimized direct organogenesis from shoot tip explants of date palm. In: Al-Khayri JM, Jain SM, Johnson DV (eds) Date Palm Biotechnology Protocols, vol I. Springer. New York, NY, pp 37–45
Simpson MG (2010) Diversity and classification of flowering plants: Amborellales, Nymphaeales, Austrobaileyales, Magnoliids, Ceratophyllales, and Monocots. In: Simpson MG (ed) Plant Systematics, 2nd edn. Academic Press, San Diego, pp 181–274
Sisunandar RA, Turquay P, Samosir Y, Adkins SW (2010) Cryopreservation of coconut (Cocos nucifera L.) zygotic embryos does not induce morphological, cytological or molecular changes in recovered seedlings. Planta 232:435–447. https://doi.org/10.1007/s00425-010-1186-x
Sisunandar NH, Mashud N, Samosir YMS, Adkins SW (2014) Embryo maturity plays an important role for the successful cryopreservation of coconut (Cocos nucifera). In Vitro Cell Dev Biol - Plant 50:688–695. https://doi.org/10.1007/s11627-014-9633-1
Sisunandar S, Alkhikmah A, Husin A, Suyadi A (2015) Embryo incision as a new technique for double seedling production of indonesian elite coconut type “kopyor.” J Math Fundam Sci 47:252–260
Sisunandar SPA, Samosir YMS, Rival A, Adkins SW (2010) Dehydration improves cryopreservation of coconut (Cocos nucifera L.). Cryobiology 61:289–296. https://doi.org/10.1016/j.cryobiol.2010.09.007
Sisunandar SPA, Samosir Y, Rival A, Adkins S (2012) Conservation of coconut (Cocos nucifera L.) germplasm at sub-zero temperature. CryoLetters 33:465–475
Solliman ME-D, Al-Khateeb AA, Mohasseb HAA, Aldaej M, Al-Khateeb SA (2019) Towards development of new technique for cryopreservation of date palm (Phoenix dactylifera L.) in vitro grown cultures. Fresenius Environ Bull 28:6256–6263
Spooner D, van Treuren R, de Vicente M (2005) Molecular markers for genebank management. IPGRI Tech Bullet 10:126
Steinmacher DA, Saldanha CW, Clement CR, Guerra MP (2007) Cryopreservation of peach palm zygotic embryos. CryoLetters 28:13–22
Su YH, Tang LP, Zhao XY, Zhang XS (2021) Plant cell totipotency: Insights into cellular reprogramming. J Integr Plant Biol 63:228–243
Subbiah A, Ramdhani S, Pammenter NW, Macdonald AH (2019) Towards understanding the incidence and evolutionary history of seed recalcitrance: an analytical review. Perspect Plant Ecol Evol Syst 37:11–19
Suranthran P, Gantait S, Sinniah UR, Subramaniam S, Alwee SSRS, Roowi SH (2012) Effect of loading and vitrification solutions on survival of cryopreserved oil palm polyembryoids. Plant Growth Regul 66:101–109. https://doi.org/10.1007/s10725-011-9633-7
Teixeira J, Söndahl M, Kirby E (1994) Somatic embryogenesis from immature inflorescences of oil palm. Plant Cell Rep 13:247–250
Towill L, Zaid A, Hughes H (1989) Cryopreservation of date palm shoot tips. In: Abstract ISHS meeting (PS II), pp 91
Uchendu EE, Muminova M, Gupta S, Reed BM (2010) Antioxidant and anti-stress compounds improve regrowth of cryopreserved Rubus shoot tips. In Vitro Cell Dev Biol - Plant 46:386–393
Umarani R, Aadhavan EK, Faisal MM (2015) Understanding poor storage potential of recalcitrant seeds. Curr Sci 108:2023–2034
Uragami A, Sakai A, Nagai M (1990) Cryopreservation of dried axillary buds from plantlets of Asparagus officinalis L. grown in vitro. Plant Cell Rep 9:328–331
Uragami A, Sakai A, Nagai M, Takahashi T (1989) Survival of cultured cells and somatic embryos of Asparagus officinalis cryopreserved by vitrification. Plant Cell Rep 8:418–421
Vineesh P, Skaria R, Mukunthakumar S, Padmesh P, Decruse S (2015) Seed germination and cryostorage of Musa acuminata subsp. burmannica from Western Ghats. S Afr J Bot 100:158–163
Virchow D (2005) In situ conservation: methods and costs. In: Cooper J, Lipper LM, Zilberman D (eds) Agricultural biodiversity and biotechnology in economic development. Springer-Verlag, New York, pp 103–125
Volk G (2010) Application of functional genomics and proteomics to plant cryopreservation. Curr Genomics 11:24–29. https://doi.org/10.2174/138920210790217945
Walters C (2015) Orthodoxy, recalcitrance and in-between: describing variation in seed storage characteristics using threshold responses to water loss. Planta 242:397–406
Wang M-R, Chen L, Zhang Z, Blystad D-R, Wang Q-C (2018) Cryotherapy: a novel method for virus eradication in economically important plant species. In: Loyola-Vargas VM, Ochoa-Alejo N (eds) Plant Cell Culture Protocols. Springer, New York, NY, pp 257–268
