Abstract
This study estimated the genotype × environment interactions for ten yield associated traits in advanced generation hybrids of several cultivars of common wheat (Triticum aestivum L.) with Aegilops kotschyi Boiss. and A. variabilis Eig. using the additive main effects and multiplicative interaction (AMMI) models. Tests were ran over five years at one location in replicated field trials. The AMMI model showed significant genotypic and environmental effects for all analysed traits. A majority of the hybrid lines were less stable in the analysed traits than their parental wheats. The older wheat cultivars, with lower environmental sensitivity, were the most stable. The best total genotype selection index, for all ten traits combined, was observed for the oldest cvs. Gama and Rusałka, and among the hybrid lines, for Ae. kotschyi/Rusałka//Smuga and Ae. kotschyi/Rusałka//Muza. The lines Ae. kotschyi/Rusałka//Smuga, Ae. kotschyi/Rusałka//Muza, Ae. kotschyi/Rusałka//Korweta, Ae. kotschyi/Rusałka//Begra///Smuga, and Ae. kotschyi/Rusałka//Begra///Turnia are recommended for inclusion in breeding programmes due to their greater stability and the good average values for the observed traits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global climate is warming. Droughts and high temperatures are the most important limiting factors for crop production in the world (Bansal and Sinha 1991). Yield reduction in wheat under heat stress can be caused by accelerated phasic development (Warrington et al. 1977), increase in respiration (Berry and Bjorkman 1980), reduction in photosynthesis (Blum 1986) and inhibition of starch synthesis in developing kernels (Jenner 1994). Common wheat (Triticum aestivum L.) is one of the most important cereals, with an estimated cultivation area of about 200 million ha (Ortiz et al. 2008). With looming negative climate change impacts on crop productivity, there is a need for biological advances in introduction of new cultivars—more productive and with more stable yield in changing climate conditions.
The economic value of common wheat is determined by a number of factors, with the most important ones being grain yield, crop quality, and yield stability. Fertility, i.e. the capacity to produce large grain yields, is a complex quantitative trait controlled by a vast number of genes, but breeders know only the role of simple traits, morphological, physiological and anatomical. How these traits are inherited is known (Slafer and Andrade 1993; Kato et al. 1998; Peng et al. 1998; Araki et al. 1999; Keller et al. 1999; Ahmed et al. 2000; Varshney et al. 2000; Li et al. 2002; Hai et al. 2005; Gupta et al. 2006; Houshmand et al. 2007; Kumar et al. 2007). The task of a breeder developing a new cultivar is to combine the largest possible number of alleles contributing to high yield. The more alleles with positive effect on yield a cultivar has, the larger the yield will be. This means it reacts less strongly to environmental changes, producing a similar yield in different growing seasons (Bedő and Láng 2015). Main spike seed set a trait with low heritability, which means that yield levels are significantly influenced by the environment. In common wheat, as in other cereals, the grain yield per land area unit depends mainly on the number of productive tillers, grain number per spike, and grain weight (Novoselovic et al. 2004).
To enrich the common wheat genepool with new, beneficial traits, it is often hybridized with related species, such as the Aegilops species which grow naturally in various parts of Asia (Kimber and Feldman 1987), Molnár-Láng et al. 2015). Such hybrids have resulted in numerous wheat-Aegilops introgression lines (with improved tolerance to diseases, nematodes, and insects, as well as to high temperatures and drought (Shimshi et al. 1982; Gorham 1990; Waines 1994; Spetsov et al. 1997; Thiele et al. 2002; Marais et al. 2005; Petersen et al. 2006; Schneider et al. 2007; Coriton et al. 2009).
The need for continuous research on new forms of wheat and the assessment of their practical value is an integral element of wheat breeding. One means of achieving this goal is to learn the relationships among individual yield-affecting traits. Quantitative traits are determined not only by genes but also by environmental factors. Hence, statistical methods are useful in evaluating these relationships. Numerous statistical methods have been developed and used to analyse the genotype-environment interactions (Becker and Léon 1988; Singh et al. 1999; Brancourt-Hulmel et al. 2003; Rharrabti et al. 2003; Mohammadi and Amri 2013; Cociu 2018). These methods have helped plant breeders to assess the stability of agronomically important traits and to predict the yield capacity of new genotypes in different environmental conditions. The fact that genotypes react differently in different environments and in different years is regarded as one of the main factors limiting breeding progress, and thus crop production (Esuma et al. 2016; Cuevas et al. 2017). Different reactions in different environments or years make predictions of future yields difficult or impossible (Yan and Kang 2002; Brancourt-Hulmel et al. 2003; Hageman et al. 2012).
The additive main effects and multiplicative interaction (AMMI) model (Zobel et al. 1988; Gauch and Zobel 1990) has been used extensively for analyses of multi-environment yield trials in order to understand complex genotype (G), environment/year (E/Y) and genotype-by-year interactions (GYI). The AMMI model has commonly been used to evaluate the genotype × environment interactions (Ghaed-Rahimi et al. 2015; Golkari et al. 2016; Paderewski et al. 2016; Shahriari 2018; Rodrigues and Paderewski 2018; Bocianowski et al. 2019b, 2020; Singh et al. 2019).
The aim of this paper was to assess the genotype-by-year interactions (GYI) for ten quantitative grain yield-affecting traits in a set of wheat-Aegilops introgression lines and their parents, using the AMMI model. The traits were: productive tillering, the length of the main tiller, the diameter of the second internode from the base, the length of spike rachis, the spikelet number per main spike, main spike density (spikelet number per 10 cm of the spike rachis length), the grain number per main spike, grain weight per main spike, main spike seed set (grain number per spikelet), and the thousand grain weight, in hybrids of Ae. kotschyi Boiss. and Ae. variabilis Eig. with Triticum aestivum L. field tested over a period of five years (2010–2014).
Material and methods
Plant material
This study involved 16 hybrid lines of Triticum aestivum L. (AABBDD) with Aegilops kotschyi Boiss. (genomic composition UUSS) and Aegilops variabilis Eig. (UUSS). The wheat cultivars used were Begra, Gama, Korweta, Monopoly, Muza, Piko, Rusałka, Smuga, Turnia and Zyta; original crosses were made in either direction, resulting in some alloplasmic lines (wheat lines with the cytoplasm of Aegilops) (Table 1).
The derivation of the materials has been described before (Prażak et al. 2017; Prażak and Molas 2017; Prażak and Krzepiłko 2018). In this study the lines were in generations F10–14 and BC1F6-10. In early generations of line development strong selection was applied for disease resistance, straw stiffness and plant height. The materials were analysed for yield and quality associated traits: plant height, lodging resistance, spike length, weight of grain from the main spike, the thousand grain weight, grain protein and micronutrients content, and resistance to leaf rust.
