Abstract
The gastrointestinal (GI) organs of the human body are responsible for transporting and extracting nutrients from food and drink, as well as excreting solid waste. Biomechanical experimentation of the GI organs provides insight into the mechanisms involved in their normal physiological functions, as well as understanding of how diseases can cause disruption to these. Additionally, experimental findings form the basis of all finite element (FE) modelling of these organs, which have a wide array of applications within medicine and engineering. This systematic review summarises the experimental studies that are currently in the literature (n = 247) and outlines the areas in which experimentation is lacking, highlighting what is still required in order to more fully understand the mechanical behaviour of the GI organs. These include (i) more human data, allowing for more accurate modelling for applications within medicine, (ii) an increase in time-dependent studies, and (iii) more sophisticated in vivo testing methods which allow for both the layer- and direction-dependent characterisation of the GI organs. The findings of this review can also be used to identify experimental data for the readers’ own constitutive or FE modelling as the experimental studies have been grouped in terms of organ (oesophagus, stomach, small intestine, large intestine or rectum), test condition (ex vivo or in vivo), number of directions studied (isotropic or anisotropic), species family (human, porcine, feline etc.), tissue condition (intact wall or layer-dependent) and the type of test performed (biaxial tension, inflation–extension, distension (pressure-diameter), etc.). Furthermore, the studies that investigated the time-dependent (viscoelastic) behaviour of the tissues have been presented.
Similar content being viewed by others
1 Introduction
The gastrointestinal (GI) tract is a muscular tube that extends from the mouth all the way to the anus (Ogobuiro et al. 2021), as can be seen in Fig. 1. The tube is hollow and allows for the passage of food and drink through the body with the aim of extracting its nutrients and expelling the waste products. The oesophagus, the first organ of the GI tract, is responsible for moving the food from the mouth to the stomach. The stomach is responsible for temporarily storing the food, breaking it down both mechanically and chemically and passing it onto the small intestine. The small intestine is the site where 90% of the absorption of nutrients from the food takes place, after which the remaining material is passed onto the large intestine. The large intestine absorbs water and electrolytes from the remaining material. The rectum then stores the solid waste product before expelling it through the anus (Ogobuiro et al. 2021). Each tissue has a slightly different microstructural composition, evolved for the specific function of each organ; for example, villi in the small intestine greatly increase its internal surface area for increased efficiency of nutrient absorption and digestive secretion (Helander and Fändriks 2014). However, all the GI organs have an innermost mucosal layer, an adjacent submucosal layer, then a muscular layer, named the muscularis propria, and, finally, an outermost adventitial (for the oesophagus) or serosal (for the stomach, small intestine, large intestine and rectum) layer. The mucosal layer also contains a thin, muscular layer called the muscularis mucosae (Wanamaker and Grimm 2004). Most collagen and elastin of the GI organs are situated in the mucosal, submucosal and outer layers (Van de Graaff 1986; Baidoo et al. 2022; Durcan et al. 2022a). For a more comprehensive outline of the anatomy of the GI organs, readers are referred to Van de Graaff (1986). Due to the alignment of the fibres in the GI tissues (collagen, elastin and muscle), it can normally be seen that their behaviour is anisotropic (Siri et al. 2020; Durcan et al. 2022a), i.e. they present different stress–strain relations depending on the direction in which the tissue is loaded.
The various organs of the gastrointestinal tract situated in the human body. Figure adapted from Cleveland Clinic (2023)
Mechanics are innate to the GI tract’s function. The transportation of food and drink through the tract is brought about by peristalsis: a mechanical process, which propels the ingested material, named fluid bolus when in the oesophagus and chyme when in the other GI organs, through sequential contractions of the muscular wall (Van de Graaff 1986). Peristalsis is also responsible for churning in the stomach, which is a form of physical digestion where the food is mechanically broken down rather than chemically such as with enzymes or stomach acid. This mechanical behaviour of the GI wall is brought about through a combination of passive distensions and active contractions, and the interaction of these with the bolus/chyme (Gregersen and Kassab 1996). The properties of the wall during the passive distensions (such as elasticity, plasticity, and viscosity) provide the stiffness (degree of force exerted by a material when it is loaded) needed along with the active force of the muscle fibres (contractility) to move the hydrodynamic bolus/chyme during peristalsis. Such passive and active properties are organ-specific, depending on their function. For example, the passive material properties of the rectal wall must possess a certain compliance (opposite of stiffness) to be able to accommodate the changing amount of faecal waste product that is temporarily stored there, while the oesophagus requires a different level of compliance to be able to adjust to various bolus sizes that enter it while not being too great as to hinder its primary goal of transporting the bolus to the stomach. However, diseases can affect the passive and active behaviour of the GI tract, disrupting the role of each organ and leading to complications within a patient’s digestive system. For instance, type-2 diabetes has been found to significantly increase the circumferential stiffness of the oesophageal wall in rats (Zhao et al. 2007).
