Abstract
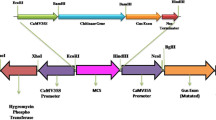
Three antifungal genes, a non-heme chloroperoxidase from Pseudomonas pyrrocinia, and an exochitinase and endochitinase from Fusarium venetanum under regulation by the CaMV 35S promoter, were used to transform Gladiolus for resistance to Fusarium oxysporum f. sp. gladioli. Gladiolus plants were confirmed to be transgenic by Southern hybridization. Semi-quantitative RT-PCR of RNA isolated from leaves and roots demonstrated expression of the Fusarium exochitinase and endochitinase genes in transgenic plants compared to controls. All transgenic plants expressing the Fusarium exochitinase or endochitinase gene had chitinase activity higher than that of the control plants. Semi-quantitative RT-PCR verified that three of the four plant lines with the chloroperoxidase gene expressed the transgene in leaves and roots while no expression was detected in control plants. Western hybridization confirmed the presence of the chloroperoxidase protein in both leaves and roots of transgenic plants. Cell extracts from one endochitinase plant line inhibited growth of germinated F. oxysporum spores more consistently than extracts from the four chloroperoxidase and three endochitinase plant lines. Three chloroperoxidase, two exochitinase, and three endochitinase transgenic plant lines sustained a significantly (P < 0.05) lower density of hyphae on roots compared to roots of non-transformed Gladiolus plants three to four days following exposure of the roots to Fusarium. Shoots from two plant lines, one containing a chloroperoxidase and the other an endochitinase gene, had less necrosis when rated on a scale of 1–3 and appeared visually to be healthier and without obvious Fusarium infection than non-transformed, regenerated Gladiolus plants 17–21 days following exposure to F. oxysporum.










Similar content being viewed by others
References
Chakrabarti A, Ganapathi TR, Mukherjee PK, Bapat VA (2003) MSI-99, a magainin analogue, imparts enhanced disease resistance in transgenic tobacco and banana. Planta 216:587–596
Church GM, Gilbert W (1984) Genome sequencing. Proc Natl Acad Sci USA 81:1991–1995
Cletus J, Balasubramanian V, Vashisht D, Sakthivel N (2013) Transgenic expression of plant chitinases to enhance disease resistance. Biotechnol Lett 35:1719–1732
Collinge DB, Jørgensen HJL, Lund OS, Lyngkjær MF (2010) Engineering pathogen resistance in crop plants: current trends and future prospects. Annu Rev Phytopathol 48:269–291
Dana de las Mercedes M, Pintor-Toro JA, Cubero B (2006) Transgenic tobacco plants overexpressing chitinases of fungal origin show enhanced resistance to biotic and abiotic stress agents. Plant Physiol 142:722–730
Dellaporta S, Wood J, Hicks J (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19–21
Di R, Blechl A, Dill-Macky R, Tortora A, Tumer NE (2010) Expression of a truncated form of yeast ribosomal protein L3 in transgenic wheat improves resistance to Fusarium head blight. Plant Sci 178:374–380
Distefano G, La Malfa S, Vitale A, Lorito M, Deng Z, Gentile A (2008) Defence-related gene expression in transgenic lemon plants producing an antimicrobial Trichoderma harzianum endochitinase during fungal infection. Trans Res 17:873–879
Emani C, Garcia JM, Lopata-Finch E, Pozo MJ, Uribe P, Kim D-J, Sunilkumar G, Cook DR, Kenerley CM, Rathore KS (2003) Enhanced fungal resistance in transgenic cotton expressing an endochitinase gene from Trichoderma virens. Plant Biotechnol J 1:321–336
Gaspar YM, McKenna JA, McGinness BS, Hinch J, Poon S, Connelly AA, Anderson MA, Heath RL (2014) Field resistance to Fusarium oxysporum and Verticillium dahliae in transgenic cotton expressing the plant defensin NaD1. J Exp Bot 65:1541–1550
Gentile A, Deng Z, La Malfa S, Distefano G, Domina F, Vitale A, Polizzi G, Lorito M, Tribulato E (2007) Enhanced resistance to Phoma tracheiphila and Botrytis cinerea in transgenic lemon plants expressing a Trichoderma harzianum chitinase gene. Plant Breed 126:146–151
