Abstract
For a better understanding of the physiological background of microspore embryogenesis (ME), the protein profile was analyzed in four winter triticale DH lines, which show extremely different embryogenic potential. The analysis were conducted with anthers at the phase of development optimal for ME induction and then after low temperature (LT, 3 weeks at 4 °C) ME-inducing tillers treatment. The sub-proteome of anthers was mapped by two-dimensional gel electrophoresis (2-DE). The protein species significantly more abundant (at least 2-fold) in responsive DH lines after LT treatment were chosen for identification by MALDI-TOF/TOF analysis. In total, 31 protein species were successfully identified as involved in the determination of microspore competence, stress response and in the regulation of ME induction. Microspore competence required sufficient energy supply and efficient system of cell protection that determine survival under prolonged LT stress treatment. LT stress was associated with increased accumulation of proteins typical for cell defence against oxidative stress (e.g., l-ascorbate peroxidase), chaperons (e.g., HSP70) and other enzymes/factors ensuring protein biosynthesis, stability and active cell divisions. Also here, effective cell defence required undisturbed energy supply. Among proteins that accumulated differentially in accordance with microspore embryogenic potential again the most important role seems to be played by the enzymes ensuring energy production and determining ability of plant stress adaptation. Two protein species (enolase, 12S storage protein), proposed earlier as candidates for markers of embryogenesis in other in vitro plant culture systems confirmed their utility for triticale anther cultures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Theoretically, every living cell possess the whole genetic information that determines the characteristics of a species as a whole and of the particular individual. Usually this information is fully expressed only in very defined cell types, namely zygotes and stem cells. In the plant kingdom, however, the genetic programme leading to the formation of the whole organism could be expressed under specific circumstances as natural (apomixis) or induced in vitro phenomenon. Microspore embryogenesis (ME) is one of the examples of such induced ‘totipotency’ taking place in male gametophytic cells triggered by stress toward sporophytic developmental pathway (Touraev et al. 1997). In the literature, the term ‘ME’ is often used synonymously with ‘androgenesis’ or ‘pollen embryogenesis’ and also in this paper it refers to the process taking place in in vitro cultured anthers in which the cells of male gametophyte were reprogrammed towards embryo-like structures (ELS) production. Such more common use of the term ‘microspore embryogenesis’ was proposed by Soriano et al. (2013) and Wędzony et al. (2015).
Regenerated haploids and the doubled haploid plants (DHs) that are produced from them are very interesting objects of studies and highly valued in many research areas (Touraev et al. 2001; Forster et al. 2007; Dunwell 2010). Moreover, incorporation of DH technology in breeding programmes increases the efficiency of new cultivar production and accelerates the breeding progress in comparison with traditional methods. However, to employ DH technology for practical purposes, the procedure of its production should be highly efficient for a wide spectrum of genotypes. Unfortunately, for many agronomically important triticale genotypes the efficiency of DH production is not sufficient (Eudes and Amundsen 2005; Lantos et al. 2014). The progressively growing economic significance of this cereal, resulting from the combination of valuable features such as high yield potential and good grain quality with high tolerance to unfavourable environmental conditions has focused much interest on the identification of genetic factors controlling its embryogenic potential and effective production of DH lines (Gonzalez et al. 2005; Krzewska et al. 2012; Żur et al. 2012, 2013).
The milestone in DH techniques development was the discovery that the efficiency of ME can be significantly increased by the application of a stress treatment (Lichter 1985; Touraev et al. 1997). In triticale, a long (2–3 weeks) low temperature (LT) treatment applied to tillers is the most effective stress factor (Immonen and Robinson 2000; Pauk et al. 2003; Wędzony 2003; Żur et al. 2008, 2009). Although this treatment is commonly used in many lab protocols, the precise mechanism of LT effect is not clear. Obviously, for winter triticale cultivated in climate conditions typical for northern and central Europe cereals, the temperature at about 4 °C that is used for ME initiation does not generate intensive stress. Moreover, a recently published report (Żur et al. 2014) confirmed that such LT treatment is not a critical prerequisite for ME induction despite it significantly increasing the effectiveness of the process in triticale anther cultures. It has been proposed that the positive effect of LT results from the induced cascade of reactions which in turn increases the tolerance to later applied stresses connected with the isolation procedure and transfer to in vitro culture conditions (Żur et al. 2008). However, as LT can impose changes in many aspects of cellular metabolism the precise identification of factors directly involved in microspore reprogramming is difficult. Among others, generation of reactive oxygen species (ROS), antioxidative system activation and altered hormonal balance have recently been indicated as the important elements of ME induction (Żur et al. 2014, 2015a, b). New molecular methods developed in the last decade, which use markers associated with a specific trait, generated novel, valuable information about molecular background of ME. Up to now, the process of microspore reprogramming has mainly been examined on genomic and transcriptomic levels with the use of four model crop species: tobacco (Nicotiana tabacum L.), rapeseed (Brassica napus L.), barley (Hordeum vulgare L.) and wheat (Triticum aestivum L.).
It was shown that during the induction of ME, some changes in DNA replication, synthesis and accumulation of mRNA and protein synthesis occurs in the competent cells (rev. by Hosp et al. 2007). First genes identified during induction of ME in barley encoded a lipid transfer protein—ECLTP, a glutathione-S-transferase—ECGST and arabinogalactan-like protein–ECA1 (Vrinten et al. 1999), but further analysis revealed that only ECA1 was expressed specifically in embryogenic cells. In barley, Maraschin et al. (2006), using microarrays containing ESTs (expressed sequence tags) identified a very distinct gene expression profile characteristic for embryogenic microspores. In comparison with developing pollen, in embryogenic microspores upregulation of genes involved in protein degradation (ubiquitin-conjugating enzyme, 20S proteasome subunit alpha-5 and alpha-2, 26S protease subunit-8, cysteine and aspartic protease and FtsH metalloprotease), starch and sugar hydrolysis (maltase, cell wall invertase), stress response (GTS, catalase), metabolism (alcohol dehydrogenase 3) and cell signalling (GTPase) was detected.
In comparison, only a limited number of studies focused on proteome changes associated with ME initiation. Proteins for ME response were identified only for the most popular crop species like maize (Vergne et al. 1993; Uváčková et al. 2012) or rapeseed (Cordewener et al. 2009). To the best of our knowledge this study is the first attempt to expand the knowledge in relation to the proteomic background that determines effective induction of ME in triticale anther cultures.
Materials and methods
Plant material
Four DH lines (DH19, DH28, DH47, DH72) of winter hexaploid triticale were chosen for the study from the mapping population of 90 DH lines ‘Saka 3006’ × ‘Modus’ (Tyrka et al. 2011), as significantly differentiated in respect of ME responsiveness (Krzewska et al. 2012). The mapping population was derived from the F1 generation of a cross between the German inbred line ‘Saka 3006’ and the Polish cultivar ‘Modus’ by the maize method (Wędzony et al. 1998). The population was derived from Hohenheim University (Stuttgart, German) and kindly provided by Dr Eva Bauer. Data received in three separate phenotyping replications makes it possible to consider DH28 and DH47 as highly responsive and DH19, DH72 as recalcitrant model objects (Krzewska et al. 2012). The procedures of seeds germination, seedlings vernalisation and plant growth conditions were described earlier by Żur et al. (2012).