From the histological images in Fig. 2, one can see that the onset of diabetes in this animal model has greatly influenced the thickness of the muscularis propria layer, and, as reported by Zhao et al. (2007), has significantly increased the amount of collagen in the mucosa-submucosa layer. These changes in morphology and fraction of microstructural components may allude to the origin of mechanical disorders of the GI tract commonly found in diabetic patients (Horowitz and Samsom 2004); due to the disease, the tissue wall is remodelled and the careful balance of forces that exist in the GI tract between the bolus and the passive/active properties of the wall, that keep the digestive system of so many humans running smoothly, has been disrupted (Frøkjær et al. 2007). Similar biomechanical changes caused by type-1 and type-2 diabetes have been found for other organs of the GI tract including the stomach (Liao et al. 2006), small intestine (Jørgensen et al. 2001; Zhao et al. 2003a) and large intestine (Zhao et al. 2009). Experimentation allows for the investigation into the origin of these disruptions to the GI tract’s mechanical function, providing the information needed to devise creative ways to treat them. As is known within the scientific method, controls, or study of the healthy tissue’s properties, are required to understand the normal function of the GI tissues, thus allowing the effects of the diseases, and potential ways to remedy them, to be properly established.
Haematoxylin and eosin (H&E) histological staining of the oesophagus of diabetic Wistar rats (realised through the Goto-Kakizaki (GK) rat model for type-2 diabetes (Goto and Kakizaki 1981)) compared to non-diabetic (normal) Wistar rats, showing the difference between muscle layer thicknesses. The thickness of the longitudinal and circular muscle layers were significantly greater in the diabetic rats compared to the normal rats (p < 0.01). Figure has been modified from the review by Zhao and Gregersen (2016) and was originally from a study by Zhao et al. (2007)
Another, potentially more advanced, way that allows for the investigation into the mechanisms of how a healthy GI tract functions, and the effect of the changes that occur under pathophysiological conditions, is the use of in silico (computational) models. The three types of computational models typically used in the field of GI biomechanics are finite element (FE) analysis (Liao et al. 2006; Panda and Buist 2019), computational fluid dynamics (CFD) (Ferrua and Singh 2010; Palmada et al. 2023) and fluid–structure interactions (FSI) (Toniolo et al. 2023; Zhang et al. 2016). Finite element models provide a numerical approximation of how the tissue or organ behaves mechanically, i.e. structurally, with consideration of its unique geometry and boundary conditions; CFD models allow for the predication of fluid flow through the digestive tract; and FSI provide a means to investigate the interplay between the fluid within and the tissue/organ material structure of the GI tract. Each of the methods has the ability to deliver understanding of the organs’ fluid or structural relations not always possible through experimentation alone (Toniolo et al. 2022), and the structural properties will be focused on in this review. For instance, using a two-layered FE model, Yang et al. (2007a) established why, in a mechanical context, mucosal folds arise within the oesophagus, presenting what would happen to the active tension required of the muscle layer to maintain normal function if these folds were not present. Physiological processes such as peristalsis (Yang et al. 2007b) and the mechanical breakdown of food in the stomach (Skamniotis et al. 2020) can be studied using FE or FSI models to provide insight into which circumstances (e.g., certain wall thickness, amount of collagen, etc.) lead to in-optimal function (Panda and Buist 2019). In addition, structural computational models can be used to establish how the organ responds when medical devices are introduced, either to assess the mechanical effects of traditional devices such as endoscopes (Lin et al. 2020), or to aid with the design of novel medical devices such as stents (Peirlinck et al. 2018; Shanahan et al. 2017), capsule endoscopes (Kim et al. 2007; Gao et al. 2010), capsule biopsy devices (Ye et al. 2019) and surgical staples (Nováček et al. 2012; Guo et al. 2021). Used in this way, models can help save time, biological test specimens and other resources needed during the design process. Further to this, FE models can be used to investigate the effects of surgical interventions, such as bariatric surgery (e.g. reduction in the size of the stomach through a partial gastrectomy) used in the treatment of patients with obesity, on the biomechanics of the GI organs (Toniolo et al. 2022), with one aim being to have patient-specific pre- and post-operative computational models of the organ prior to the procedure to provide a means to assess the best surgical intervention and predict potential post-procedural complications. Moreover, surgical simulations are a growing technology which can utilise FE models to provide haptic force feedback information to a surgeon (Chakravarthy et al. 2014), allowing them to practise and hone their skills before conducting surgery on a patient (Badash et al. 2016). In essence, computational models allow us to predict and numerically assess the complex mechanical behaviour of the GI organs under a wide variety of conditions and thus have valuable applications throughout engineering and medicine.
The equations underpinning the type of FE models mentioned above, as well as the structure portion of FSI models, are conservation and constitutive laws, which describe the mechanical behaviour of the tissue according to Newton’s principles and the individual composition of the material, respectively (Patel et al. 2022). Constitutive laws, originating in this case from the domain of continuum solid mechanics, provide a mathematical representation of the tissue’s behaviour and are based on the well-informed theory that each component (constituent) of the material contributes to its overall behaviour, and thus, its material response can be modelled through a summation of the behaviour of each part. This type of modelling, specifically microstructural based constitutive models, allows for the investigation of the effect of different constituents on the material behaviour of the tissue (Holzapfel and Ogden 2020). Due to the different types of fibres in each of the GI organs, and the differing fractions of mechanically-influential fibres such as collagen and elastin, the individual layers tend to present distinct material behaviour, bearing different loads when forces are applied to the whole tissue structure (Dargar et al. 2019). Due to the soft nature of the GI tissues, which allow easily for large deformations of the organ, the stress–strain response is linear at very small strains but quickly becomes nonlinear when deformed further (Egorov et al. 2002; Rosen et al. 2008; Natali et al. 2009; Christensen et al. 2015; Borsdorf et al. 2021). Therefore, nonlinear elastic laws, rather than linear elastic (which are used for traditional engineering materials such as metals and concrete, or for hard tissue like bone), are often used to describe the behaviour of such tissues (and more modern engineering materials such as polymers) (Holzapfel and Ogden 2006; Chagnon et al. 2015). Additionally, the arrangement of the microstructural components of the tissue, such as collagen and elastin fibres, results in the GI organs exhibiting an anisotropic material response. For this reason, anisotropic constitutive models are often employed when representing the behaviour of the GI tissues. Other, more complex behaviour can also be considered in the constitutive model, such as the time-dependent (viscoelastic) and history-dependent (stress-softening) response of the tissue. Constitutive laws can be used to simulate both the passive and active behaviour of the GI tissues. For a comprehensive review on the constitutive laws used to model the GI tract, readers are referred to Patel et al. (2022).