Ghag SB, Shekhawat UKS, Ganapathi TR (2012) Petunia floral defensins with unique prodomains as novel candidates for development of Fusarium wilt resistance in transgenic banana plants. PLoS ONE 7:e39557. doi:10.1371/journal.pone0039557
Ghag SB, Shekhawat UKS, Ganapathi TR (2014) Native cell-death genes as candidates for developing wilt resistance in transgenic banana plants. AoB Plants 6:plu037. doi:10.1093/aobpla/plu037
Girhepuje PV, Shinde GB (2011) Transgenic tomato plants expressing a wheat endochitinase gene demonstrate enhanced resistance to Fusarium oxysporum f. sp. lycopersici. Plant Cell, Tissue Organ Cult 105:243–251
Han J, Lakshman DK, Galvez LC, Mitra S, Baenziger PS, Mitra A (2012) Transgenic expression of lactoferrin imparts enhanced resistance to head blight of wheat caused by Fusarium graminearum. BMC Plant Biol 12:33. http://www.biomedicentral.com/1471-2229/12/33
Hartl L, Zach S, Seidl-Seiboth V (2012) Fungal chitinases: diversity, mechanistic properties and biotechnological potential. Appl Microbiol Biotechnol 93:533–543
Jacks TJ, de Lucca AJ, Rajasekaran K, Stromberg K, van Pée KH (2000) Antifungal and peroxidative activities of nonheme chloroperoxidase in relation to transgenic plant protection. J Agric Food Chem 48:4561–4564
Jacks TJ, Rajasekaran K, Stromberg KD, de Lucca AJ, van Pée KH (2002) Evaluation of peracid formation as the basis for resistance to infection in plants transformed with haloperoxidase. J Agric Food Chem 50:706–709
Jongedijk E, Tigelaar H, van Roekel JSC, Bres-Vloemans SA, Dekker I, van den Elzen PJM, Cornelissen BJC, Melchers LS (1995) Synergistic activity of chitinases and β-1,3-glucanases enhances fungal resistance in transgenic tomato plants. Euphytica 85:173–180
Kamo K, Lakshman D, Bauchan G, Rajasekaran K, Cary J, Jaynes J (2015) Expression of a synthetic antimicrobial peptide, D4E1, in Gladiolus plants for resistance to Fusarium oxysporum f. sp. gladioli. Plant Cell Tissue Organ Cult 121:459–467
Kumar V, Parkhi V, Kenerley CM, Rathore KS (2009) Defense-related gene expression and enzyme activities in transgenic cotton plants expressing an endochitinase gene from Trichoderma virens in response to interaction with Rhizoctonia solani. Planta 230:277–291
Kumar DP, Singh RK, Anupama PD, Solanki MK, Kumar S, Srivastava AK, Singhal PD, Arora DK (2011) Studies on exo-chitinase production from Trichoderma asperellum UTP-16 and its characterization. Ind J Microbiol 52:388–395
Larone DH (1995) Medically important fungi, a guide to identification, 3rd edn. American Society for Microbiology Press, Washington
Li WL, Faris JD, Muthukrishnan S, Liu DJ, Chen PD, Gill BS (2001) Isolation and characterization of novel cDNA clones of acidic chitinases and β-1,3-glucanases from wheat spikes infected by Fusarium graminearum. Theor Appl Genet 102:353–362
Li HP, Zhang JB, Shi RP, Huang T, Fischer R, Liao YC (2008) Engineering Fusarium head blight resistance in wheat by expression of a fusion protein containing a Fusarium-specific antibody and an antifungal peptide. Mol Plant Microbe Interact 21:1242–1248
Li Z, Zhou M, Zhang Z, Ren L, Du L, Zhang B, Xu H, Xin Z (2011) Expression of a radish defensing in transgenic wheat confers increased resistance to Fusarium graminearum and Rhizoctonia cerealis. Funct Integr Genomics 11:63–70
Lorito M, Woo SL, Fernandez IG, Colucci G, Harman GE, Pintor-Toro JA, Filippone E, Muccifora S, Lawrence CB, Zoina A, Tuzun S, Scala F (1998) Genes from mycoparasitic fungi as a source for improving plant resistance to fungal pathogens. Proc Natl Acad Sci USA 95:7860–7865
Makandar R, Essig JS, Schapaugh MA, Trick HN, Shah J (2006) Genetically engineered resistance to Fusarium head blight in wheat by expression of Arabidopsis NPR1. MPMI 19:123–129
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 368–369
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Niu C, Akasaka-Kennedy Y, Faustinelli P, Joshi M, Rajasekaran K, Yang H, Chu Y, Cary J, Ozias-Akins P (2009) Antifungal activity in transgenic peanut (Arachis hypogaea L.) conferred by a nonheme chloroperoxidase gene. Peanut Sci 36:126–132