Anther culture protocol
The protocol for anther culture has been described earlier by Wędzony (2003) and used with some modifications according to Krzewska et al. (2012) and Żur et al. (2012). In short, tillers harvested when the majority of microspores were at mid- to late-uninucleate stage of development were wrapped in plastic bags, placed in jars containing Hoagland’s salt solution and stored at 4 °C, in the dark, for 3 weeks. Subsequently, the spikes were sterilized with 96% ethanol. Aseptically excised anthers were placed in 60 × 15 mm Petri dishes (100 anthers from spike per dish) containing the induction medium C17 (Wang and Chen 1983) modified according to Krzewska et al. (2012). The cultures were stored in the dark at 28 ± 1 °C. Embryo-like structures (ELS) were transferred successively starting from the 6th week of culture, in 3-week intervals. The structures were put into 90 × 20 mm Petri dishes (30 ELS per a dish) with the regeneration medium 190-2R (Zhang and Xu 1983), containing 30 mg/l sucrose, 0.5 mg/l kinetin, 0.5 mg/l NAA and 0.6% agar, pH 6.0. The regeneration phase took place at 26 °C, in the light (at about 30 µmol m−2 s−1 for the first week, then increased to 80–100 µmol m−2 s−1) with 16/8 h (day/night) photoperiod.
Evaluation of microspore embryogenesis effectiveness in anther cultures
Five parameters were chosen in order to describe the ME effectiveness:
-
ELS/100A—the number of embryo-like structures (ELS) per 100 anthers (A),
-
GR/100ELS—the number of green regenerants (GR) per 100 embryo-like structures,
-
AR/100ELS—the number of albino regenerants (AR) per 100 embryo-like structures,
-
GR/100A—the number of green regenerants per 100 anthers,
-
AR/100A—the number of albino regenerants per 100 anthers.
Each dish containing 100 anthers collected from one spike was assumed to be a replicate. Mean values were calculated from at least six replicates.
To evaluate the effect of low temperature (3 weeks at 4 °C) on ME effectiveness, anthers excised from freshly cut (FC) tillers were used as the control. Mean values were calculated from at least six replicates.
Protein extraction
Anthers collected from freshly cut tillers and from tillers subjected to ME-inducing treatment (3 weeks at 4 °C) were immediately frozen in liquid nitrogen and stored at −80 °C until protein extraction.
The protocol for protein isolation was performed according to Klubicova et al. (2011). Subsequently, anthers (ca 1 g fresh weight) were ground to a fine powder in liquid nitrogen using a mortar and pestle. After adding 10 ml of phenol based extraction buffer and centrifugation, proteins were precipitated from the phenol phase by using 0.1 M ammonium acetate in methanol and collected by centrifugation (5000×g for 15 min at 4 °C.). Then a protein pellet was washed twice with 0.1 M ammonium acetate in methanol, next twice with 80% acetone and once with 70% ethanol. The pellet was finally dissolved in 200 µl isoelectric focusing (IEF) sample solution (8 M urea, 2 M thiourea, 2% (w/v) CHAPS, 2% (v/v) Triton X-100, 50 mM DTT) and then the protein concentration was determined by the Bradford method (Bradford 1976). Protein quantification was performed in triplicate against a standard curve of bovine serum albumin (BSA). Aliquots of proteins (500 µg) were stored at −80 °C pending further analysis.
Two-dimensional gel electrophoresis
The portions were defrosted, mixed with 3.2 µl of ampholytes (pH 5–8), adjusted to the final volume of 315 µl with the IEF sample solution and then loaded onto immobilized pH gradient (IPG) strips (pH 5–8, 17 cm, linear gradient, Bio-Rad) in an isoelectric focusing unit (Protean IEF Cell, Bio-Rad). The IEF running conditions were described by Klubicova et al. (2011). After the first dimension IPG strips were equilibrated for 15 min in 5 ml of SDS equilibration buffer (1.5 M Tris–HCl pH 6.8, 6 M urea, 30% (v/v) glycerol, 5% (w/v) SDS) with 2% (w/v) DTT followed for 15 min with the same buffer but containing 2.5% (w/v) iodoacetamide (IAA) instead of DTT. The strips were then transferred onto 12% SDS–polyacrylamide gels and overlayed with 0.5% (w/v) agarose in SDS running buffer with some addition of bromophenol blue as tracking dye. The second dimension was conducted at 10 mA/gel until the dye front reached the bottom of the gel (approx. 16 h) by using Protean II xi Cell (Bio-Rad).
Gels (in triplicates) were stained overnight in Colloidal Coomassie Brilliant Blue (CBB G-250), digitalised using ChemiDoc MP System (Bio-Rad) and subjected to Image Lab Program ver 4.1 (Bio-Rad). Image analyses (normalization, spot matching, expression analyses, and statistics) were performed with PDQuest 8.0 software (Bio-Rad). Firstly, images of gels were inverted, centralized and cropped according the same anchor spot, then the correlation coefficient between replicates was checked. The Master Gel was selected automatically and used for all bioinformatics analysis. The spot relative intensities were normalized according to total density in the gel images. One-way ANOVA statistical analysis was performed with a 95% significance level to determine which protein species were differentially abundant between the samples collected from control and LT-treated plants. On the basis of the above calculations, spots showing a statistically significant (p ≤ 0.05) increase in abundance (at least 2-fold) after and before ME inducing treatment in responsive DH lines were selected and manually picked for digestion and identification.
Mass spectrometric identification of protein species
Proteins were in-gel digested with trypsin according to the protocol described by Shevchenko et al. (1996). The extracts of tryptic peptides obtained were then spotted onto an AnchorChip target plate (MTP AnchorChip 384 T F, Bruker) and left to dry at ambient temperature. The anchors were subsequently covered with a solution of α-cyano-4-hydroxycinnamic acid (0.7 mg CHCA in 85% ACN, 15% H2O, 0.1% TFA and 1 mM NH4H2PO4) and again left to dry. All spectra were collected with the use of an ultrafleXtreme MALDI-TOF/TOF mass spectrometer and Compass 1.3 software for instrument control and data processing (both from Bruker Daltonik, Bremen, Germany). MS spectra were acquired in positive reflectron mode and externally calibrated using Peptide Calibration Standard II (Bruker). Fragment spectra were obtained by post-source decay (PSD) and internally calibrated on immonium ions. Protein identification was based on peptide mass fingerprint confirmed by fragment spectra (PMF + MS/MS). MS and PSD spectra were peak-picked in flexAnalysis 3.3, sent to BioTools 3.2 (both Bruker software packages) and submitted to database search with the use of Mascot 2.4 (Matrix Science, London, England, http://www.matrixscience.com) in-house server. Five custom databases were created for this purpose based on the protein databases for Triticosecale (120 entries), Secale (945 entries), Triticum (50,862 entries), Aegilops (39,589 entries) and Hordeum (38,447 entries), taken from NCBI Taxonomy Browser. MS and MS/MS mass tolerance was 50 ppm and 0.5 Da, respectively. The identification results obtained were examined in terms of the score level (greater than 64) and number of matched peptides (more than 2).