The parameters, i.e. constants, of the constitutive model are specific to the material in question. This, along with the formulation of the constitutive model based on knowledge of the material’s microstructure and the observed experimental behaviour, distinguishes one material from another for, for example, use in multi-material FE simulations. The parameters also allow for a quantitative comparison between different materials, particularly if the same constitutive law is used. To determine these parameters, the model must be compared with experimental data of the tissue (Weizel et al. 2022). Then, the parameters that provide a mathematical simulation closest to that of the experimental data are determined through an optimisation method (Patel et al. 2022). Different types of experiments are required to establish the various aspects of the material’s behaviour, e.g. active or passive, anisotropic, hyperelastic, viscoelastic, stress-softening. Therefore, to be able to determine the effects of disease on the function of the GI organs (experimentally and in silico), to model their constitutive behaviour and further understand the contribution of each component, and to be able to model using the FE method the behaviour of the organ as a function of its geometry and boundary conditions, experimental data are required.
This review paper considers this topic, providing a comprehensive, systematic review of the experimental studies currently available in the literature on the biomechanical behaviour of the GI organs. The articles found in the search are presented for each GI organ in terms of their test condition (ex vivo or in vivo), the origin of tissue tested (human, rodent, porcine, etc.), type of experiment conducted (uniaxial tension, compression, zero-stress state, etc.), and in terms of whether the direction-dependent and layer-dependent behaviour of the organ was studied. Furthermore, the articles investigating the time-dependent behaviour of the GI organs are shown, and those studying the active or diseased state are mentioned. The proportion of experiments conducted on different species for each GI organ are also illustrated, highlighting, in particular, which organs are lacking experimental data on human tissue. Additionally, the most common experimental techniques to characterise the GI organs are outlined, and the prominence within literature of certain experimental practices, such as preconditioning and the use of a physiological saline solution bath, are displayed. This review aims to bring awareness to the experimental data that exists in regard to the mechanical characterisation of the GI organs and highlight what is currently absent as a call for further experimentation in this area. The information presented here can also be used to direct readers to studies in their particular area of interest, for instance, to provide further understanding or experimental data for their own constitutive and FE modelling.
2 Review strategy
The systematic search for this review was carried out using the PubMed database. The search was conducted using key terms associated with biomechanical experimentation, such as “biomechanical”, “mechanical”, “properties”, “behaviour”, “response”, “stress”, “strain”, that could be found in the title or abstract of an article in combination with terms for each of the organs studied: oesophagus, stomach, small intestine, large intestine and rectum. The terms used for each organ can be found in Table 1. Even though the rectum is part of the large intestine; it has been treated as a separate organ here due to its unique function in comparison with the remaining large intestine; the rectum is responsible for the storage and excretion of faeces, whereas the other regions of the large intestine absorb water and electrolytes from the consumed material. The results of the search for each organ were then screened according to certain criteria; these included articles published in peer-reviewed journals, i.e. no pre-prints or conference proceedings, that provided novel (original) experimental data on the macrostructural mechanical properties of the organs in question, in particular experimental data that presented/allowed for the establishment of the stress–strain relations of the tissue or provided the pressure–volume relationship of the organ structure. Experimental studies on the sphincters of the GI tract were not included. There was no lower date limit for the articles; however, studies available online after 15 October 2022 were not included. Any articles not retrieved from the search but known by the authors were added to the pool of articles included in this review.
3 Experimental techniques
A variety of techniques are used to mechanically characterise the GI tissues. The type of test chosen should be in line with the proposed research question, e.g. are physiological or supraphysiological loading conditions more suitable to quantify the material properties of the GI tissues in the setting/application that we are interested in? In this section, we will outline some of the most common experimental techniques used to quantify the biomechanical behaviour of the GI tract.
For the interpretation of data obtained from such experimental techniques, it is commonly assumed that tissues of the GI tract are incompressible. That is to say that during experimental loading, the volume of the tissue does not change (Nolan and McGarry 2016). While this, physically, is not completely true, the high water content of soft tissues means that they often exhibit properties close to incompressibility (Gilchrist et al. 2014); therefore, the assumption is sufficient in producing meaningful results and is valuable in that it provides a simplification that reduces computational cost.
