Abstract
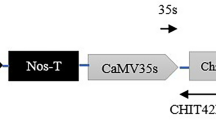
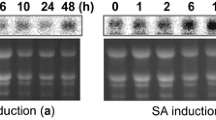
Various chitinases have been shown to inhibit the growth of fungal pathogens in in vitro as well as in planta conditions. chi194, a wheat chitinases gene encoding a 33-kDa chitinase protein, was overexpressed in tomato plants (cv. Pusa Ruby) under the control of maize ubiquitin 1 promoter. The integration of transgene in tomato plants was confirmed with polymerase chain reaction (PCR) and Southern blot analysis. The inheritance of the transgene in T1 and T2 generations were shown by molecular analysis and the hygromycin sensitivity test. The broad range of chitinase activity was observed among the transgenic lines in T0 and a similar range was retained in the T1 and T2 generations. Most importantly, the transgenic tomato lines with high chitinase activity were found to be highly resistant to the fungal pathogen Fusarium oxysporum f. sp. lycopersici. Thus, the results demonstrated that the expression of the wheat endochitinase chi194 in tomato plants confers resistance against Fusarium wilt disease caused by the fungal pathogen Fusarium oxysporum f. sp. lycopersici.






Similar content being viewed by others
References
Afroz A, Chaudhry Z, Rashid U, Muhammad Ali G, Nazir F, Iqbal J, Khan MR (2010) Enhanced resistance against bacterial wilt in transgenic tomato (Lycopersicon esculentum) lines expressing the Xa21 gene. Plant Cell Tissue Organ Cult. doi:10.1007/s11240-010-9825-2
Bartnicki-Garcia S (1968) Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol 22:87–108
Bhargava A, Osusky M, Forward BS, Hancock RE, Kay WW, Misra S (2007) Expression of a polyphemusin variant in transgenic tobacco confers resistance against plant pathogenic bacteria, fungi and a virus. Plant Cell Tissue Organ Cult 88(3):301–312
Boller T, Gehri A, Mauch F, Vögeli U (1983) Chitinase in bean leaves: induction by ethylene, purification, properties, and possible function. Planta 157:22–31
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brogue K, Chet I, Holliday M, Cressman R, Biddle P, Knowlton S, Mauvais CJ, Broglie R (1991) Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science 254(5035):1194–1197
Chang CT, Lo HF, Wu CJ, Sung HY (1992) Purification and properties of chitinase from cabbage. Biochem Int 28:707–715
Chen SC, Liu AR, Wang FH, Ahammed GJ (2009) Combined overexpression of chitinase and defensin genes in transgenic tomato enhances resistance to Botrytis cinerea. Afr J Biotechnol 8(20):5182–5188
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19–21
Fiocchetti F, D’Amore R, De Palma M, Bertini L, Caruso C, Caporale C, Testa A, Cristinzio G, Saccardo F, Tucci M (2008) Constitutive over-expression of two wheat pathogenesis-related genes enhances resistance of tobacco plants to Phytophthora nicotianae. Plant Cell Tissue Organ Cult 92:73–84
Ganesan M, Bhanumathi P, Ganesh Kumari K, Lakshmi Prabha A, Song P-S, Jayabalan N (2009) Transgenic Indian cotton (Gossypium hirsutum) harboring rice chitinase gene (Chi II) confers resistance to two fungal pathogens. Am J Plant Biochem Biotechnol 5(2):63–74
Gaynor JJ (1988) Primary structure of an endochitinase mRNA from Solanum tuberosum. Nucleic Acids Res 16:5210
Gleason ML, Edmunds BA (2006) Tomato diseases and disorders. Available online at: http://www.extension.iastate.edu/Publications/PM1266.pdf
Hellens R, Mullineaux P, Klee H (2000) Technical focus: a guide to Agrobacterium binary Ti vectors. Trends Plant Sci 5:446–451
Huang JK, Wen L, Swegle M, Tran HC, Thin TH, Naylor HM, Muthukrishnan S, Reeck GR (1991) Nucleotide sequence of a rice genomic clone that encodes a class I endochitinase. Plant Mol Biol 16:479–480
Jones JB Jr (1999) Tomato plant culture: in the field, greenhouse, and home garden. CRC Press, Boca Raton, pp 136
Jongedijk E, Tigelaar H, van Roekel JSC, Bres-Vloemans SA, Dekker I, van den Elzen PJM, Cornelissen BJC, Melchers LS (1995) Synergistic activity of chitinases and beta-1,3-glucanases enhances fungal resistance in transgenic tomato plants. Euphytica 85:173–180
Kumar V, Parkhi V, Kenerley CM, Rathore KS (2009) Defense-related gene expression and enzyme activities in transgenic cotton plants expressing an endochitinase gene from Trichoderma virens in response to interaction with Rhizoctonia solani. Planta 230(2):277–291
Kurosaki F, Tashiro N, Nishi A (1987) Secretion of chitinase from cultured carrot cells treated with fungal mycelial walls. Physiol Mol Plant Pathol 31:211–216