Results
Microspore embryogenesis effectiveness in anther culture
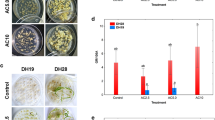
Among four DH lines chosen for the study, two responsive genotypes: DH28 and DH47 were characterized by significantly higher values of ELS/100A in comparison to two recalcitrant genotypes: DH19 and DH72 (Fig. 1a). Moreover, the total regeneration ability (R/100ELS) in responsive DH lines was at least 2.5-fold higher than in recalcitrant DH lines: 6.0 for DH47 and 2.3 for DH72. It has been revealed that although low temperature treatment (LT, 3 weeks at 4 °C) of tillers was not an obligatory requirement for ME induction in responsive DH lines it significantly improved ME effectiveness (Fig. 1a, c). The mean number of ELS in non-stressed cultures gained 50 and 74 per 100 anthers respectively for DH28 and DH47 and was almost doubled after LT treatment (Fig. 1a). Furthermore, LT treatment increased green plant regeneration ability (GR/100ELS) in responsive DH lines almost 3-fold and reduced the number of albino regenerants at least 2-fold (Fig. 1b). However, LT did not significantly influence any ME effectiveness parameters in recalcitrant DH lines (Fig. 1a–c).
a–c The effect of low temperature tillers treatment (LT, 3 weeks at 4 °C) on the androgenic effectiveness in anther culture of four selected DH lines of winter triticale (×Triticosecale Wittm.). a Microspore embryogenesis induction effectiveness (ELS/100A); b Total regeneration ability (R/100ELS: GR/100ELS and AR/100ELS); c Final regeneration effectiveness (R/100A: GR/100A and AR/100A). Presented data are the means of 6 biological replications. Mean values marked with asterisk showed significant difference between the cultures started from freshly cut and low temperature treated tillers according to Duncan’s multiple range test (p ≤ 0.05). ELS/100A—the number of embryo-likestructures (ELS) produced per 100 anthers (A), R/100ELS—the total number of regenerants (R) per 100 embryo-like structures transferred to regeneration medium (ELS): GR/100ELS—the number of green regenerants (GR) per 100 embryo-like structures (ELS), AR/100ELS—the number of albino regenerants (AR) per 100 embryo-like structures (ELS), R/100A—the total number of regenerants (R) per 100 isolated anthers (A): GR/100A—the number of green regenerants (GR) per 100 anthers (A), AR/100A—the number of albino regenerants (AR) per 100 anthers (A); LT treated—low temperature treatment (3 weeks at 4 °C)
2-DE analysis of anther proteins after cold treatment
Total soluble proteins were extracted first from the anthers isolated from freshly cut tillers at the moment optimal for ME induction and then from LT-treated tillers in which ME had been initiated. The total protein yields obtained with phenol based protocol was similar for all studied DH lines and treatments studied, 1.9–2.0 mg per 1 g of fresh weight (FW). In total, out of the 2561 differential protein spots which were detected with the use of two-dimensional gel electrophoresis, 194 revealed changes in their abundance. Among them, 102 protein spots were accumulated in anthers with at least a 2-fold change after the application of stress treatment in all DH lines studied (Table 1) and 13 of them were successfully identified (Table 2). All of them had 25% or higher protein sequence cover (Table 2). LT treatment did not induce any significant qualitative changes in anthers sub-proteome of triticale DH lines selected for the study. The majority of the protein species identified were classified as involved in stress response reactions like defence against reactive oxygen species (l-ascorbate peroxidase 1) and cold-induced membrane stabilization (low temperature-responsive RNA-binding protein). A relatively big representation of proteins with chaperone activity (elongation factor Tu and three isoforms of heat shock protein HSP70) was also detected. Other proteins accumulated after LT stress were identified as associated with specific metabolic processes: glucose metabolism (glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase), tricarboxylic acid cycle (isocitrate dehydrogenase) and both anabolic (beta-galactosidase 1) and catabolic (xylulose kinase) processes of carbohydrate metabolism. The last two protein species were associated with cell divisions (subtilisin-like protease SDD1) and protein synthesis (protein disulfide isomerase family protein 1–2). Two protein species showed the highest change in abundance: disulfide isomerase family protein 1–2 and low temperature-responsive RNA-binding protein, 7.1 and 6.8-fold respectively (Table 2).
Differentially abundant protein species in responsive genotypes and mass spectrometry-based identification of chosen protein spots
The changes in protein abundance as the effect of LT treatment in association with the embryogenic potential of particular DH line were presented in Table 3. In all comparisons between responsive and recalcitrant DH lines of triticale before LT treatment, 179 differentially expressed proteins were detected. Among them 86 protein species revealed higher abundance in the anthers of responsive DH lines at the phase of development ideal for ME-induction. Six were successfully identified (Table 2) as involved in various metabolic processes like methionine synthesis (5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase), ammonia detoxification (aspartate aminotransferase), carbohydrate metabolism (phosphoribulokinase) and mitochondrial respiration (mitochondrial-processing peptidase subunit alpha). One of the protein species significantly more abundant in responsive DH lines was elongation factor 1-gamma, which regulate peptide bond formation and ensure the accuracy of protein synthesis on the ribosome. The last protein species that could be responsible for acquisition of microspore competence in triticale was V-type proton ATPase subunit B1, involved in ion transport across cell membranes.
Protein species more abundant in responsive DH line after LT treatment were considered as crucial for microspore embryogenesis induction in triticale anther cultures. Among 155 protein species with changed abundance after LT treatment, 68 were up-regulated in responsive DH lines in comparison with recalcitrant ones (Table 3). From them 12 were successfully identified (Table 2; Fig. 2). The majority of them were associated with cell metabolism like glycolysis (enolase) and polysaccharides hydrolysis (beta-amylase). Three of the identified proteins were involved in photosynthesis: oxygen-evolving enhancer protein 1, ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit and ATP synthase CF1 beta subunit (chloroplast), which is involved in ATP production in the presence of a proton gradient across the membrane. The other two detected proteins were associated with biosynthesis of S-adenosylmethionine (S-adenosylmethionine synthase 1) and carbohydrate metabolism (fructokinase-2). Proteins characteristic for biotic/abiotic stress responses were represented by elicitor-responsive protein 3, heat shock cognate 70 kDa protein 4 and putative aconitate hydratase, cytoplasmic. Two storage protein species (11S globulin seed storage protein 2 and 12S seed storage globulin 1) were also accumulated after LT treatment in responsive DH lines.
Representative 2-DE gel image of triticale anthers protein map after low temperature treatment (3 weeks at 4 °C). Circles indicate the position of identified differentially abundant protein species. Spots are described in Table 2
Generally, selected protein spots were identified as homologues of proteins from related e plant species as Triticum urartu Thum. ex Gandil. or Aegilops tauschii Coss.(Table 2). The highest number of identified proteins in analysed DH lines of triticale was associated with cell metabolism (47%) and stress response (28%) (Fig. 3).