Mechanical experimentation of human or animal soft tissues can be separated into three categories: in vivo, in situ and ex vivo. In vivo experimentation is carried out in the natural environment of the organ, while the human/animal is still living. For organs such as the skin, these experiments can be conducted on the surface of the body. However, for the GI organs, as they are inside the body, a device must be inserted into the body to obtain biomechanical measurements. In situ tests are those conducted, whilst the tissue is still connected to the body but is not in its completely natural state, such as experiments conducted on an organ accessed via a surgical opening to the chest. In situ experiments can be carried out both while the human/animal is alive or post-mortem. Ex vivo (sometimes called “in vitro”, although “ex vivo” is technically more accurate in regard to the macromechanical characterisations of soft tissues) experimentation is when the organ is removed via dissection from its natural environment and, thus, is no longer alive during the mechanical tests. Tissue can be taken from either alive or deceased subjects, however when the tissue is tested, it is always deceased. Firstly, we will describe the ex vivo experimental techniques commonly used to characterise the GI tissues, and secondly, we will summarise the in vivo techniques. In situ tests are the same as those used for either ex vivo or in vivo experimentation and therefore have not been given their own section.
3.1 Ex vivo
Ex vivo experiments are those performed on naturally grown tissues taken outside of their physiological environment, i.e. excised via dissection from alive or deceased subjects. When the experiments are conducted, the tissue is deceased; therefore, measures should be taken to test the tissue as soon as possible to reduce the time-dependent effects of death, such as ischaemia, on the mechanical properties of the tissue (Marie et al. 2019). In addition, measures are also taken within the test set-up to simulate a more physiological environment in terms of moisture, temperature and, sometimes, carbon dioxide and oxygen concentrations (Liu et al. 2017a; Sokolis et al. 2011), reducing these factors as ones that can cause a discrepancy between in vivo and ex vivo material behaviour (as in vivo is often the environment of interest).
3.1.1 Uniaxial tension
Uniaxial tensile tests are the most basic tension test in which a specimen of a planar material is loaded along its length, often until failure. For a uniaxial tensile test, the specimen must have a length-to-width ratio of at least 4:1 (ASTM International 2016) (which can be an issue when working with small organs such as the rabbit oesophagus (Jensen et al. 1987)), and the specimens can either be dogbone-shaped (Yang et al. 2006c; Sommer et al. 2013) or rectangular, as seen in Fig. 3. Dogbone samples are more ideal as they encourage rupture to take place in the middle of the specimen rather than at the grip (though this is not guaranteed, and specimens that rupture at the grip should be discarded from analysis); however, it can be difficult to punch consistent dogbone specimens from soft tissues and so in the field of soft tissue biomechanics, it is common to use rectangular-shaped specimens (Egorov et al. 2002; Zhao et al. 2005; Gao et al. 2008; Carniel et al. 2014a; Christensen et al. 2015; Ivakhov et al. 2020).
A uniaxial tensile test experimental set-up used to investigate the small intestine of pigs. The bottom clamp (grip) is fixed, while the upper clamp is moved in a displacement-controlled way a. Sample preparation of strips of small intestinal tissue for uniaxial tensile testing; to investigate anisotropy (direction-dependent behaviour) of the tissue, specimens can be cut in the longitudinal and circumferential directions, as well as at various angles b (Nagaraja et al. 2021)
Uniaxial tensile tests are commonly employed for isotropic materials, such as some metals and polymers (Khan and Liu 2012; Mehnert and Steinmann 2019); however, they can be used to study the anisotropy of a GI tissue by testing strips from the longitudinal (axial) and circumferential directions, and also from various angles in-between these two directions, as seen in Fig. 3b. They cannot, however, be used to determine the radial stress–strain relation of the tissue. Often the grips used to secure the tissue for uniaxial tensile testing have serrated edges or sand paper attached to their inner surfaces to prevent the sample from slipping during testing (Davis et al. 2018; Nagaraja et al. 2021). Sometimes the grips are tightened to a pre-established torque level to find the optimal balance between preventing slippage during testing and not causing the sample to rupture at the grip because they are too tightly secured. Furthermore, tightening the grips to a specific torque provides consistency and reduces the influence of one factor that could affect the repeatability of the results (Davis et al. 2018; Durcan et al. 2022b).
The strain-rate-independent (elastic) behaviour of a tissue can be established under uniaxial tension by loading a sample until failure at a quasi-static strain rate; that is, a strain rate slow enough to allow, theoretically, the viscous relaxation to take place during loading; thus, the material is close to its equilibrium state (material properties once all viscous effects have disappeared). Some experimental studies that perform tests like these precondition their sample first (more on preconditioning in Sect. 4.6.2), removing some of the history- and time-dependent effects that occur during initial loading of a soft biological tissue. Moreover, experiments such as stress-relaxation tests may be carried out to determine the equilibrium stress–strain of the sample (Carniel et al. 2020). Sometimes also called ramp and hold tests, stress-relaxation tests consist of very quickly stretching a sample to a certain strain and holding it there for a considerable amount of time. For soft tissues, it is expected that the stress within the tissue when held will decrease. The length of time that the material is held depends on its relaxation time: for some soft tissues it can take as little as 5 min for the stress to plateau during relaxation (Zhao et al. 2003b; Jia et al. 2015; Carniel et al. 2020), while for some polymers it can take around 30 min (Hossain et al. 2012). When carried out over various stretch levels, the stress after the relaxation period plotted against the strain at which the sample was stretched provides the equilibrium stress–strain relation of the material and, in the context of large strain, can be used to model its hyperelastic behaviour. Creep tests are similar to stress-relaxation tests in that the equilibrium stress–strain relation of the material can be established; however, creep tests are load-controlled rather than strain-controlled. For creep tests, a certain stress is applied to the material and the stress is held at that level while the strain of the sample changes due to viscous effects (Zhao et al. 2003b; Jia et al. 2015). For soft tissues, it can normally be expected that the strain will increase as the sample is held at a certain stress. The maximum deformation (strain) after the creep period can then be plotted against the stress level the sample was held at. Doing this for several stress levels and plotting them on the same graph can provide a picture of the equilibrium stress–strain relationship of the material.