Field experiments
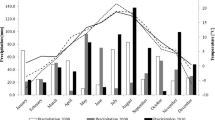
The field study was carried out over a period of five years, from 2010 to 2014, in an experimental field of University of Life Sciences in Lublin, Poland, located in Zamość (50°42′36.7″ N, 23°12′47.3″ E) in a randomized complete block design, with two replicates. Plots were sown in a single day between Sept 21 and 26 in different years. The plots were 2.0 × 1.0 m, with 20 × 10 cm row/plant spacing (100 seeds per plot). Soil was brown classified as good wheat complex and standard recommended cultivation and fertilization practices were applied (NPK = 90–60–90 kg/ha). In each year, seeds for nest year’s experiment were multiplied on separate, adjacent plots, ensuring purity. For laboratory evaluation, plants were collected from the field at the beginning of August, when fully ripe, and ten quantitative characteristics were analysed in ten plants from each replicate: productive tillering, the main tiller length (cm), the diameter of the second internode from the base (cm), the spike rachis length (cm), the spikelet number per main spike, the main spike density (spikelet number per 10 cm of the spike rachis length), the grain number per main spike, grain weight per main spike (g), grain number per spikelet of the main spike, and the thousand grain weight (g), following the Methodological instructions for conducting varietal experiments, 1978, COBORU, Słupia Wielka). Weather data (Table 2) were obtained from the Meteorological Station in Zamość, Płoskie 1 (50°42′36.7″ N, 23°12′47.3″ E).
Statistical analysis
Variances and covariances among observed traits were tested. The relationships between the observed traits were estimated using the Pearson’s correlation coefficients on the basis of mean values for genotypes. The data were analysed using the additive main effects and multiplicative interaction (AMMI) model (Gauch and Zobel 1990), for each trait independently. The AMMI model first fits the additive effects for the main effects of genotypes (G) and years (Y), followed by multiplicative effects for GYI by PCA. The results of the AMMI analysis are presented as biplot graphs. The AMMI model (Nowosad et al. 2016) is expressed by the following formula:
where yge is the trait mean of a genotype g in year e, μ is the grand mean, αg is the mean genotype deviation, βe is the mean year deviation, N is the number of PCA axes retained in the adjusted model, λn is the eigenvalue of the PCA axis n, γgn is the genotype score for the PCA axis n, δen is the score eigenvector for the PCA axis n, and Qge is the residual, including the AMMI noise and pooled experimental error. The AMMI stability value (ASV) was used to compare the stability of genotypes as described by Purchase et al. (2000):
where SSIPCA1 is the sum of squares for IPCA1, SSIPCA2 is the sum of squares for IPCA2, and the IPCA1 and IPCA2 scores are the genotype scores in the AMMI model. A lower ASV score indicates a more stable genotype across years (Nowosad et al. 2017).
The genotype selection index (GSI), calculated for each genotype, incorporates both the trait mean and the ASV index in a single criterion (GSIi), as follows (Farshadfar and Sutka 2003):
where RMi is the rank of the trait mean [from maximum to minimum for productive tillering, the diameter of the second internode from the base, the length of spike rachis, the spikelet number per main spike, main spike density, the grain number per main spike, grain weight per main spike, main spike seed set (grain number per spikelet) and the thousand grain weight, and from minimum to maximum for the length of the main tiller] for i-th genotype, and RAi is the rank of the ASV for the i-th genotype. Finally, the total genotype selection index (TGSI) was calculated for each genotype as the sum of the GSIs for all ten traits.
All analyses were conducted using the GenStat v. 18 statistics software.
Results
Variances, covariances and correlations
Variances and covariances between observed traits are presented in Table 3. The variance of the traits ranged from 0.05 (for the spike rachis length) to 458.03 (for the main tiller length) (Table 3). Covariances between productive tillering and other traits were negative and ranged from − 123.09 (the main tiller length) to − 0.8 (grain number per spikelet of the main spike). Other covariances were positive and ranged from 0.04 (between the spike rachis length and the grain number per spikelet in main spike) to 191.45 (between the main tiller length and the grain number per main spike). All pairs of observed traits were correlated, except for the grain number per spikelet of main spike with: productive tillering, the spikelet number per main spike, main spike density (spikelet number per 10 cm of the spike rachis length) and the grain weight per main spike (Table 3).
Analysis of variance
The three sources of variation (genotype, year, and the G × Y interaction) were highly significant for all ten traits (Table 4) of wheat. In the ANOVA, the sum of squares for the main genotype effect ranged from 20.69% (for the main spike seed set) to 87.77% (for the spikelet number per main spike) of the total trait variation, and this factor had the highest effect on all observed traits except the main spike seed set (Table 4). The differences between years explained from 0.62% (for the spikelet number per main spike) to 16.81% (for grain weight per main spike) of the total trait variation, while GYI explained from 5.09% (for the spikelet number per main spike) to 25.97% (for main spike seed set) (Table 4). Values for the first three principal components were also significant (at the 0.001 level) for all observed traits, together representing from 64.05% (for the diameter of the second internode from the base) to 85.46% (for the main spike density) of the entire effect (Table 4). The first principal component (IPCA 1) accounted for from 38.54% (for grain weight per main spike) to 72.76% (for the main spike density) of variation caused by the interaction (Table 4).
Productive tillering
The numbers of productive tillers per plant ranged from 3.2 (for VR5 in 2012) to 71.0 (for Ae. variabilis in 2013), with the average of 15.21 (Table S1). Ae. variabilis had the highest average productive tillering (52.56), while cv. Muza had the lowest (10.22). The average productive tillering in individual years ranged from 12.45 in 2011 to 17.30 in 2013 (Table S1). Ae. variabilis adapted in environments with similar conditions to these in 2013, and not adapted in 2011 (Fig. S1). VR5 adapted in environments with similar conditions to these in 2014, and not adapted in 2012. KRKo4 and KRMu4 were the most stable, with the ASV of 0.283 and 0.443, respectively, while Ae. variabilis (8.269) and Ae. kotschyi (7.300) were the least stable (Table S1). Cvs. Piko and Monopoly, with high average productive tillering (13.66 and 13.90, respectively) and the ASV equal to 0.450 and 1.050, respectively, had the best genotype selection indices (11 and 14, respectively).