Discussion
Proteomics is a powerful tool for studying complex biological systems and developmental processes such as microspore embryogenesis. Moreover, it can provide not only data about general metabolic activity of cells and tissues, but also information about the stability of the protein, post-translational modifications and sub-cellular localisation.
It is well known that during induction of ME numerous changes at different levels, from overall morphology to gene expression, occur (Pauls et al. 2006; Segui-Simarro and Nuez 2008). Microspore reprogramming not only implies the expression of an embryogenic program, but also a stress-related cellular response and a repression of the gametophytic program. Numerous studies focused on searching for the gene(s) responsible for efficient ME induction with the use of model plant species: rapeseed (Joosen et al. 2007), wheat (Sánchez-Díaz et al. 2013) and barley (Muñoz-Amatriaín et al. 2009). The first report for molecular regulation of ME in triticale was published recently (Żur et al. 2013). However, gene expression data alone do not reveal the full complexity of the ME mechanism. Molecular systems biology and bioinformatics studies of various organisms have revealed that transcript levels do not always correlate with protein quantity (Ghazalpour et al. 2011; Baerenfaller et al. 2012). Therefore, the data obtained from protein profiles analysis is a highly expected supplement widening our understanding of the molecular regulation of ME in anther cultures of studied winter triticale DH lines.
Protein species involved in the acquisition of microspore competence in triticale anthers
The higher abundance of some protein species in responsive DH lines before LT treatment seems to be crucial for microspore competence. The protein species identified were mainly involved in metabolic processes, which seems to be in agreement with Uváčková et al. (2012). Similarly, like in the case of triticale (5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase; spot no 14), protein species involved in methionine metabolism was indicated as possibly involved in maize ME induction. Another protein supposed to be important for acquisition of microspore competence both in tested triticale DH lines (spot no 19) as well as in maize (Uváčková et al. 2012) is V-type proton ATPase involved in ion transport across cell membranes.
Detected differentially expressed phosphoribulokinase (PRK, spot no 16) suggest that efficient regeneration of ribulose-1,5-bisphosphate could also be important in respect of microspore competence in DH lines of triticale studied. This protein catalyses the ATP-dependent phosphorylation of ribulose-5-phosphate to ribulose-1,5-phosphate, a key step in the pentose phosphate pathway where carbon dioxide is assimilated. Some data showed that PRK synthesis could be regulated by such factors like light, drought stress or ABA (Hu et al. 2012).
Aspartate aminotransferase (spot no 15) is possibly involved in microspore competence, regulating metabolic coupling of ammonia detoxification. Similarly, Zhang et al. (2009) also reported high levels of this protein in embryogenic callus of grapevine.
One of the most important roles seems to be that played by elongation factor 1-gamma (spot no 18), which regulate peptide bond formation and ensure the accuracy of protein synthesis on the ribosome. Its accumulation in responsive DH lines of triticale outnumbered the level characteristic for recalcitrant genotypes by almost 4 times. However, as its function is also associated with stress response (Olarewaju et al. 2004), the precise mode of its action is unclear.
Protein species associated with stress-defence reaction
Cell response to stress factors depends on two main parameters: the cells physiological state and the stress intensity. When the stress level exceeds cellular tolerance, the cells die. Yet, less intensive stress induce adaptation mechanisms modifying certain aspects of cell physiology and the gene expression programs by intracellular signaling networks (Fehér et al. 2003). The LT treatment (3 weeks at 4 °C), which is used as a trigger in triticale ME (Immonen and Robinson 2000; Pauk et al. 2003; Wędzony, 2003; Żur et al. 2008, 2009), is accompanied by various morphological, cytological and physiological rearrangements in plant cells. It slows down the degradation processes, affects the cytoskeleton, activates Ca2+ pathways, and stimulates expression of small heat shock proteins (Touraev et al. 1997; Zoriniants et al. 2005; Dubas et al. 2010). Among protein species that were accumulated in anthers of the triticale DH lines studied after LT treatment were those involved in carbon metabolism. For example beta-galactosidases (spot no 1) constitute a widespread family of glycosyl hydrolases in plants and are thought to be involved in the metabolism of cell wall polysaccharides. The higher level of this protein could be explained by intensive cell wall synthesis associated with increased cell division activity in embryogenic tissue (Chugh and Khurana 2002). The other protein species which showed a significant increase in abundance after LT treatment was glyceraldehyde-3-phosphate dehydrogenase (spot no 2), an enzyme of the glycolysis pathway which catalyzes main steps in energy metabolism and in partitioning reducing power in the form of NADPH to the cytosol. In Arabidopsis the higher enzyme’s transcript level was also observed in response to oxidative stress, and its overexpression suppressed cell death (Bustos et al. 2008). The importance of sufficient energy supply for adaptation to LT stress is confirmed by accumulation of phosphoglycerate kinase. Regulation of the abundance of proteins involved in glycolysis might provide additional energy also for processes important for ME induction.
Another protein that plays important role in stress defence by supplying NADH for antioxidant systems is isocitrate dehydrogenase. Its accumulation (spot no 3) was detected in triticale anthers after LT treatment. The suppression of its activity enhances the susceptibility of animal cells to apoptosis induction (Jung and Park 2011). Acting as ROS scavenging enzymes, these proteins play a role in regulating the cellular redox status. In several recently published reports ROS and redox status were suggested as factors regulating ME induction (Jacquard et al. 2009; Żur et al. 2014).
LT treatment induced also increased accumulation of 3 isoforms of molecular chaperone, heat shock protein HSP70 (spots no 6–8), involved in correct folding of new or misfolded polypeptides (Mayer and Bukau 2005). Their function is very important under stress conditions where misfolding of polypeptides occurs more commonly. One of these isoforms (spot no 27) was more abundant in responsive DH lines, which suggests its possible direct role in ME induction. Similarly, transcriptome analysis revealed changes in the expression of genes encoding HSPs in response to microspore reprogramming inducing factors such as: heat, starvation, colchicine and gamma radiation and during induction of ME in isolated microspore cultures of rapeseed (Pechan et al. 1991). Its direct involvement in ME has not been confirmed, but its presence is a constant element associated with this process. Testillano et al. (2000) observed increased synthesis of HSP70 and HSP90 in isolated microspore cultures of Brassica napus L., Nicotiana tabacum L. and Capsicum annuum L. Further studies revealed the presence of these proteins in nucleus and cytoplasm after high temperature shock (Seguí-Simarro et al. 2003) and indicated that whereas HSP90 is typical for stress response, HSP70 with high possibility participate in induction of ME process. Moreover, Cordewener et al. (2009), by using anti-HSP antibodies, were able to observe the sub-cellular localisation of HSP70 in microspores of rapeseed. They showed that in embryogenic microspores HSP70 were accumulated in the nucleus of a vegetative cell, which was associated with the re-entry of the vegetative cell into the cell cycle, which is necessary for the induction of embryogenesis (Binarova et al. 1993). The conclusion that in uni-cellular vacuolated microspores, the level of HSP70 is high enough to initiate embryogenesis, while in later stages of microspores stress treatment and an increase in HSP level is required to support embryo induction (Cordewener et al. 2009), seems to be in agreement with our results.