In order to provide a complete picture of the viscoelasticity of a tissue, the time-dependent (viscous) behaviour of the material should be investigated alongside the time-independent properties. The time-dependent behaviour can be studied by conducting uniaxial tensile tests at several different strain rates, including those within and above the quasi-static range and ideally an order of magnitude apart, e.g. 0.1 mm/s, 1.0 mm/s and 10 mm/s (due to the variable nature of soft tissues and thus their mechanical response, an order of magnitude between the strain rates provides a big enough range to be able to experimentally observe the strain rate effects. Tensile tests can also be carried out at dynamic strain rates to establish the behaviour of the tissue under impact. Additionally, cyclic tests can be performed to investigate the differences between the loading and unloading curves. If the sample has been preconditioned, the difference between the loading–unloading curve that remains is thought to be mainly due to the time-dependent relaxation of the specimen. Uniaxial tensile tests are popular in determining the active properties of soft tissues. In this case, the sample is held at zero strain, or other strain levels, and is either activated using a compound, such as potassium chloride, which activates muscle contraction or via electrical stimulation (Jiang et al. 2017; Tomalka et al. 2017). The measured force and change in length of the sample are then used to establish the stress–strain relation under active conditions.
3.1.2 Biaxial tension
Biaxial tensile tests are similar to uniaxial tensile tests in that they are performed on planar materials under tension; however, biaxial tests consist of stretching a square sample of a material along two orthogonal directions simultaneously, as seen in Fig. 4; hence, with each individual tissue sample, biaxial tests allow the direction-dependent properties of the tissue to be studied. On this note, biaxial tensile tests are often preferred to uniaxial tensile tests in the domain of hollow soft tissue mechanics as, by stretching the tissue in two directions at the same time rather than testing isolated strips in only one direction, biaxial tension is closer to the in vivo loading environment of the organ wall. The stretching in two directions can either be to the same degree, which is called equibiaxial tension, or by different amounts per direction. The choice of this will depend on the application, e.g. during physiological loading conditions, the tissue may undergo differing amounts of stretch in the circumferential and longitudinal directions, thus it may be of value to prescribe different amounts of loading to the circumferential and longitudinal directions to match those typically experienced in vivo.
A biaxial tensile test experimental set-up used to investigate the small intestine of pigs. Deformation is applied to a square sample (10 mm × 10 mm) through hooks attached to each side. Four graphite markers were placed on the surface of the sample to optically track its displacement during testing (Bellini et al. 2011)
For biaxial tension, the samples must be square, but the size is not critical as long as it is well supported by the testing machine (Bellini et al. 2011; Sommer et al. 2013). This freedom with size can be useful in particular for soft tissue specimens where the number of samples available is either often severely limited, e.g. with human testing, or should be kept to a minimum due to ethical considerations, e.g. with animal testing. The square sample size can be adjusted to allow for as many test samples as possible from a single excised organ. As shown in Fig. 4, the gripping mechanism for biaxial tensile tests is different to that for a uniaxial tension system. Here, several hooks placed equidistantly along each side of the square sample are used to secure and then stretch the specimen. When the specimen is set-up, the time-independent and time-dependent behaviour of the tissue can be studied using similar methods for uniaxial tension, e.g. cyclic testing, varying strain rates, stress-relaxation, etc., as outlined in Sect. 3.1.1.
3.1.3 Pure shear
Pure shear tests, sometimes called planar tensile tests, are similar to uniaxial tension tests in that rectangular samples are stretched in only one direction. With pure shear tests, however, the width of the sample is much larger than its length, as can be seen in Fig. 5, for which the length-to-width ratio must be at least 1:2 (Mulvihill and Walsh 2013). This ensures that no significant contraction can take place along the width during loading, making it that the tension in one direction is equal to the orthogonal direction’s compression, producing no rigid body rotation and thus only shear strains within the specimen. Furthermore, pure shear tests are similar to uniaxial tensile tests in that the grips are often serrated or have sand-paper attached to them to reduce slippage of the sample during testing (Davis et al. 2018), and similar tests can be conducted to establish the time-independent and time-dependent behaviour (Sect. 3.1.1).
Schematic diagram of planar tension (pure shear) sample preparation and experimental set-up. Figure modified from the work of Masri et al. (2018) on the human male urethra. Although the urethra is not part of the GI tract, it has similar anatomical characteristics and physiological roles as the GI organs in that it is tubular and enacts peristalsis to excrete a waste product (urine). The hashed lines depict a fixed lower grip, while the arrow shows the direction the upper grip moves to apply tension to the sample. Note that Masri et al. (2018) studied the anisotropic properties of the human urethra under planar tension by testing samples in both the longitudinal and circumferential directions
3.1.4 Simple shear
In the domain of small deformation, simple shear differs from pure shear in that in the simple shear strain state, rotation can occur; this was found to be not fully the case in large deformation; however, as divergence in stress–strain behaviour between the two states can occur at large strain values (Moreira and Nunes 2013). A typical simple shear test set-up is like that which can be seen in Fig. 6, where the sample’s bottom and top surfaces are translated relative to each other. Simple shear tests provide the opportunity to determine the behaviour of the tissue under non-normal forces (those applied parallelly to the tissue surface) as well as the material’s shear modulus, which is useful when considering the types of deformations that exist during normal function of the GI tract (Mishra and Rao 2005).