The main tiller length
The length of the main tiller ranged from 17.40 cm (for Ae. kotschyi in 2010) to 123.10 cm (for KRT4 in 2011) over the the five years, with the average of 89.78 cm (Table S2). The average the length of the main tiller was the highest in KRT4 (117.18 cm) and the lowest in Ae. kotschyi (27.54 cm). The average the main tiller length in individual years ranged from 86.99 cm in 2010 to 92.79 cm in 2021. KRP4 adapted in environments with similar conditions to these in years 2012 and 2014, and not adapted in 2010 and 2011 (Fig. S2). The KR4 and KRBP4 adapted in environments with similar conditions to these in 2013, and not adapted in 2011 and 2014. Cv. Zyta and alloplasmic introgression line KRBS4, with the ASV of 0.224 and 0.279, respectively, were the most stable, while KRMo4 (4.467) and KRP4 (3.303) were the least stable (Table S2). RK4, with the average the main tiller length ranging from 66.9 to 76.6 cm and the ASV of 0.374, had the best genotype selection index (6).
The diameter of the second internode from the base
The diameter of the second internode from the base ranged from 0.036 cm (Ae. kotschyi in 2012) to 0.508 cm (Begra in 2013) over the five years, with the average of 0.339 cm (Table S3). The average the diameter of the second internode from the base was highest in KRBT4 (0.414 cm) and lowest in Ae. kotschyi (0.073 cm). The averages individual years ranged from 0.326 cm in 2010 to 0.362 cm in 2011. The stability of parental cultivars as well as euplasmic and alloplasmic introgression lines is illustrated on Fig. S3. Ae. kotschyi, KRZ4 and KRP4 adapted in environments with similar conditions to these in 2014, and not adapted in 2011 (Fig. S3). Begra, KRG6, KRMu4 and KRS4 adapted in environments with similar conditions to these in 2013, and not adapted in 2010. Cv. Monopoly and Ae. variabilis, with the ASV of 0.132 and 0.174, respectively, were the most stable, cv. Begra (1.547) and euplasmic introgression line RV5 (0.853) were the least stable (Table S3). Cv. Monopoly with the highest average diameter (0.388 cm) and the ASV equal to 0.132, had the best genotype selection index (7) (Table S3).
The length of spike rachis
The length of the spike rachis ranged from 2.37 cm (for Ae. kotschyi in 2013) to 13.8 cm (for KRBT4 in 2010) over the five years, with the average of 8.839 cm (Table S4). It was highest in KRBT4 (12.236 cm) and the lowest in Ae. kotschyi (3.264 cm). The averages in individual years ranged from 8.156 cm in 2011 to 9.454 cm in 2014. The stability of genotypes can be evaluated on the biplot (Fig. S4). KRZ4, KR4 and RV5 adapted in environments with similar conditions to these in years 2012 and 2013, and not adapted in 2010 (Fig. S4). KRP4, RK4 and VR5 adapted in environments with similar conditions to these in 2014, and not adapted in 2011. Alloplasmic introgression lines KRBS4, KRMo4 and KRS4, with the ASV of 0.017, 0.025 and 0.030, respectively, were the most stable, while KRB4 (0.696) and KRP4 (0.661) were the least stable (Table S4). Cv. Turnia, with a high average spike rachis length (11.186 cm) and the ASV of 0.043, had the best genotype selection index (7).
The spikelet number per main spike
The spikelet number per main spike ranged from 2.7 (for Ae. kotschyi in 2017) to 23.2 (for KRG6 in 2010) over the five years, with the average of 18.05 (Table S5). Cv. Monopoly had the highest average the spikelet number (22), while Ae. variabilis had the lowest (3). The average number in individual years ranged from 17.67 in 2012 to 18.59 in 2014. The stability of the genotypes can be evaluated on the biplot (Fig. S5). Alloplasmic introgression lines VR5, KRG6 and KRMu4 adapted in environments with similar conditions to these in years 2010 and 2014, and not adapted in 2013 (Fig. S5). KR4 adapted in environments with similar conditions to these in 2011, and not adapted in 2012. Cv. Rusałka, with the ASV of 0.068, was the most stable, while introgression lines RV5 (5.402) and VR5 (3.412) were the least stable (Table S5). Cv. Korweta, with the average the spikelet number per main spike of 20.24 and a high ASV (0.249), had the best genotype selection index (11), while introgression lines VR5 and RV5 had the worst genotype selection index (50).
Main spike density
The main spike density of the genotypes ranged from 5.44 (for Ae. variabilis in 2013) to 30.83 (for RV5 in 2011) over the five years, with the average of 19.08 (Table S6). Alloplasmic introgression line KRBP4 had the highest average density (25.62), and Ae. variabilis had the lowest (6.04). The averages ranged in individual years from 18.35 in 2014 to 20.51 in 2011. The stability of the character is presented on the biplot, Fig. S6. Among the introgression lines KR4 and KRZ4 adapted in environments with similar conditions to these in years 2010 and 2011, not adapted in 2013 and 2014 (Fig. S6). Ae. variabilis was the most stable, with the ASV of 0.260, while introgression lines RV5 (15.139) and KR4 (9.528) were the least stable (Table S6). Alloplasmic introgression line KRBP4, with the highest average main spike density (25.62) and the good ASV (0.779), had the best genotype selection index (5), while alloplasmic introgression line KR4 had the worst genotype selection index (50).
The grain number per main spike
The grain number per main spike ranged from 2.6 (for Ae. kotschyi in 2012) to 60.5 (for KRBS4 in 2013) over the five years, with the average of 35.7 (Table S7). The average the grain number per main spike was highest for the cv. Turnia (47.86) and lowest for Ae. kotschyi had (4.4). The average the grain number per main spike in individual years ranged from 30.93 in 2010 to 44.39 in 2014. Introgression lines RV5, KR4 and KRG6 adapted in environments with similar conditions to these in years 2011 and 2014, and not adapted in 2013 (Fig. S7). Alloplasmic introgression lines KRB4 and KRMo4 as well as cvs. Zyta and Piko adapted in environments with similar conditions to these in 2012, and not adapted in 2010. Cv. Korweta, with the ASV of 0.197, was the most stable, while introgression lines VR5 (10.949) and RV5 (9.967) were the least stable (Table S7). Alloplasmic introgression line KRMu4, with an the average the grain number per main spike of 47.66 and the ASV of 0.724, had the best genotype selection index (6).
Grain weight per main spike
The grain weight per main spike of the genotypes ranged from 0.019 g (for Ae. kotschyi in 2012) to 3.023 g (for KRT4 in 2011) over the five years, with the average of 1.291 g (Table S8). Alloplasmic introgression line KRMu4 had the highest average grain weight per main spike (1.901 g), and Ae. kotschyi had the lowest (0.034 g). The average grain weight per main spike in individual years ranged from 1.007 g in 2010 to 1.898 g in 2014. Alloplasmic introgression lines KRP4 and KRMo4, and cvs. Muza and Piko adapted in environments with similar conditions to these in years 2010, 2012 and 2013, and not adapted in 2011 and 2014 (Fig. S8). Cv. Zyta, with the ASV of 0.072, was the most stable, while euplasmic introgression line RV5 (1.128) was the least stable (Table S8). Cv. Zyta, with a high average grain weight per main spike (1.535 g) and the best ASV, had the best genotype selection index (11).