It was proved that many stress factors like low temperature, heat shock, mechanical stress, and starvation, which are the main ME triggers highly intensify ROS accumulation (Mittler 2002). Therefore, the effective antioxidative system is crucial for the microspore viability. The hypothesis has been put forward (Żur et al. 2014), claiming that the effectiveness of ME in triticale anther cultures depends on both cell tolerance to/defence ability against oxidative stress and ROS-induced signal transduction associated with the stress treatment used for microspore reprogramming. Similarly to our study, one of the antioxidative enzyme—l-ascorbate peroxidase 1 (spot no 9) was accumulated after ME-inducing treatment in maize and rapeseed (Joosen et al. 2007; Cordewener et al. 2009; Uváčková et al. 2012). Its role can be more complex as in various plant cell culture systems high ascorbate levels stimulate cell division (de Pinto et al. 1999).
Another protein identified connected with stress response and detected in this experiment as more abundant after LT-treatment is low temperature-responsive RNA-binding protein (spot no 10). Low temperature-responsive (LTR) genes control membrane and protein stabilization in the process of freezing tolerance acquisition. One of such genes codes RNA-binding proteins (RBPs) which play crucial roles in all aspects of post-transcriptional gene regulation. They are involved in developmental processes and in adaptation of plants to various environmental conditions (Lorkovic 2009; Ambrosone et al. 2012).
One of the most important proteins playing a role in the elongation phase of protein synthesis in plant mitochondria and plastids is EF-Tu-elongation factor Tu (Fu et al. 2012). Its accumulation (spot no 11) was observed in analysed triticale anthers after ME-inducing treatment. EF-Tu expression has been studied in several plant species in response to different environmental stresses for example cold stress in barley and maize (Dunn et al. 1993; Berberich et al. 1995). It was revealed that under stress, it also acts as a chaperone displaying a protein disulfide isomerase activity and facilitating renaturation of proteins when conditions return to normal (Fu et al. 2012).
The same role can be played by another differentially accumulated protein, disulfide isomerases (PDIs) (spot no 12), belonging to the family of thiol-disulfide oxidoreductases catalysing disulfide bond formation. PDIs are suggested to play a role as chaperones because of their ability to bind to unfolded or partially folded proteins (Wang and Tsou 1993). In cereals these proteins are important in multiple metabolic functions, including secretory protein folding and redox signalling (d’Aloisio et al. 2010).
Protein species associated with efficient induction of microspore embryogenesis in triticale anther culture
The largest number of protein species (47%) identified in our study was involved in metabolic processes, which is in agreement with results obtained by Uváčková et al. (2012). It could be explained by enormous metabolic changes which accompanied microspore reprogramming and ME induction (Fehér et al. 2003). The precise mechanism of this process is not defined what seems important is the alkalinisation of the cytoplasm which coincides with upregulation of genes coding for different vacuolar ATPase subunits. This phenomenon was proposed (Pauls et al. 2006) to be behind the observed cytoskeletal rearrangements and/or initiation of protein phosphorylation cascades (Segui-Simarro and Nuez 2008).
Generally, the majority of differentially abundant proteins was involved in carbon metabolism. One of the examples is beta-amylase (spot no 20) involved in the turnover of starch, an energy and carbon source necessary for cell division and differentiation (Andriotis et al. 2010). In accordance with our data, upregulation of genes responsible for starch hydrolysis was detected during induction of ME in barley (Maraschin et al. 2006). Another important protein of primary metabolism, which was indicated as involved in ME induction is fructokinase (spot no 22), which catalyzes the transfer of a phosphate group from ATP to fructose resulting in the production of fructose-6-phosphate. Some published data (Imin et al. 2004; Pan et al. 2009) suggest that this enzyme plays an important role in somatic embryogenesis in Medicago truncatula Gaertn and Citrus sinensis (L.) Osbeck.
Another group of genes upregulated during somatic and microspore embryogenesis code Auxin- and ABA-inducible factors. It was supposed that some elements of ABA signalling pathways could be involved in the activation of gene expression programs leading directly or indirectly to ME (Maraschin et al. 2005; Tsuwamoto et al. 2007). For example gene encoding cystein-labeled class II metallothionine protein—EcMt (Reynolds and Crawford 1996) in wheat and ABA-regulated alcohol dehydrogenase (Maraschin et al. 2006) in barley were upregulated during ME. Another of the ABA-regulated genes encodes S-adenosyl-l-methionine synthase (Kim et al. 2015) detected in this study as associated with ME induction (SAMS, spot no 25). This is a key enzyme in the biosynthesis of ethylene and polyamines, involved also in methylation reactions (Van de Poel et al. 2013). The data showing a role of gaseous plant hormone—ethylene in in vitro callus growth, organo- and embryogenesis has been reported quite frequently (Kumar et al. 2009). Additionally, it was proven that changes in the SAMS expression level strongly affected genes associated with defense response to abiotic stress, ethylene and jasmonic acid synthesis (Yu et al. 2012). Therefore higher levels of SAMS in anthers isolated from responsive DH lines after LT treatment are possibly not only the reaction to LT stress but also impose direct effect on ME induction.
The importance of plant adaptation ability to biotic/abiotic stress is confirmed by the differential expression of elicitor responsive protein 3 (spot no 26). In our study, a 2-fold change in the abundance of this protein was observed in responsive DH lines after LT treatment. The role of such a protein is elusive, although its accumulation could be caused by mechanical damage during the anthers isolation. Wounding itself could be a significant signal for dedifferentiation, causing the change in mRNA content for example in tobacco mesophyll protoplast cultures (Grosset et al. 1990).
Another protein which discriminated studied DH lines of triticale with various embryogenic potential after LT treatment was enolase (spot no 21). Similarly, enolase isoenzymes displayed differential expression in developing somatic embryos of spruce (Lippert et al. 2005). Authors suggested that its accumulation was caused by oxidative stress and proposed that it could be used as a molecular marker for embryogenic maturation. Studies on tomato and Arabidopsis demonstrated that enolase expression is more prevalent in non-green tissues, which could be associated with higher demand for glycolysis-produced energy (Van Der Straeten et al. 1991) in tissues devoid of chlorophyll. These data suggest that higher accumulation of enolase in triticale anthers of responsive DH lines reflects its better adaptation to ME-inducing treatment performed at 4 °C in the dark. ME-inducing treatment also caused higher accumulation of 12S storage protein in responsive DH lines of triticale. This result agrees with data published by Crouch (1982), who suggested that this protein could be another potential marker of ME.
Summarizing the results, 31 protein species were successfully identified as involved in the determination of microspore competence, stress response and in the regulation of ME induction. It is suggested that microspore competence was associated with sufficient energy supply and an efficient system of cell protection that determine survival under prolonged LT stress treatment (3 weeks at 4 °C) used for triggering ME in triticale anther cultures. Generally, LT stress was associated with increased accumulation of proteins typical for cell defense against oxidative stress, chaperons and other enzymes/factors ensuring protein biosynthesis, stability and active cell divisions. Also, effective cell defence required undisturbed energy supply. Among proteins that accumulated differentially in accordance with microspore embryogenic potential, again the most important role seems to be played by the enzymes ensuring energy production and determining the ability of plant stress adaptation. Two protein species (enolase, 12S storage protein) proposed earlier as candidates for molecular marker of embryogenesis in other in vitro plant culture systems confirmed their utility for triticale anther cultures.