Schematic diagram of a simple shear test being conducted on the rectum from pigs. The arrows indicate the direction the plates move during testing (Qiao et al. 2005)
3.1.5 Uniaxial compression
Uniaxial compression tests are carried out by pressing a sample of tissue between two plates, as seen in Fig. 7a. These tests involve subjecting a uniform sample, either a square or a short cylinder, to compressive deformation in order to study the behaviour of the tissue and its ability to bear load under compressive strains. The tests used to establish the time-independent and time-dependent behaviour of a soft tissue outlined in Sect. 3.1.1, such as creep, stress-relaxation and cyclic tests, can also be applied to compression tests; however, instead of stretching the material, the applied load will be a compression.
a Schematic diagram of a uniaxial compression test being conducted on the rectum from pigs. The arrow indicates the direction the top plate moves during testing (Qiao et al. 2005). b Semi-spherical indenter used to investigate the large intestine from rats. The indenter is rigid and has a 3-mm-diameter, which comes into contact with the tissue during testing. b modified from Stewart et al. (2016)
3.1.6 Indentation
Similarly to compression tests, indentation tests also prescribe compressive strains to a material; however, the indenter causing the displacement is not a plate covering the entirety of the sample, rather a probe with a compression area that is much smaller than the surface of the sample where the compression is taking place. The shape of the indenter attached the probe can be a more unusual shape compared to the flat plate used for traditional compression testing, for instance a semi-sphere as seen in Fig. 7b, allowing for more nuanced loading regimes (Toniolo et al. 2022). As the only constraints are that the surface where the test takes place is much larger than the size of the indenter and is relatively flat, the tissue specimens for indentation testing can be almost any shape. This is useful for tissues where it can be difficult to cut uniform specimens.
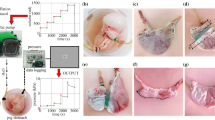
3.1.7 Distension
Distension tests, also called inflation tests, for the GI organs, or other hollow organs, involve the stretching of the organ from its inside. A schematic diagram of a distension test conducted both ex vivo and in situ on the small and large intestines is shown in Fig. 8a, and an example of an experimental set-up for a distension test on the stomach can be seen in Fig. 8b. Note that in Fig. 8a the fluid being injected into one end of the oesophagus flows out the other end of the oesophagus. In this study, the authors recorded the pressure exerted by the fluid on the wall of the organ and measured the intestinal diameter (Lu et al. 2012). Contrarily, the fluid injected into the stomach seen in Fig. 8b is not able to pass out of the other side; for this study, the authors measured the circumferential and longitudinal deformations using three-dimensional ultrasound imaging (Liao et al. 2006). These studies show just two examples of how a distension test can be carried out, in which there are many variations. The essence of the test is the same, however, in that a fluid (liquid or gas) is injected into the hollow organ creating a pressure on the organ wall. The pressure is recorded along with a strain measure (diameter, cross-sectional area (CSA), wall thickness, arc length, three-dimensional imaging) and/or the volume of fluid. Usually ex vivo distension tests are performed on passive tissue; however, it is possible to quantify the contractility of the specimen and thus calculate the contribution of the passive and active stress on the organ’s mechanical behaviour.
a Schematic diagram of both in vitro, i.e. ex vivo, and in situ experimental set-ups for distension testing. Distension and contractility were studied in regard to the small and large intestines of mice (Lu et al. 2012). b A distension test experimental set-up used to investigate the stomach of diabetic and non-diabetic rats. A range of luminal pressures were applied to the organ specimen, and the displacements were measured through three-dimensional ultrasound imaging. b modified from Liao et al. (2006)
3.1.8 Inflation–extension
While distension tests measure the stress–strain relation of an organ in one loading condition, inflation–extension tests measure it in two. Inflation–extension tests, as the name suggests, involve both distension of the tissue in the circumferential direction and stretch in the axial/longitudinal direction, allowing for characterisation of the tissue’s anisotropic properties in a state closer to in vivo conditions compared to uniaxial or biaxial tensile testing, i.e. with the organ structure intact. An example of the experimental set-up for an inflation–extension test can be seen in Fig. 9.
Schematic diagram of an inflation–extension experimental set-up a and a close-up of a segment of rodent (Wistar rat) large intestine held in the grips prior to testing b (Sokolis et al. 2011)
3.1.9 Zero-stress state
It can be the case that the no-load state of a tissue is different from its zero-stress state. In 1983, both Vaishnav and Vossoughi (1983) and Fung (1984) demonstrated this to be the case with arteries, and since then it has been determined that many other soft biological tissues also possess residual stresses and strains in their no-load configuration, including the GI tract (Gregersen et al. 2000). The purpose of these residual strains has been attributed to providing a more balanced stress distribution within the organ wall (Aggarwal et al. 2016). Figure 10 shows a schematic diagram of how a ring segment of a residually stressed tubular tissue deforms between its no-load state and its zero-stress state; the ring specimen deforms into a sector when, in its no-load state, it is cut radially, producing a parameter by which the degree of residual strains within a tubular tissue can be defined: the opening angle, \(\alpha\). The greater the opening angle, the greater the residual strains in the tissue specimen. Therefore, the opening angle can be used to compare the varying degree of residual strains throughout an organ (e.g. along its axial length) or between organs. To determine the residual stresses, however, the residual strains must be quantified. For this, the morphology of the tissue, i.e. the inner and outer circumferences of the different layers within the ring specimens, before and after deforming to the zero-stress state can be used to establish the residual strains present. From here, the residual stresses can be calculated via a constitutive law.