Main spike seed set (grain number per spikelet)
The main spike seed set of the genotypes ranged from 0.307 (for VR5 in 2012) to 3.566 (for Ae. kotschyi in 2013) over the five years, with the average of 1.934 (Table S9). Alloplasmic introgression line KRMu4 had the highest average main spike seed set (2.455 g), and alloplasmic introgression line KR4 had the lowest (1.351 g). The average main spike seed set in individual years ranged from 1.663 g in 2010 to 2.318 g in 2014. Ae. kotschyi and Ae. variabilis adapted in environments with similar conditions to these in 2013, and not adapted in 2011 and 2014 (Fig. S9). Alloplasmic introgression line VR5 adapted in environments with similar conditions to these in 2010, and not adapted in 2012. Cv. Rusałka was the most stable, with the ASV of 0.095, while alloplasmic introgression line KR4 (2.864) was the least stable (Table S9). Cv. Rusałka had the best genotype selection index (7), while Ae. kotschyi had the worst (53).
The thousand grain weight (TGW)
The TGW values ranged from 6.95 g (for Ae. variabilis in 2011) to 50.21 g (for Rusałka in 2014), with the average of 31.22 g (Table S10). Cv. Rusałka had the highest average TGW (42.81 g), and Ae. kotschyi had the lowest (7.9 g). The average TGW in individual years ranged from 28.75 g in 2011 to 36.63 g in 2014. Ae. variabilis, alloplasmic introgression line KRMo4, and cvs. Begra and Smuga adapted in environments with similar conditions to these in years 2012 and 2013, and not adapted in 2011 (Fig. S10). Ae. kotschyi, cv. Gama, and alloplasmic introgression lines KR4 and KRBP4 adapted in environments with similar conditions to these in 2010, and not adapted in 2014. The ASV ranged from 0.183 (for alloplasmic introgression line KRKo4) to 3.120 (for alloplasmic introgression line KRP4). The GSI ranged from 8 (for alloplasmic introgression line KRKo4) to 52 (for alloplasmic introgression line KRBP4).
Total genotype selection index
The best total genotype selection index (for all ten traits combined) was observed for cvs. Gama (TGSI = 194), Rusałka (TGSI = 209), and Korweta (TGSI = 223) and for alloplasmic introgression lines KRS4 (TGSI = 222) and KRMu4 (TGSI = 229), and the worst for alloplasmic introgression line KR4 (TGSI = 433). Alloplasmic introgression lines KRMu4, KRS4, KRKo4, KRBS4, and KRBT4 are recommended for further inclusion in breeding programmes due to their better stability in comparison with other lines and the good average values for the observed traits.
Discussion
Crop breeding among others aims at producing new, starting materials for breeding, with desirable levels of resistance to diseases, pests, and unfavourable abiotic conditions. In wheat breeding, the difficulty in achieving these goals is in part a consequence of low genetic variation within the species Triticum aestivum L. (Pilch 2011). Wild species related to wheat, e.g. genus Aegilops, are a valuable source of alleles for agronomic traits useful in breeding, and their use as source material for cross-breeding significantly increases the gene pool of wheat. The species Ae. kotschyi Boiss. and Ae. variabilis Eig. are highly resistant to abiotic stressors, including drought, high temperatures, and soil salinity (Shimshi et al. 1982; Kimber and Feldman 1987). For this reason, hybrids of common wheat with these species were chosen to test the effect of changes in weather conditions in consecutive years on their yield traits.
The experimental field located near Zamość was perfectly suitable for this type of experiment due to its extreme summer and winter conditions. The climate in Zamość area is characterized by dry and hot summers and cold winters. It is also characterized by a large number of sunny days a year (38%) and at times there are up to 60 frost days per year, and with temperature below freezing for up to 130 days. The snow cover time ranges from 60 to 80 days (Reszel 1992). Global warming has resulted in greater variation in weather conditions in recent years, with less frequent rainfall and resulting droughts.
The atmospheric conditions in 2010–2014 were variable (Table 2). In 2010–2013, between April and July, the rainfall deficit was 59.7%, 66.1%, 59.8% and 29.8%, respectively. Only in 2014 did the precipitation total exceed the long-term average, by 2.8%. In all years of the study, the average monthly temperature from April to July exceeded the long-term average, by 6.1 °C in 2010, 5.1 °C in 2011, 5.5 °C in 2012 and 6.6 °C in 2013 and 2014.
For the experiments described here the plots were sown at 20 × 10 cm spacing. This permitted the assessment of tillering, but also promoted it to some degree. However, at times similar experiments are planted at even greater spacing, e.g. 20 × 15 cm (Ćwiklińska et al. 2009). Under these conditions, the tillering of the introgression lines was similar to that of wheat. In both groups, the number of productive tillers were significantly lower than in the Aegilops parents. It is clear that selection in early generations removed much of excessive tillering from the introgression lines. Among the latter, Ae. kotschyi/Rusałka//Korweta and Ae. kotschyi/Rusałka//Muza lines were the most stable in terms of the number of tillers. Cvs. Piko and Monopoly, with the best tillering, had the best genotype selection index. The number of productive tillers per plant is highly variable and largely determined by the environment (Nawracała 2004). Araki et al. (1999) located QTLs linked to the tiller number on chromosome arm 4AS of common wheat. Among the introgression lines, the average the length of the main tiller range more widely than among the parental wheat cultivars. The shortest main tillers were in Ae. kotschyi Boiss., Ae. variabilis Eig. and lines Rusałka/Ae. kotschyi and Ae. kotschyi/Rusałka//Begra///Piko. Short-straw forms are more resistant to lodging. Keller et al. (1999) identidied nine loci responsible for lodging resistance in wheat, on chromosome arms 1BS, 2AS, 2D, 3AS, 4AS, 5AL, 5BL, 6BL and 7BL. Ahmed et al. (2000) found seven QTLs and RFLP markers associated with plant height on chromosomes 1A, 1D, 2B, 2D and 4B of common wheat. Genes influencing stem stiffness in wheat have been located on chromosomes 1A, 1BS, 3AS, 4AS, 5AL, 3BL, 5BL, 2D (Nawracała 2004; Hai et al. 2005; Houshmand et al. 2007).
The numerical values of morphological features were probably affected by the amount of precipitation during individual growing season. In 2014, when the April to July rainfall total was the highest, the spikes were the longest with the highest numbers of spikelets and grains, grain weight per spike, and the thousand grain weight. Yield traits are influenced by multiple QTLs located on most chromosomes. Some of the characters measured are known to be controlled by numerous chromosome regions, scattered over many chromosomes (Kato et al. 1998; Peng et al. 1998; Varshney et al. 2000; Li et al. 2002; Nawracała 2004; Kumar et al. 2006; Nalam et al. 2006). Mollasadeghi et al. (2012) reported that wheat plant height, productive tiller number and grain number showed more genetic variation than other features.