Ntui VO, Thirukkumaran G, Azadi P, Khan RS, Nakamura I, Mii M (2010) Stable integration and expression of wasabi defensing gene in “Egusi’ melon (Colocynthis citrullus L.) confers resistance to Fusarium wilt and Alternaria leaf spot. Plant Cell Rep 29:943–954
Okubara PA, Dickman MB, Blechl AE (2014) Molecular and genetic aspects of controlling the soilborne necrotrophic pathogens Rhizoctonia and Pythium. Plant Sci 228:61–70
Paul J-Y, Becker DK, Dickman MB, Harding RM, Khanna HK, Dale JL (2011) Apoptosis-related genes confer resistance to Fusarium wilt in transgenic ‘Lady Finger’ bananas. Plant Biotech J 9:1141–1148
Popowich EA, Firsov AP, Mitiouchkina TY, Filipenya VL, Dolgov SV, Reshetnikov VN (2007) Agrobacterium-mediated transformation of Hyacinthus orientalis with thaumatin II gene to control fungal diseases. Plant Cell, Tissue Organ Cult 90:237–244
Rajasekaran K, Cary JW, Jacks TJ, Stromberg KD, Cleveland TE (2000) Inhibition of fungal growth in planta and in vitro by transgenic tobacco expressing a bacterial nonheme chloroperoxidase gene. Plant Cell Rep 19:333–338
Ruhlman TA, Rajasekaran K, Cary JW (2014) Expression of chloroperoxidase from Pseudomonas pyrrocinia in tobacco plastids for fungal resistance. Plant Sci 228:98–106
Sanford J, Smith FD, Russell JA (1993) Optimizing the biolistic process for different biological applications. Methods Enzymol 217:483–510
Shin S, Mackintosh CA, Lewis J, Heinen SJ, Radmer L, Dill-Macky R, Baldridge GD, Zeyen RJ, Muehlbauer GJ (2008) Transgenic wheat expressing a barley class II chitinase gene has enhanced resistance against Fusarium graminearum. J Exp Bot 59:2371–2378
Somssich IE, Bollmann J, Hahlbrock K, Kombrink E, Schulz W (1989) Differential early activation of defense-related genes in elicitor-treated parsley cells. Plant Mol Biol 12:227–234
van Pée K-H (1996) Biosynthesis of halogenated metabolites by bacteria. Ann Rev Microbiol 50:375–399
Volpi C, Janni M, Lionetti V, Bellincampi D, Favaron F, D’Ovidio R (2011) The ectopic expression of a pectin methyl esterase inhibitor increases pectin methyl esterification and limits fungal diseases in wheat. MPMI 24:1012–1019
Wiesner W, van Pee K-H, Lingens F (1986) Detection of a new chloroperoxidase in Pseudomonas pyrrocinia. FEBS Lett 209:321–324
Wilfret GJ (1992) Gladiolus. In: Larson RA (ed) Introduction to floriculture. Academic Press, San Diego, pp 143–157
Wolfgang W, van Pee K-H, Lingens F (1986) Detection of a new chloroperoxidase in Pseudomonas pyrrocinia. FEBS 209:321–324
Wolframm C, Lingens F, Mutzel R, van Pée K-H (1993) Chloroperoxidase-encoding gene from Pseudomonas pyrrocinia: sequence expression in heterologous hosts, and purification of the enzyme. Gene 130:131–135
Wu L, Zhang H, Zhang J, Wu L, Xi Z, Chen Y (2015) Overexpression of Zm-HINT1 in Arabidopsis thaliana enhances resistance to Fusarium graminearum. Plant Cell, Tissue Organ Cult 121:511–518
Acknowledgments
Mention of a trademark, proprietary product or vendor does not imply its approval to the exclusion of other products or vendors that may also be suitable. Siobhan O’Connor provided excellent technical assistance with the DNA blot and RNA analysis and Sarah Wantoch with the Western blot.
Author’s contributions
KK developed the study, wrote the manuscript and did the antifungal challenge experiments with DL and RP. MAG and RJ did the Western blot. PO provided the two chitinase genes. KR and JC provided the CPO gene that JC had subcloned for bombardment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interest with this research.
Rights and permissions
About this article
Cite this article
Kamo, K., Lakshman, D., Pandey, R. et al. Resistance to Fusarium oxysporum f. sp. gladioli in transgenic Gladiolus plants expressing either a bacterial chloroperoxidase or fungal chitinase genes. Plant Cell Tiss Organ Cult 124, 541–553 (2016). https://doi.org/10.1007/s11240-015-0913-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0913-1