The results increased our knowledge concerning the mechanism of microspore embryogenesis induction in winter triticale anther cultures. It provides the opportunity for optimization of the method in order to increase its efficiency, especially in the case of recalcitrant agronomically important genotypes. Some of the identified proteins/genes could be proposed as molecular markers for triticale microspore embryogenesis. Nevertheless, before practical application in breeding programs their utility should be validated on a wider population of triticale genotypes and with the use of isolated microspore culture system.
References
Ambrosone A, Costa A, Leone A, Grillo S (2012) Beyond transcription: RNA-binding proteins as emerging regulators of plant response to environmental constraints. Plant Sci 182:12–18. doi:10.1016/j.plantsci.2011.02.004
Andriotis VM, Pike MJ, Kular B, Rawsthorne S, Smith AM (2010) Starch turnover in developing oilseed embryos. New Phytol 187:791–804. doi:10.1111/j.1469-8137.2010.03311.x
Baerenfaller K, Massonnet C, Walsh S, Baginsky S, Bühlmann P, Hennig L, Hirsch-Hoffmann M, Howell KA, Kahlau S, Radziejwoski A, Russenberger D, Rutishauser D, Small I, Stekhoven D, Sulpice R, Svozil J, Wuyts N, Stitt M, Hilson P, Granier C, Gruissem W (2012) Systems-based analysis of Arabidopsis leaf growth reveals adaptation to water deficit. Mol Syst Biol. doi:10.1038/msb.2012.39
Berberich T, Sugawara K, Harada M, Kusano T (1995) Molecular cloning, characterization and expression of an elongation factor 1 alpha gene in maize. Plant Mol Biol 29:611–615
Binarova P, Straatman K, Hause B, Hause G, Van Lammeren AA (1993) Nuclear DNA synthesis during the induction of embryogenesis in cultured microspores and pollen of Brassica napus L. Theor Appl Genet 87:9–16. doi:10.1007/bf00223736
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Bustos DM, Bustamante CA, Iglesias AA (2008) Involvement of non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase in response to oxidative stress. J Plant Physiol 165:456–461. doi:10.1016/j.jplph.2007.06.005
Chugh A, Khurana P (2002) Gene expression during somatic embryogenesis—recent advances. Curr Sci 88:715–730
Cordewener J, Van Der Wal F, Joosen R, Boutilier K, America T (2009) Proteomics in rapeseed microspore embryogenesis. In: Touraev A, Forster B, Jain SM (eds) Advances in haploid production in higher plants. Springer, Netherlands, pp 135–146
Crouch M (1982) Non-zygotic embryos of Brassica napus L. contain embryo-specific storage proteins. Planta 156:520–524. doi:10.1007/BF00392774
D’aloisio E, Paolacci AR, Dhanapal AP, Tanzarella OA, Porceddu E, Ciaffi M (2010) the protein disulfide isomerase gene family in bread wheat (T. aestivum L.). BMC Plant Biol 10:101. doi:10.1186/1471-2229-10-101
De Pinto MC, Francis D, De Gara L (1999) The redox state of the ascorbate-dehydroascorbate pair as a specific sensor of cell division in tobacco BY-2 cells. Protoplasma 209:90–97
Dubas E, Wędzony M, Petrovska B, Salaj J, Żur I (2010) Cell structural reorganization during induction of androgenesis in isolated microspore cultures of triticale (×Triticosecale Wittm.). Acta Biol Crac Ser Bot 52(1):73–86. doi:10.1007/s00299-009-0730-2
Dunn MA, Morris A, Jack PL, Hughes MA (1993) A low-temperature-responsive translation elongation factor 1 alpha from barley (Hordeum vulgare L.). Plant Mol Biol 23:221–225
Dunwell JM (2010) Haploids in flowering plants: origins and exploitation. Plant Biotechnol J 8:377–424. doi:10.1111/j.1467-7652.2009.00498.x
Eudes F, Amundsen E (2005) Isolated microspore culture of Canadian 6x triticale cultivars. Plant Cell Tiss Organ Cult 82:233–241
Fehér A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult 74:201–228
Forster BP, Heberle-Bors E, Kasha KJ, Touraev A (2007) The resurgence of haploids in higher plants. Trends Plant Sci 12:368–375. doi:10.1016/j.tplants.2007.06.007
Fu J, Momčilović I, Prasad PVV (2012) Roles of protein synthesis elongation factor EF-Tu in heat tolerance in plants. J Bot 2012:1–8. doi:10.1155/2012/835836
Ghazalpour A, Bennett B, Petyuk VA, Orozco L, Hagopian R, Mungrue IN, Farber CR, Sinsheimer J, Kang HM, Furlotte N, Park CC, Wen P-Z, Brewer H, Weitz K, Camp DG, Pan C, Yordanova R, Neuhaus I, Tilford C, Siemers N, Gargalovic P, Eskin E, Kirchgessner T, Smith DJ, Smith RD, Lusis AJ (2011) Comparative analysis of proteome and transcriptome variation in mouse. PLoS Genet 7:e1001393. doi:10.1371/journal.pgen.1001393
Gonzalez JM, Muniz LM, Jouve N (2005) Mapping of QTLs for androgenetic response based on a molecular genetic map of ×Triticosecale Wittmack. Genome 48:999–1009. doi:10.1139/g05-064
Grosset J, Marty I, Chartier Y, Meyer Y (1990) mRNAs newly synthesized by tobacco mesophyll protoplasts are wound-inducible. Plant Mol Biol 15:485–496
Hosp J, De Maraschin SF, Touraev A, Boutilier K (2007) Functional genomics of microspore embryogenesis. Euphytica 158:275–285. doi:10.1007/s10681-006-9238-9
Hu X, Wu X, Li C, Lu M, Liu T, Wang Y, Wang W (2012) Abscisic acid refines the synthesis of chloroplast proteins in maize (Zea mays) in response to drought and light. PLoS ONE 7:e49500. doi:10.1371/journal.pone.0049500
Imin N, De Jong F, Mathesius U, Van Noorden G, Saeed NA, Wang XD, Rose RJ, Rolfe BG (2004) Proteome reference maps of Medicago truncatula embryogenic cell cultures generated from single protoplasts. Proteomics 4:1883–1896. doi:10.1002/pmic.200300803
Immonen S, Robinson J (2000) Stress treatments and ficoll for improving green plant regeneration in triticale anther culture. Plant Sci 150:77–84. doi:10.1016/S0168-9452(99)00169-7
Jacquard C, Mazeyrat-Gourbeyre F, Devaux P, Boutilier K, Baillieul F, Clément C (2009) Microspore embryogenesis in barley: anther pre-treatment stimulates plant defence gene expression. Planta 229:393–402. doi:10.1007/s00425-008-0838-6
Joosen R, Cordewener J, Supena ED, Vorst O, Lammers M, Maliepaard C, Zeilmaker T, Miki B, America T, Custers J, Boutilier K (2007) Combined transcriptome and proteome analysis identifies pathways and markers associated with the establishment of rapeseed microspore-derived embryo development. Plant Physiol 144:155–172. doi:10.1104/pp.107.098723