The ASVs indicated that a few alloplasmic introgression lines with Ae. kotschyi were the most stable in terms of the all analysed morphological traits, but from parental wheats some were the most stable in the case of some traits such as the length of the main tiller (Zyta), the diameter of the second internode from the base (Monopoly), the spikelet number per main spike (Rusałka), the grain number per main spike (Korweta), the grain weight per main spike (Zyta), the main spike seed set (Rusałka), the thousand grain weight (Turnia, Monopoly).
The alloplasmic introgression lines Ae. kotschyi/Rusałka//Piko, Ae. kotschyi/Rusałka//Monopoly, and Begra, Gama, Muza, Piko, Smuga parental wheats were adapted in environments with similar conditions to these in 2010, 2012 and 2013, in terms of the grain weight per main spike and thousand grain weight. The alloplasmic introgression line Ae. variabilis/Rusałka was adapted in environments with similar conditions to these only in year 2010, in terms of main spike seed set.
The best total genotype selection index for all ten traits combined was observed for the cvs. Gama and Rusałka, and among hybrid lines, for Ae. kotschyi/Rusałka//Smuga and Ae. kotschyi/Rusałka//Muza. Due to their stability and the good average values for the observed traits, the lines Ae. kotschyi/Rusałka//Smuga, Ae. kotschyi/Rusałka//Muza, Ae. kotschyi/Rusałka//Korweta, Ae. kotschyi/Rusałka//Begra///Smuga, and Ae. kotschyi/Rusałka//Begra///Turnia can be recommended for further inclusion in breeding programmes.
In a study by Rodrigues and Paderewski (2018), the AMMI analysis was applied to phenotypic data for the location × management × year combination (treated as environmental conditions). In wheat cvs. Alcazar, Rapsodia, Boomer, Anthus, Kris and Batuta, the interaction positively affected yield in 2008, but negatively in 2009. The remaining cultivars (Mewa, Legenda, Sukces, Smuga, Turnia, and Zyta) had positive or near-zero interaction effects in 2009, but negative effects in 2008. Ibrahim and Said (2020) assessed 42 genotypes of common wheat in eight environments in Egypt, with two locations and two planting dates in the two growing seasons. They estimated the grain yield, spike number per plant, the thousand grain weight, and plant height. The combined analysis showed that most of the means squares were assigned to environmental effects, indicating that the environments were diverse, with large differences between environmental means causing most of the variation in grain yield and other features. According to the authors, six genotypes, showed better overall adaptability in different environments.
Mohammadi (2017) used the AMMI model to analyse the genotype, year and GYI effects for the grain yield and drought-adaptive traits of 25 wheat genotypes during five seasons. The results showed that GYIs lead to different rankings of genotypes in different environments. According to the author, these breeding lines are unstable because they are more adapted to favourable growing conditions. On the other hand, old wheat cultivars with less sensitivity to environmental conditions were the most stable. Similarly, in our research, the oldest cvs., Rusałka and Gama, were the most stable. Cv. Rusałka, because it crosses well with rye, has long been used in wide hybridization (Stefanowska 1986). Winter cvs. Gama and Begra were placed on the Polish National List of Varieties of Agricultural Plants in 1982, and Korweta in 1997, Zyta in 1999, Turnia in 2001, Piko in 2002, Muza and Smuga in 2004 (COBORU 1982, 1997, 1999, 2001, 2002, 2004). Monopoly it is Canadian wheat cultivar grown in 1990s.
In contrast to the old wheat cultivars, the introgression lines showed less stability, probably due to their hybrid character. The importance of GYIs in plant breeding programmes has been a focus of attention for wheat breeders (Brancourt-Hulmel et al. 2003; Mohammadi and Amri 2013; Farshadfar et al. 2015; Golkari et al. 2016; Paderewski et al. 2016; Semcheddinne et al. 2017; Mohammadi 2017; Rodrigues and Paderewski 2018; Roostaei et al. 2018; El-Sherbeny et al. 2019; Singh et al. 2019; Ibrahim and Said 2020; Eltaher et al. 2021).
The frequently used the AMMI model provides a useful tool for diagnosing GEI patterns and improving the accuracy of response estimates (Rharrabti et al. 2003; Paderewski et al. 2016; Fotso et al. 2018; Hassani et al. 2018; Bocianowski et al. 2018, 2019a, 2019c, 2021). The AMMI stability value revealed high stability among the cultivars. According to Yang et al. (2009), a biplot based on the AMMI is a useful visualization technique for identifying similarity or dissimilarity among genotypes or environments. At the same time, Yang et al. (2009) caution against the use of a biplot as more than a visual descriptive tool, as the method utilizes only a sample of the full data set and does not involve any statistical hypothesis testing.
The best total genotype selection index for all ten traits combined was obtained for the cvs. Gama and Rusałka, and among the introgression lines, for KRS4 and KRMu4.
Conclusions
We hypothesized that wheat lines with introgressions from Aegilops kotschyi Boiss. and Ae. variabilis Eig. would be less sensitive to variable environmental conditions and more stable in terms of the yield traits analysed during the years of the study. However, their responses were varied. For some of these lines, Morphological traits and the genotype selection indexes were higher than or comparable to those of the parental wheats, while for other lines, the values were lower. All of the first group lines have Ae. kotschi in the pedigree, used as female in the original cross. Hence, all are alloplasmic lines but at this point, in the absence of reciprocal hybrids, no association between the cytoplasm and line performance can be made. Regardless, based on the results colleted in this study, these lines appear as the most suitable for inclusion in breeding programmes due to their stability and favourable mean values for the observed traits.