Wang M-R, Lambardi M, Engelmann F, Pathirana R, Panis B, Volk GM, Wang Q-C (2021) Advances in cryopreservation of in vitro-derived propagules: technologies and explant sources. Plant Cell Tiss Org Cult 144:7–20. https://doi.org/10.1007/s11240-020-01770-0
Wang Q, Valkonen JP (2009) Cryotherapy of shoot tips: novel pathogen eradication method. Trends Plant Sci 14:119–122
Watanabe K, Yamada Y, Ueno S, Mitsuda H (1985) Change of freezing resistance and retention of metabolic and differentiation potentials in cultured green Lavandula vera cells which survived repeated freeze-thaw procedures. Agric Biol Chem 49:1727–1731
Weckx S, Inzé D, Maene L (2019) Tissue culture of oil palm: finding the balance between mass propagation and somaclonal variation. Front Plant Sci 10:722
Welewanni I, Bandupriya D (2017) Coconut cryopreservation: present status and future prospects. CORD 33:21–21
Welewanni I, Perera C, Jayasekera A, Bandupriya D (2020) Recovery, histological observations and genetic integrity in coconut (Cocos nucifera L.) embryogenic calli cryopreserved using encapsulation-dehydration procedure. J Natl Sci Found Sri Lanka 48:175. https://doi.org/10.4038/jnsfsr.v48i2.9538
Wen B, Cai C, Wang R, Song S, Song J (2012) Cytological and physiological changes in recalcitrant Chinese fan palm (Livistona chinensis) embryos during cryopreservation. Protoplasma 249:323–335. https://doi.org/10.1007/s00709-011-0283-4
Wen B, Song S (2007) Acquisition of cryotolerance in maize embryos during seed development. CryoLetters 28:109–118
Wilms H, De Bièvre D, Longin K, Swennen R, Rhee J, Panis B (2021) Development of the first axillary in vitro shoot multiplication protocol for coconut palms. Sci Rep 11:18367. https://doi.org/10.1038/s41598-021-97718-1
Wilms H, Rhee JH, Rivera RL, Longin K, Panis B (2019) Developing coconut cryopreservation protocols and establishing cryo-genebank at RDA; a collaborative project between RDA and Bioversity International. Acta Hortic 1234:343–348. https://doi.org/10.17660/ActaHortic.2019.1234.45
Wowk B (2010) Thermodynamic aspects of vitrification. Cryobiology 60:11–22
Wu Y, Zhao Y, Engelmann F, Zho M (2001) Cryopreservation of kiwi shoot tips. CryoLetters 22:277–284
Xie H, Konate M, Sai N, Tesfamicael KG, Cavagnaro T, Gilliham M, Breen J, Metcalfe A, Stephen JR, De Bei R (2017) Global DNA methylation patterns can play a role in defining terroir in grapevine (Vitis vinifera cv. Shiraz). Front Plant Sci 8:1860
Yamamoto S, Rafique T, Priyantha WS, Fukui K, Matsumoto T, Niino T (2011) Development of a cryopreservation procedure using aluminium cryo-plates. CryoLetters 32:256–265
Yang Z, Sheng J, Lv K, Ren L, Zhang D (2019) Y2SK2 and SK3 type dehydrins from Agapanthus praecox can improve plant stress tolerance and act as multifunctional protectants. Plant Sci 284:143–160. https://doi.org/10.1016/j.plantsci.2019.03.012
Yarra R, Jin L, Zhao Z, Cao H (2019) Progress in tissue culture and genetic transformation of oil palm: An overview. Int J Mol Sci 20:5353
Zamecnik J, Faltus M, Bilavcik A (2021) Vitrification Solutions for Plant Cryopreservation: Modification and Properties. Plants 10:2623
Zhao C, Zhu J-K (2016) The broad roles of CBF genes: From development to abiotic stress. Plant Signal Behav 11:e1215794–e1215794. https://doi.org/10.1080/15592324.2016.1215794
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was supported by Vietnam National Science and Technology Research Programs, grant number: DTDL.CN-12/19.
Author information
Authors and Affiliations
Contributions
VAN, SWA, NPT, and QTN designed the outline of the paper. VAN composed the manuscript, figures, and tables. MAL and AB contributed to the tables and citations. SS and SK contributed to “Fidelity assessment approaches for cryopreservation in palms” section. AB contributed to “Explant choice” and “Dehydration approaches” sections. SWA, QTN, and SS provided grammatical and style corrections.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nguyen, VA., Nguyen, P.T., Le, MA. et al. A practical framework for the cryopreservation of palm species. In Vitro Cell.Dev.Biol.-Plant 59, 425–445 (2023). https://doi.org/10.1007/s11627-023-10330-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-023-10330-y