Jung KH, Park J-W (2011) Suppression of mitochondrial NADP+-dependent isocitrate dehydrogenase activity enhances curcumin-induced apoptosis in HCT116 cells. Free Radical Res 45:431–438. doi:10.3109/10715762.2010.540574
Kim SH, Kim SH, Palaniyandi SA, Yang SH, Suh JW (2015) Expression of potato S-adenosyl-l-methionine synthase (SbSAMS) gene altered developmental characteristics and stress responses in transgenic Arabidopsis plants. Plant Physiol Biochem 87:84–91. doi:10.1016/j.plaphy.2014.12.020
Klubicova K, Danchenko M, Skultety L, Berezhna VV, Hricova A, Rashydov NM, Hajduch M (2011) Agricultural recovery of a formerly radioactive area: II. Systematic proteomic characterization of flax seed development in the remediated Chernobyl area. J Proteomics 74:1378–1384. doi:10.1016/j.jprot.2011.02.029
Krzewska M, Czyczylo-Mysza I, Dubas E, Golebiowska-Pikania G, Golemiec E, Stojalowski S, Chrupek M, Zur I (2012) Quantitative trait loci associated with androgenic responsiveness in triticale (×Triticosecale Wittm.) anther culture. Plant Cell Rep 31:2099–2108. doi:10.1007/s00299-012-1320-2
Kumar V, Parvatam G, Ravishankar GA (2009) AgNO3—a potential regulator of ethylene activity and plant growth modulator. Electron J Biotechnol. doi:10.2225/vol12-issue2-fulltext-1
Lantos C, Bona L, Boda K, Pauk J (2014) Comparative analysis of in vitro anther- and isolated microspore culture in hexaploid Triticale (×Triticosecale Wittmack) for androgenic parameters. Euphytica 197:27–37. doi:10.1007/s10681-013-1031-y
Lichter R (1985) From microspores to rape plants: a tentative way to low glucosinolate strains. In: Sorensen H (ed) Cruciferous crops: production, utilization, description, vol II. Nijhoff/Junk, Boston, pp 268–277
Lippert D, Zhuang J, Ralph S, Ellis DE, Gilbert M, Olafson R, Ritland K, Ellis B, Douglas CJ, Bohlmann J (2005) Proteome analysis of early somatic embryogenesis in Picea glauca. Proteomics 5:461–473. doi:10.1002/pmic.200400986
Lorkovic ZJ (2009) Role of plant RNA-binding proteins in development, stress response and genome organization. Trends Plant Sci 14:229–236. doi:10.1016/j.tplants.2009.01.007
Maraschin SF, De Priester W, Spaink HP, Wang M (2005) Androgenic switch: an example of plant embryogenesis from the male gametophyte perspective. J Exp Bot 56:1711–1726. doi:10.1093/jxb/eri190
Maraschin SDF, Caspers M, Potokina E, Wulfert F, Graner A, Spaink HP, Wang M (2006) cDNA array analysis of stress-induced gene expression in barley androgenesis. Physiol Plant 127:535–550. doi:10.1111/j.1399-3054.2006.00673.x
Mayer MP, Bukau B (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62:670–684. doi:10.1007/s00018-004-4464-6
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Muñoz-Amatriaín M, Svensson JT, Castillo AM, Cistué L, Close TJ, Vallés MP (2009) Expression profiles in barley microspore embryogenesis. In: Touraev A, Jain SM, Forster BP (eds) Advances in haploid production in higher plants. Springer, New york, pp 127–134
Olarewaju O, Ortiz PA, Chowdhury WQ, Chatterjee I, Kinzy TG (2004) The translation elongation factor eEF1B plays a role in the oxidative stress response pathway. RNA Biol 1:89–94
Pan Z, Guan R, Zhu S, Deng X (2009) Proteomic analysis of somatic embryogenesis in Valencia sweet orange (Citrus sinensis Osbeck). Plant Cell Rep 28:281–289. doi:10.1007/s00299-008-0633-7
Pauk J, Mihály R, Monostori T, Puolimatka M (2003) Protocol of triticale (×Triticosecale Wittmack) microspore culture. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants. Springer, Netherlands, pp 129–134
Pauls KP, Chan J, Woronuk G, Schulze D, Brazolot J (2006) When microspores decide to become embryos—cellular and molecular changes. This review is one of a selection of papers published in the Special Issue on Plant Cell Biology. Can J Bot 84:668–678. doi:10.1139/b06-064
Pechan PM, Barteis D, Brown DCW, Schell J (1991) Messenger-RNA and protein changes associated with induction of Brassica microspore embryogenesis. Planta 184:161–165
Reynolds TL, Crawford RL (1996) Changes in abundance of an abscisic acid-responsive, early cysteine-labeled metallothionein transcript during pollen embryogenesis in bread wheat (Triticum aestivum). Plant Mol Biol 32:823–829
Sánchez-Díaz RA, Castillo AM, Vallés MP (2013) Microspore embryogenesis in wheat: new marker genes for early, middle and late stages of embryo development. Plant Reproduction 26:287–296. doi:10.1007/s00497-013-0225-8
Segui-Simarro JM, Nuez F (2008) How microspores transform into haploid embryos: changes associated with embryogenesis induction and microspore-derived embryogenesis. Physiol Plant 134:1–12. doi:10.1111/j.1399-3054.2008.01113.x
Seguí-Simarro JM, Testillano PS, Risueño MC (2003) Hsp70 and Hsp90 change their expression and subcellular localization after microspore embryogenesis induction in Brassica napus L. J Struct Biol 142:379–391. doi:10.1016/s1047-8477(03)00067-4
Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68:850–858
Soriano M, Li H, Boutilier K (2013) Microspore embryogenesis: establishment of embryo identity and pattern in culture. Plant Reprod 26:181–196. doi:10.1007/s00497-013-0226-7
Testillano PS, Coronado MJ, Segui JM, Domenech J, Gonzalez-Melendi P, Raska I, Risueno MC (2000) Defined nuclear changes accompany the reprogramming of the microspore to embryogenesis. J Struct Biol 129:223–232. doi:10.1006/jsbi.2000.4249
Touraev A, Vicente O, Heberle-Bors E (1997) Initiation of microspore embryogenesis by stress. Trends Plant Sci 2:285–303
Touraev A, Pfosser M, Heberle-Bors E (2001) The microspore: a haploid multipurpose cell. In: Advances in botanical research. Academic Press, Cambridge, pp 53–109
Tsuwamoto R, Fukuoka H, Takahata Y (2007) Identification and characterization of genes expressed in early embryogenesis from microspores of Brassica napus. Planta 225:641–652. doi:10.1007/s00425-006-0388-8
Tyrka M, Bednarek PT, Kilian A, Wędzony M, Hura T, Bauer E (2011) Genetic map of triticale compiling DArT, SSR, and AFLP markers. Genome 401:391–401. doi:10.1139/G11-009