References
Ahmed TA, Tsujimoto H, Sasakuma T (2000) QTLs associated with plant height and related characters in hexaploid wheat. Breed Sci 50(4):267–273. https://doi.org/10.1270/jsbbs.50.267
Araki E, Miura H, Sawada S (1999) Identification of genetic loci affecting amylose content and agromomic traits on chromosome 4A of wheat. Theor Appl Genet 98:977–984. https://doi.org/10.1007/s001220051158
Bansal KC, Sinha SK (1991) Assessment of drought resistance in 20 accessions of Triticum aestivum and related species I. Total dry matter and grain yield stability. Euphytica 56:7–14. https://doi.org/10.1007/BF00041738
Becker HC, Léon J (1988) Stability analysis in plant breeding. Plant Breed 101:1–23. https://doi.org/10.1111/j.1439-0523.1988.tb00261.x
Bedő Z, Láng L (2015) Wheat breeding: current status and bottlenecks. In: Molnár-Láng M, Ceoloni C, Doležel J (eds) Alien introgression in wheat. Springer International Publishing, Cham, pp 77–101
Berry J, Bjorkman O (1980) Photosynthetic response and adaptations to temperature in higher plants. Ann Rev Plant Physiol 31:491–532. https://doi.org/10.1146/annurev.pp.31.060180.002423
Blum A (1986) The effect of heat stress on wheat leaf and spike photosynthesis. J Exp Bot 37:111–118. https://doi.org/10.1093/jxb/37.1.111
Bocianowski J, Księżak J, Nowosad K (2019) Genotype by environment interaction for seeds yield in pea (Pisum sativum L.) using additive main effects and multiplicative interaction model. Euphytica 215:191. https://doi.org/10.1007/s10681-019-2515-1
Bocianowski J, Niemann J, Nowosad K (2019b) Genotype-by-environment interaction for seed quality traits in interspecific cross-derived Brassica lines using additive main effects and multiplicative interaction model. Euphytica 215:7. https://doi.org/10.1007/s10681-018-2328-7
Bocianowski J, Nowosad K, Szulc P (2019) Soil tillage methods by years interaction for harvest index of maize (Zea mays L.) using additive main effects and multiplicative interaction model. Acta Agric Scand Sect 69:75–81. https://doi.org/10.1080/09064710.2018.1502343
Bocianowski J, Szulc P, Nowosad K (2018) Soil tillage methods by years interaction for dry matter of plant yield of maize (Zea mays L.) using additive main effects and multiplicative interaction model. J Integr Agr 17:2836–2839. https://doi.org/10.1016/S2095-3119(18)62085-4
Bocianowski J, Tratwal A, Nowosad K (2020) Genotype by environment interaction for area under the disease-progress curve (AUDPC) value in spring barley using additive main effects and multiplicative interaction model. Aust Plant Pathol 49:525–529. https://doi.org/10.1007/s13313-020-00723-7
Bocianowski J, Tratwal A, Nowosad K (2021) Genotype by environment interaction for main winter triticale varieties characteristics at two levels of technology using additive main effects and multiplicative interaction model. Euphytica 217:26. https://doi.org/10.1007/s10681-020-02756-x
Brancourt-Hulmel M, Doussinault G, Lecomte Ch, Bérard P (2003) Genetic improvement of agronomic traits of winter wheat cultivars released in France from 1946 to 1992. Crop Sci 43(1):37–45. https://doi.org/10.2135/cropsci2003.0037
COBORU (1982) The Polish National List of Varieties of Agricultural Plants. Słupia Wielka.
COBORU (1997) The Polish National List of Varieties of Agricultural Plants. Słupia Wielka.
COBORU (1999) The Polish National List of Varieties of Agricultural Plants. Słupia Wielka.
COBORU (2001) The Polish National List of Varieties of Agricultural Plants. Słupia Wielka.
COBORU (2002) The Polish National List of Varieties of Agricultural Plants. Słupia Wielka.
COBORU (2004) The Polish National List of Varieties of Agricultural Plants. Słupia Wielka.
Cociu AI (2018) Relationship among yield and yield components of winter wheat (Triticum aestivum L.) cultivars, as affected by tillage systems. Rom Agric Res 35:155–161
Coriton O, Barloy D, Huteau V, Lemoine J, Tanguy AM, Jahier J (2009) Assignment of Aegilops variabilis Eig. chromosomes and translocations carrying resistance to nematodes in wheat. Genome 52(4):338–346. https://doi.org/10.1139/g09-011
Cuevas J, Crossa J, Montesinos-López OA, Burgueño J, Pérez-Rodríguez P, de los Campos G (2017) Bayesian genomic prediction with genotype × environment interaction kernel models. G3: Genes Genomes Genetics 7(1):41–53. https://doi.org/10.1534/g3.116.035584
Ćwiklińska A, Broda Z, Bocianowski J (2009) Comparative analysis of the features of wild species within the genus Secale for widening genetic variability to be utilized in breeding. Biul IHAR 252:119–137 ((In Polish))
El-Sherbeny GAR, Khaled GAA, Elsayed HMA (2019) Inheritance of gene action for field component traits in bread wheat under normal and druoght stress conditions. Int J Mod Agric 8(2):18–26. https://doi.org/10.17762/ijma.v8i2.82
Eltaher S, Baenziger PS, Belamkar V, Emara HA, Sallam A, Nower AA, Salem KFM, Alqudah AM, Salam A (2021) GWAS revealed effect of genotype × environment interactions for grain yield of Nebraska winter wheat. BMC Genomics 22(1):2. https://doi.org/10.1186/s12864-020-07308-0
Esuma W, Kawuki RS, Herselman L, Labuschagne MT (2016) Stability and genotype by environment interaction of provitamin A carotenoid and dry matter content in cassava in Uganda. Breed Sci 66(3):434–443
Farshadfar E, Mohammadi R, Amri A (2015) Interpreting genotype × environment interactions for grain yield of rainfed durum wheat in Iran. Crop J 3(6):526–535. https://doi.org/10.1016/j.cj.2015.08.003
Farshadfar E, Sutka J (2003) Locating QTLs controlling adaptation in wheat using AMMI model. Cereal Res Commun 31(3):249–256. https://doi.org/10.1007/BF03543351
Fotso AK, Hanna R, Kulakow P, Parkes E, Iluebbey P, Ngome FA, Suh C, Massussi J, Choutnji I, Wirnkar VL (2018) AMMI analysis of cassava response to contrasting environments: case study of genotype by environment effect on pests and diseases, root yield, and carotenoids content in Cameroon. Euphytica 214:155. https://doi.org/10.1007/s10681-018-2234-z
Gauch HG, Zobel RW (1990) Imputing missing yield trial data. Theor Appl Genet 79:753–761. https://doi.org/10.1007/BF00224240