Schematic diagram of how a circumferential ring segment of a residually stressed tubular organ deforms from its no-load state to its zero-stress state, including a schematic definition of the opening angle (\(\alpha\)) (Gao et al. 2000)
To determine the circumferential residual strains of a tubular tissue, the usual protocol is that described in Fung and Liu (1989) where ring-like specimens of the tissue, 1–2 mm in length, are cut. The cross-section of these specimens are photographed, as seen in the pictures on the left in and in the centre of Fig. 11, then a radial cut is made to the wall of the ring. Usually this causes the specimens to open into an sector, as seen in Fig. 10 and on the right in Fig. 11. The specimens are given time to equilibrate, allowing any viscous effects to dissipate, and are then photographed again. The difference in lengths of the inner and outer circumferences of the specimens from the closed ring to the open sector are used to calculate the residual strains of the tissue. The closed ring is when the tissue is in the no-load state, i.e. no external loads such as luminal pressures are exerted on the wall, and the open sector is considered the zero-stress state, when all the internal, residual stresses of the material have been released. This method is based on some assumptions such as that the ring specimen is a perfect circle, though in reality this is not often the case. Recently, in 2019 and 2021, respectively, Sigaeva et al. (2019) and Lefloch et al. (2021) developed novel ways of assessing residual strains without this perfect circle assumption to make the measurement of tissue rings more accurate, particularly when the tissue being investigated is diseased (as these specimens are often more irregular compared to healthy tissue). However, within the literature, currently most zero-stress state studies still use the method outlined in Fung and Liu (1989), which is reasonably accurate when the samples keep their mainly circular geometric formation. As can be seen in Fig. 11, this technique can be carried out on intact wall specimens or on ring-like specimens separated into their different composite layers, e.g. the mucosa-submucosa layer and the muscularis propria layer. It is also possible to study how the ring segments open over time, thus including the viscous effects (i.e. time-dependent effects) in the residual stress/strain analysis.
Longitudinal prestretch can be determined by measuring the difference between the length of the tubular tissue in situ and comparing this to its length ex vivo. In addition, longitudinal strips can be cut free from the wall and allowed to equilibrate. Similarly to the circumferential samples, the deformations of these longitudinal strips can be used to determine the residual strains in the longitudinal direction.
Experimental results showing the no-load and zero-stress state of circumferential ring specimens from the oesophagus of pigs, investigating the residual strains of the intact wall as well as the separated layers (mucosa-submucosa, circular muscle and longitudinal muscle). Figure modified from Zhao et al. (2007)
3.2 In vivo
In vivo experimentation is that carried out in the organ’s natural environment on a subject which is alive. While ex vivo experimentation is often very similar to standard engineering material characterisation tests, in vivo tests on the GI organs pose an added complication of needing to measure the deformations of a material, which cannot be seen with the naked eye (Dargar et al. 2016).
3.2.1 Distension
Distension tests conducted in vivo are similar to those carried out ex vivo (Sect. 3.1.7); however, while a balloon is sometimes used when testing ex vivo, it is always used in vivo in order to keep the fluid contained. Unlike ex vivo distension testing, the outer diameter cannot be simply measured using a camera to determine the strain of the sample. Therefore, modalities such as impedance planimetry and ultrasound must be employed to determine the strain of the wall in relation to the pressure exerted by the volume of fluid injected into the organ’s lumen (Drewes et al. 2001; Takeda et al. 2002; Petersen et al. 2003; Takeda et al. 2003).
3.2.2 Elastography
Elastography is a technique that can be used to non-destructively determine the mechanical properties of the GI tract in vivo, including its layer-dependent properties (Dargar et al. 2019) and thus can be used to quantify a tissue’s material behaviour in its physiological environment (Li and Cao 2017). Furthermore, elastography can be used clinically to identify the health state of soft tissues (Evans et al. 2010; Venkatesh et al. 2013; Kennedy et al. 2017). There are many different types of elastography, and their type depends on how the strains are measured; however, in essence, firstly a stimulus is applied to the tissue, for instance a vibration (Evans et al. 2010) or a compression (Dargar et al. 2019), the deformation is then tracked via an imaging modality such as ultrasound, magnetic resonance or optics, and, finally, the tissue’s mechanical properties are determined computationally through inverse analysis (Li and Cao 2017). For a comprehensive understanding of ultrasound, optical and magnetic resonance elastography, readers are referred to the reviews by Li and Cao (2017), Kennedy et al. (2013) and Low et al. (2016), respectively.
4 Review findings
The number of search results, articles screened from the search and articles added by the authors for each organ can be found in Table 2. Out of all the articles, the proportion of studies collected for the oesophagus was 33%, for the small intestine 29%, for the large intestine 18%, for the stomach 11% and for the rectum 9%. Figure 12 shows the number of publications for each organ as a function of year in which they were published. The results for each organ were organised into whether the experimentation was conducted ex vivo or in vivo, for which the number of articles for each state can be seen in Fig. 13.
It should be noted that in this review, experiments conducted in situ on alive subjects have been considered as in vivo, and in situ experiments conducted on deceased subjects have been regarded as ex vivo. There were so few in situ experiments that they did not warrant a results table of their own. This explains how an “indentation test” may be conducted in vivo (Table 4); in actuality it was conducted in situ while the subject was still alive, i.e. there was still blood flow in the organs.