Uváčková Ľ, Takáč T, Boehm N, Obert B, Šamaj J (2012) Proteomic and biochemical analysis of maize anthers after cold pretreatment and induction of androgenesis reveals an important role of anti-oxidative enzymes. J Proteom 75:1886–1894. doi:10.1016/j.jprot.2011.12.033
Van De Poel B, Bulens I, Oppermann Y, Hertog ML, Nicolai BM, Sauter M, Geeraerd AH (2013) S-adenosyl-l-methionine usage during climacteric ripening of tomato in relation to ethylene and polyamine biosynthesis and transmethylation capacity. Physiol Plant 148:176–188. doi:10.1111/j.1399-3054.2012.01703.x
Van Der Straeten D, Rodrigues-Pousada RA, Goodman HM, Van Montagua M (1991) Plant enolase: gene structure, expression, and evolution. Plant Cell 3:719–735
Vergne P, Riccardi F, Beckert M, Dumas C (1993) Identification of a 32-kDa anther marker protein for androgenic response in maize, Zea mays L. Theor Appl Genet 86:843–850
Vrinten PL, Nakamura T, Kasha KJ (1999) Characterization of cDNAs expressed in the early stages of microspore embryogenesis in barley (Hordeum vulgare L.). Plant Mol Biol 41:455–463
Wang P, Chen Y (1983) Preliminary study on prediction of height of pollen H2 generation in winter wheat grown in the field. Acta Agron Sin 9:283–284
Wang C-C, Tsou C-L (1993) Protein disuffide isomerase is both an enzyme and a chaperone. FASEB J. 7:1515–1517
Wędzony M (2003) Protocol for doubled haploid production in hexaploid triticale (×Triticosecale Wittm.) by crosses with maize. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants. Springer, New York, pp 135–140
Wędzony M, Marcińska I, Ponitka A, Ślusarkiewicz-Jarzina A, Woźna J (1998) Production of doubled haploids in triticale (×Triticosecale Wittm.) by means of crosses with maize (Zea mays L.) using picloram and dicamba. Plant Breed. 117:211–215. doi:10.1111/j.1439-0523.1998.tb01928.x
Wędzony M, Żur I, Krzewska M, Dubas E, Szechyńska-Hebda M, Wąsek I (2015) Doubled haploids in triticale. In: Eudes F (ed) triticale. Springer, New York, pp 111–128
Yu J, Lee G, Park Y (2012) Physiological role of endogenous S-adenosyl-L-methionine synthetase in Chinese cabbage. Hortic Environ Biotechnol 53:247–255. doi:10.1007/s13580-012-0021-7
Zhang LY, Xu J (1983) Increasing differentiation frequencies in wheat pollen callus. In: Hu H, Vega MR (eds) Cell and tissue culture techniques for cereal crop improvement. Science Press, Beijing, pp 431–432
Zhang J, Ma H, Chen S, Ji M, Perl A, Kovacs L, Chen S (2009) Stress response proteins’ differential expression in embryogenic and non-embryogenic callus of Vitis vinifera L. cv. Cabernet Sauvignon—a proteomic approach. Plant Sci 177:103–113. doi:10.1016/j.plantsci.2009.04.003
Zoriniants S, Tashpulatov AS, Heberle-Bors E, Touraev A (2005) The role of stress in the induction of haploid microspore embryogenesis. In: Don Palmer CE, Keller W, Kasha K (eds) Haploids in crop improvement II. Springer, Berlin, pp 35–52
Żur I, Dubas E, Golemiec E, Szechyńska-Hebda M, Janowiak F, Wędzony M (2008) Stress-induced changes important for effective androgenic induction in isolated microspore culture of triticale (×Triticosecale Wittm.). Plant Cell Tissue Organ Cult 94:319–328. doi:10.1007/s11240-008-9360-6
Żur I, Dubas E, Golemiec E, Szechyńska-Hebda M, Golębiowska G, Wędzony M (2009) Stress-related variation in antioxidative enzymes activity and cell metabolism efficiency associated with embryogenesis induction in isolated microspore culture of triticale (×Triticosecale Wittm.). Plant Cell Rep 28:1279–1287. doi:10.1007/s00299-009-0730-2
Żur I, Krzewska M, Dubas E, Gołębiowska-Pikania G, Janowiak F, Stojałowski S (2012) Molecular mapping of loci associated with abscisic acid accumulation in triticale (×Triticosecale Wittm.) anthers in response to low temperature stress inducing androgenic development. Plant Growth Regul 68:483–492. doi:10.1007/s10725-012-9738-7
Żur I, Dubas E, Krzewska M, Sánchez-Díaz RA, Castillo AM, Vallés MP (2013) Changes in gene expression patterns associated with microspore embryogenesis in hexaploid triticale (×Triticosecale Wittm.). Plant Cell Tissue Organ Culture (PCTOC) 116:261–267. doi:10.1007/s11240-013-0399-7
Żur I, Dubas E, Krzewska M, Janowiak F, Hura K, Pociecha E, Bączek-Kwinta R, Płażek A (2014) Antioxidant activity and ROS tolerance in triticale (×Triticosecale Wittm.) anthers affect the efficiency of microspore embryogenesis. Plant Cell Tissue and Organ Cult (PCTOC) 119:79–94. doi:10.1007/s11240-014-0515-3
Żur I, Dubas E, Krzewska M, Janowiak F (2015a) Current insights into hormonal regulation of microspore embryogenesis. Front Plant Sci 6:424. doi:10.3389/fpls.2015.00424
Żur I, Dubas E, Krzewska M, Waligórski P, Dziurka M, Janowiak F (2015b) Hormonal requirements for effective induction of microspore embryogenesis in triticale (×Triticosecale Wittm.) anther cultures. Plant Cell Rep 34:47–62. doi:10.1007/s00299-014-1686-4
Acknowledgements
The research was supported by National Science Centre (NCN); Project No. 2011/01/N/NZ9/02541 financed by Polish Ministry of Science and Higher Education. The protein identification was carried out with the equipment purchased thanks to the financial support of the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (Contract No. POIG.02.01.00-12-023/08). The authors are grateful to Prof. Martin Hajduch and Dr. Katarína Klubicová (Department of Reproduction and Developmental Biology, Institute of Plant Genetics and Biotechnology, Slovak Academy of Sciences, Nitra, Slovakia) who provided insight and expertise that greatly assisted the research, although they may not agree with all of the interpretations/conclusions of this paper.
Author information
Authors and Affiliations
Contributions
MK co-performed the experiments, co-analyzed the data and co-wrote the manuscript. GG-P, ED, MG performed the experiments and collected the raw data. IŻ designed the experiments and co-wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Krzewska, M., Gołębiowska-Pikania, G., Dubas, E. et al. Identification of proteins related to microspore embryogenesis responsiveness in anther cultures of winter triticale (×Triticosecale Wittm.). Euphytica 213, 192 (2017). https://doi.org/10.1007/s10681-017-1978-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-017-1978-1