Ghaed-Rahimi L, Heidari B, Dadkhodaie A (2015) Genotype × environment interactions for wheat grain yield and antioxidant changes in association with drought stress. Arch Agron Soil Sci 61(2):153–171. https://doi.org/10.1080/03650340.2014.926004
Golkari S, Haghparast R, Roohi E, Mobasser S, Ahmadi MM, Soleimani K, Khalilzadeh G, Abedi-Asl G, Babaei T (2016) Multi-environment evaluation of winter bread wheat genotypes under rainfed conditions of Iran-using AMMI model. Crop Breed J 6(2):17–31. https://doi.org/10.22092/cbj.2016.107104
Gorham J (1990) Salt Tolerance in the Triticeae: K/Na discrimination in Aegilops species. J Exp Bot 41(226):615–621. https://doi.org/10.1093/jxb/41.9.1095
Gupta PK, Rustgi S, Kumar N (2006) Genetic and molecular basis of grain size and grain number and its relevance to grain productivity in higher plants. Genome 49:565–571. https://doi.org/10.1139/g06-063
Hageman JA, Malosetti M, van Eeuwijk F (2012) Two-mode clustering of genotype by trait and genotype by environment data. Euphytica 183(3):349–359. https://doi.org/10.1007/s10681-010-0236-6
Hai L, Guo H, Xiao S, Jiang G, Zhang X, Yan C, Xin Z, Jia J (2005) Quantitative trait loci (QTL) of stem strength and related traits in a doubled-haploid population of wheat (Triticum aestivum L.). Euphytica 141(1–2):1–9. https://doi.org/10.1007/s10681-005-4713-2
Hassani M, Heidari B, Dadkhodaie A, Stevanato P (2018) Genotype by environment interaction components underlying variations in root, sugar and white sugar yield in sugar beet (Beta vulgaris L.). Euphytica 214:79. https://doi.org/10.1007/s10681-018-2160-0
Houshmand S, Knox RE, Clarke FR, Clarke JM (2007) Microsatellite markers flanking a stem solidness gene on chromosome 3BL in durum wheat. Mol Breeding 20(3):261–270. https://doi.org/10.1007/s11032-007-9088-8
Ibrahim K, Said AA (2020) Grain yield stability of new bread wheat genotypes (Triticum aestivum L.) under normal and heat stress conditions. Egypt J Agron 42(2):171–184. https://doi.org/10.21608/AGRO.2020.32118.1216
Jenner CF (1994) Starch synthesis in the kernel of wheat under high temperature conditions. Aust J Plant Physiol 21:791–806. https://doi.org/10.1071/PP9940791
Kato K, Miura H, Akiyama M, Kuroshima M, Sawada S (1998) RFLP mapping of the three major genes, Vrn1, Q and B1, on the long arm of chromosome 5A of wheat. Euphytica 101(1):91–95. https://doi.org/10.1023/A:1018372231063
Keller M, Karutz Ch, Schmid JE, Stamp P, Winzeler M, Keller B, Messmer MM (1999) Quantitative trait loci for lodging resistance in a segregating wheat × spelt population. Thear Appl Genet 98(6/7):1171–1182. https://doi.org/10.1007/s001220051182
Kimber G, Feldman M (1987) Wild wheat: an introduction. Coll. Agric. Univ. of Missouri, Columbia. Spec Rep 353:1–146
Kumar N, Kulwal PL, Balyan HS, Gupta PK (2007) QTL mapping for yield and yield contributing traits in two mapping populations of bread wheat. Mol Breed 19:163–177. https://doi.org/10.1007/s11032-006-9056-8
Kumar N, Kulwal PL, Gaur A, Tyagi AK, Khurana JP, Khurana P, Balyan HS, Gupta P (2006) QTL analysis for grain weight in common wheat. Euphytica 151(2):135–144. https://doi.org/10.1007/s10681-006-9133-4
Li WL, Nelson JC, Chu CY, Shi LH, Huang SH, Liu DJ (2002) Chromosomal locations and genetic relationships of tiller and spike characters in wheat. Euphytica 125:357–366. https://doi.org/10.1023/A:1016069809977
Marais GF, McCallum B, Snyman JE, Pretorius ZA, Marais AS (2005) Leaf rust and stripe rust resistance genes Lr54 and Yr37 transferred to wheat from Aegilops kotschyi. Plant Breed 143:115–123. https://doi.org/10.1007/s10681-006-9092-9
Methodological instruction for conducting varietal experiments (1978) COBORU, Słupia Wielka. (In Polish)
Mohammadi R (2017) Interpretation of genotype × year interaction in rainfed durum wheat under moderate cold conditions of Iran. New Zeal J Crop Hort 45(1):55–74. https://doi.org/10.1080/01140671.2016.1231126
Mohammadi R, Amri A (2013) Genotype × environment interaction and genetic improvement for yield and yield stability of rainfed durum wheat in Iran. Euphytica 192(2):227–249. https://doi.org/10.1007/s10681-012-0839-1
Mollasadeghi V, Elyasi S, Mirzamasoumzadeh B (2012) Genetic variation of 12 bread wheat genotypes based on number of phonological and morphological traits. Ann Biol Res 3:4734–4740
Molnár-Láng M, Ceoloni C, Doležel J (2015) Alien introgression in wheat. Springer International Publishing, Cham, p 381
Nalam VJ, Vales MI, Watson CJW, Kianian SF, Riera-Lizarazu O (2006) Map-based analysis of genes affecting the brittle rachis character in tetraploid wheat (Triticum turgidum L.). Theor Appl Genet 112(2):373–381. https://doi.org/10.1007/s00122-005-0140-y
Nawracała J (2004) Genetic basis of wheat (Triticum aestivum L.) breeding. In: Outline of Cereal Genetics. Editor A. Górny, Institute of Plant Genetics, Polish Academy of Sciences in Poznań, pp. 183–327. (In Polish)
Novoselovic D, Baric M, Drezner G, Gunjaca J, Lalic A (2004) Quantitative inheritance of some wheat plant traits. Genet Mol Biol 27(1):92–98. https://doi.org/10.1590/S1415-47572004000100015
Nowosad K, Liersch A, Popławska W, Bocianowski J (2016) Genotype by environment interaction for seed yield in rapeseed (Brassica napus L.) using additive main effects and multiplicative interaction model. Euphytica 208:187–194. https://doi.org/10.1007/s10681-015-1620-z
Nowosad K, Liersch A, Poplawska W, Bocianowski J (2017) Genotype by environment interaction for oil content in winter oilseed rape (Brassica napus L.) using additive main effects and multiplicative interaction model. Indian J Genet Pl Br 77:293–297. https://doi.org/10.5958/0975-6906.2017.00039.6
Ortiz R, Sayre K, Govaerts B, Gupta R, Subbarao GV, Ban T, Hodson D, Dixon JM, Ortiz Monasterio JI, Reynolds M (2008) Climate change: Can wheat beat the heat? Agr Ecosyst Environ 126:46–58. https://doi.org/10.1016/j.agee.2008.01.019
Paderewski J, Gauh HG, Mądry W, Gacek ES (2016) AMMI analysis of four-way genotype × location × management × year data from a wheat trial in Poland. Crop Sci 56(5):2157–2164. https://doi.org/10.2135/cropsci2015.03.015
Peng ZS, Yen C, Yang JL (1998) Chromosomal location of genes for supernumerary spikelet in bread wheat. Euphytica 103(1):109–114. https://doi.org/10.1023/A:1018323310621