Lan HY, Tian YC, Wang CH, Liu GZ, Zhang LH, Wang LL, Chen ZH (2000) Studies of transgenic tobacco plants expressing beta-1,3-glucanase and chitinase genes and their potential for fungal resistance. Acta Genetica Sinica 27:70–77
Leah R, Tommerup H, Svendsen I, Mundy J (1991) Biochemical and molecular characterization of three barley seed proteins with antifungal properties. J Biol Chem 226:1564–1573
Lin ZF, Wu D, Luo A, Zhang W (1992) Chitinases from seeds of Zea mays and Coix lachryma-jobi L. purification and some properties. Process Biochem 27:83–88
Marchant R, Davey MR, Lucas JA, Lamb CJ, Dixon RA, Power JB (1998) Expression of a chitinase transgene in rose (Rosa hybrida L.) reduces development of blackspot disease (Diplocarpon rosae Wolf). Mol Breed 4(3):187–194
Mauch F, Hadwiger LA, Boller T (1984) Ethylene: symptom, not signal for the induction of chitinase and β-1,3-glucanase in pea pods by pathogens and elicitors. Plant Physiol 76:607–611
Molano J, Polacheck I, Duran A, Cabib E (1979) An endochitinase from wheat germ. Activity on nascent and preformed chitin. J Biol Chem 254:4901–4907
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Neuhaus JM, Sticher L, Meins F Jr, Boller T (1991) A short C-terminal sequence is necessary and sufficient for the targeting of chitinases to the plant vacuole. Proc Natl Acad Sci USA 88:10362–10366
Ouyang B, Li HX, Ye ZB (2003) Increased resistance to Fusarium wilt in transgenic tomato expressing bivalent hydrolytic enzymes. J Plant Physiol Mol Biol 29:179–184
Ouyang B, Chen YH, Li HX, Qian CJ, Huang SL, Ye ZB (2005) Transformation of tomatoes with osmotin and chitinase genes and their resistance to Fusarium wilt. J Horticult Sci Biotechnol 80(5):517–522
Punja ZK (2001) Genetic engineering of plants to enhance resistance to fungal pathogens—a review of progress and future prospects. Can J Plant Pathol 23:216–235
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York, pp 6.39–6.64
Sarowar S, Kim YJ, Kim EN, Kim KD, Choi JY, Hyung NI, Shin JS (2006) Constitutive expression of two pathogenesis-related genes in tomato plants enhanced resistance to oomycete pathogen Phytophthora capsic. Plant Cell Tissue Organ Cult 86(1):7–14
Shimahara K, Takiguchi Y (1988) Preparation of crustacean chitin. Methods Enzymol 161:417–423
Shin S, Mackintosh CA, Lewis J, Heinen SJ, Radmer L, Dill-Macky R, Baldridge GD, Zeyen RJ, Muehlbauer GJ (2008) Transgenic wheat expressing a barley class II chitinase gene has enhanced resistance against Fusarium graminearum. J Exp Bot 59(9):2371–2378
Singh A, Kirubakaran SI, Sakthivel N (2007) Heterologous expression of new antifungal chitinase from wheat. Protein Expr Purif 56:100–109
Swegle M, Huang J-K, Lee G, Muthukrishnan S (1989) Identification of an endochitinase cDNA clone from barley aleurone cells. Plant Mol Biol 12:403–412
Tohidfar M, Mohammadi M, Ghareyazie B (2005) Agrobacterium-mediated transformation of cotton (Gossypium hirsutum) using a heterologous bean chitinase gene. Plant Cell Tiss Organ Cult 83(1):83–96
Tsuyoshi T, Koga D, Ide A, Ishibashi T, Horino-Matsushige M, Yagishita K, Imoto T (1984) Purification and some properties of chitinases from yam, Dioscorea opposita Thumb. Agric Biol Chem 48:931–939
Veluthambi K, Gupta AK, Sharma A (2003) The current status of plant transformation technologies. Curr Sci 84(3):368–380
Wadsworth SA, Zikakis JP (1984) Chitinase from soybean seeds: purification and some properties of the enzyme system. J Agric Food Chem 32:1284–1288
Wessels JGH, Sietsma JH (1981) Fungal cell walls: a survey. In: Tanner W, Loewus FA (eds) Encyclopedia of plant physiology, new series, vol 13B: plant carbohydrates II. Springer, Berlin, pp 352–394
Yamagami T, Funatsu G (1993) Purification and some properties of three chitinases from the seeds of rye (Secale cereale). Biosci Biotechnol Biochem 57:643–647
Zhu Q, Lamb CJ (1991) Isolation and characterization of a rice gene encoding a basic chitinase. Mol Gen Genet 226:289–296
Acknowledgments
We thank Prof. N. Sakthivel, Department of Biotechnology, Pondicherry University, India, and Dr. S. Muthukrishnan, Department of Biochemistry, Kansas State University, USA, for kindly providing the plasmids for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Girhepuje, P.V., Shinde, G.B. Transgenic tomato plants expressing a wheat endochitinase gene demonstrate enhanced resistance to Fusarium oxysporum f. sp. lycopersici . Plant Cell Tiss Organ Cult 105, 243–251 (2011). https://doi.org/10.1007/s11240-010-9859-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9859-5