Petersen G, Seberg O, Yde M, Berthelse K (2006) Phylogenetic relationshipe of Triticum and Aegilops and evidence for the origin of the A, B and D genomes of common wheat (Triticum aestivum). Mol Phylogenet Evol 39:70–82. https://doi.org/10.1016/j.ympev.2006.01.023
Pilch J (2011) Introgressions of genes from taxonomically related species in improvement of wheat Triticum aestivum L. and other cultivated plants. Biul IHAR 260/261: 21–42. (In Polish)
Prażak R, Kociuba W, Kramek A (2017) Evaluation of Aegilops variabilis Eig. and Aegilops kotschyi Boiss. with Triticum aestivum L. hybrid lines regarding field resistance to brown rust. Agronomy Science Annales UMCS sectio E Agricultura VOL. LXXII 4:113–121. https://doi.org/10.24326/as.2017.4.11(InPolish)
Prażak R, Krzepiłko A (2018) Evaluation of iron and zinc content in grain of Aegilops L. × Triticum aestivum L. hybrid lines. J Elem 23(2):545–557. https://doi.org/10.5601/jelem.2017.22.3.1486
Prażak R, Molas J (2017) Evaluation of protein content in grain Aegilops L. × Triticum aestivum L. hybrid lines. Polish J Agron 29:35–42 (In Polish)
Purchase JL, Hatting H, van Deventer CS (2000) Genotype × environment interaction of winter wheat (Triticum aestivum L.) in South Africa: II. Stability analysis of yield performance. S Afr J Plant Soil 17:101–107. https://doi.org/10.1080/02571862.2000.10634878
Reszel R (1992) The natural environment of the Zamość province. Ed. "Emma Art" Lublin, p. 1–17. (In Polish)
Rharrabti Y, Villegas D, Royo C (2003) Durum wheat quality in Mediterranean environments: III. Stability and comparative methods in analysing G×E interaction. Field Crops Res 80(2):141–146. https://doi.org/10.1016/S0378-4290(02)00178-8
Rodrigues PC, Paderewski J (2018) Constrained AMMI model: application to Polish winter wheat post-registration data. Crop Sci 58(4):1458–1469. https://doi.org/10.2135/cropsci2017.06.0347
Roostaei M, Kamali MRJ, Roohi E, Mohammadi R (2018) Evaluation of Sardari bread wheat ecotypes under rainfed cold conditions of Iran. J Agric Sci 156(4):504–514. https://doi.org/10.1017/S0021859618000564
Schneider A, Molnár I, Molnár-Láng M (2007) Utilisation of Aegilops (goatgrass) species to widen the genetic diversity of cultivated wheat. Euphytica 163:1–19. https://doi.org/10.1007/s10681-007-9624-y
Semcheddinne N, Guendouz A, Oulmi A, Hafsi M (2017) Screening of Wheat (Triticum durum) for drought tolerance in semiarid conditions. Int J Biosci 10(6):166–178. https://doi.org/10.12692/ijb/10.6.166-178
Shahriari Z (2018) Dissection of genotype × environment interactions for mucilage and seed yield in Plantago species: application of AMMI and GGE biplot analyses. PLoS ONE 13(5):e0196095. https://doi.org/10.1371/journal.pone.0196095
Shimshi D, Mayoral ML, Atsmon D (1982) Response to water stress in wheat and related wild species. Crop Sci 22:123–128. https://doi.org/10.2135/cropsci1982.0011183X002200010028x
Singh C, Gupta A, Gupta V, Singh GP (2019) Genotype × environment interaction analysis of multi-environment wheat trials in India using AMMI and GGE biplot models. Crop Breed Appl Biot 19(3):309–318. https://doi.org/10.1590/1984-70332019v19n3a43
Slafer GA, Andrade FH (1993) Physiological attributes to the generation of grain yield in bread wheat cultivars released at different eras. Field Crops Res 31:351–367. https://doi.org/10.1016/0378-4290(93)90073-V
Spetsov P, Mingeot D, Jacquemin JM, Samardjieva K, Marinova E (1997) Transfer of powdery mildew resistance from Aegilops variabilis into bread wheat. Euphytica 93:49–54. https://doi.org/10.1023/A:1002904123885
Stefanowska G (1986) Influence of Triticum aestivum L. and Secale cereale L. on some genetic and breeding features of Triticale. Publishing series - Scientific dissertations (Poland). Agricultural Academy, Lublin, no. 96. Availability: Centralna Biblioteka Rolnicza, POB 360, 00–950 Warszawa 40 – Poland. (In Polish)
Thiele A, Schumann E, Peil A, Weber WE (2002) Eyespot resistance in wheat × Aegilops kotschyi backcross lines. Plant Breed 121:29–35. https://doi.org/10.1046/j.1439-0523.2002.00669.x
Varshney RK, Prasad M, Roy JK, Kumar N, Harjit-Singh DHS, Balyan HS, Gupta PK (2000) Identification of eight chromosomes and a microsatellite marker on 1AS associated with QTL for grain weight in bread wheat. Theor Appl Genet 100:1290–1294. https://doi.org/10.1007/s001220051437
Waines JG (1994) High temperature stress in wild wheats and spring wheats. Aust J Plant Physiol 21:705–715. https://doi.org/10.1071/PP9940705
Warrington IJ, Dunstone RL, Green LM (1977) Temperature effects at three development stages on the yield of the wheat ear. Aust J Agric Res 28:11–27. https://doi.org/10.1071/AR9770011
Yan W, Kang MS (2002) GGE Biplot analysis—a graphical tool for breeders geneticists, and agronomists. T&F eBooks CRS Press, Boca Raton, pp 1–288
Yang RC, Crossa J, Cornelius PL, Burgueno J (2009) Biplot analysis of genotype × environment interaction: proceed with caution. Crop Sci 49:1564–1576. https://doi.org/10.2135/cropsci2008.11.0665
Zobel RW, Wright MJ, Gauch HG (1988) Statistical analysis of yield trial. Agron J 80:388–393. https://doi.org/10.2134/agronj1988.00021962008000030002x
Acknowledgements
The authors would like to thank the Editor and the anonymous Reviewers whose inputs have greatly improved the quality of the manuscript.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
JB: Conceptualization, JB and RP: Methodology, JB: Software, JB and RP: Validation, JB: Formal analysis, JB and RP: Investigation, RP: Rresources, JB and RP: Data curation, JB and RP: Writing—original draft preparation, JB and RP: Writing—review and editing, JB: Visualization, JB: Supervision, All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bocianowski, J., Prażak, R. Genotype by year interaction for selected quantitative traits in hybrid lines of Triticum aestivum L. with Aegilops kotschyi Boiss. and Ae. variabilis Eig. using the additive main effects and multiplicative interaction model. Euphytica 218, 11 (2022). https://doi.org/10.1007/s10681-022-02967-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-022-02967-4