In some studies, different types of experiments were conducted, either using various techniques, e.g. ex vivo inflation–extension and ex vivo zero-stress state analysis, and/or different organs, e.g. large intestine and rectum, and/or different species, e.g. pig and human. From this point forward, each test situation (i.e. species, organ and experimental technique) will be treated as separate even if they are presented within the same article, and will, therefore, be referred to as individual “experiments”.
4.1 Oesophagus
The oesophagus had the greatest number of experimental studies out of all the GI organs (Table 2). The experiments conducted on the oesophagus ex vivo are summarised in Table 3. Of these studies, several looked into the effects of pathological conditions on the organ’s mechanical properties, including oesophageal varices in rabbits (Jensen et al. 1987; Gregersen et al. 1991), osteogenesis imperfecta in mice (Gregersen et al. 2001), oesophagitis in humans (Vanags et al. 2003), diabetes in rats (Yang et al. 2004; Zeng et al. 2004a, b; Yang et al. 2006; Zhao et al. 2007; Liu et al. 2012; Jiang et al. 2019) and cancer in pigs (Aho et al. 2016a). Zeng et al. (2004b) looked at how diabetes affects the material behaviour of rodent oesophagi over time. As a treatment for diabetic GI disorder, Liu et al. (2012) studied the effect of Tangweian Jianji (a Chinese medicinal compound) on the mechanical properties of the oesophagus in diabetic rats. Others looked at the effects of epidermal growth factor (EGF) to investigate how abnormal growth may affect the function of the oesophagus in rats (Zhao et al. 2003a), while some investigated the effect of ageing on the mechanical properties of the oesophagus in humans (Vanags et al. 2003) and Wistar rats (Gregersen et al. 2004; Zhao and Gregersen 2015).
Most ex vivo studies of the oesophagus investigated its passive material properties; however, some studied its active properties: Tøttrup et al. (1990) looked at the active properties of human oesophageal muscle, and Wareham and Whitmore (1982) investigated the active mechanical properties of the muscularis propria of guinea pig oesophagi. As can be seen in Fig. 14a, ex vivo experimentation on the oesophagus was conducted using a wide variety of animals. Experiments conducted on oesophagi from rats were the most prevalent, while ex vivo experimentation conducted on human tissue accounted for only 5%.
The oesophagus had the most in vivo studies of all the organs considered (Fig. 13), a summary of which can be found in Table 4. Several conditions were studied in regard to their effect on the mechanical properties of the oesophagus in vivo, including oesophageal varices in rabbits (Gregersen et al. 1991, 1988), nutcracker oesophagus (i.e. abnormal peristalsis) in humans (Mujica et al. 2001), chest pain of oesophageal origin (sometimes referred to as functional chest pain (FCP)) in humans (Rao et al. 2001, 2002; Drewes et al. 2006; Nasr et al. 2010), systemic sclerosis in humans (Villadsen et al. 1997, 2001; Gregersen et al. 2011) and type-1 diabetes in humans (Frøkjær et al. 2007). Gregersen et al. (1992) studied the mechanical changes that occur in the oesophagi of opossums that have been obstructed. Juhl et al. (1994) investigated the effect of damage caused by endoscopic sclerotherapy on the mechanical properties of minipig oesophagi, while Vinter-Jensen et al. (1995) studied the potential viability of EGF as a treatment (therapeutic potential) for this damage, also using oesophagi from minipigs. Drewes et al. (2002, 2003, 2005) conducted several studies on pain perception in relation to distension of the oesophagus in humans. Takeda et al. (2003) studied the active and passive properties of the human oesophagus in vivo through the use of a muscle relaxant, atropine. As can be seen in Fig. 14b, the majority of in vivo experimentation of the oesophagus was carried out on humans.
4.2 Stomach
Only 11% of all the articles collected in this review investigated the mechanical properties of the stomach (Table 2). A summary of the experiments conducted ex vivo on the stomach can be found in Table 5. Of these studies, Liao et al. (2006) looked into the effects of disease on the stomach’s material behaviour, in particular the impact of type-2 diabetes on the mechanical properties of stomach tissue from rats. Notably, Carniel et al. (2020) and Toniolo et al. (2022) studied stomach tissue removed from patients (humans) suffering with morbid obesity who had undergone a laparoscopic sleeve gastrectomy, while Marie et al. (2019) investigated how sleeve gastrectomies affect the biomechanical behaviour of the stomach using specimens from pigs for which the surgical procedure had been performed ex vivo. In terms of the active behaviour of the stomach, Merlo and Cohen (1989) evaluated the active mechanical properties of its muscle layers with tissue excised from cats, and Tomalka et al. (2017) electrically stimulated the smooth muscle of pig stomachs to assess their behaviour. Furthermore, Klemm et al. (2020) studied both the intact wall of the stomach from pigs (mucosal and muscular layers) and just its muscle layer to determine the contribution of each layer in the tissue’s active behaviour, while Borsdorf et al. (2021) investigated the active response of the combined muscle layer (oblique, longitudinal and circular muscle) and just the circular gastric smooth muscle layer to compare their influence on the mechanical behaviour of the stomach from domestic pigs.
In vivo experimentation on the stomach was the least common compared to the other GI organs (Fig. 13), for which only the healthy, passive properties were investigated. A summary of the experiments carried out in vivo on the stomach can be found in Table 6. Stomach tissue originating from porcine was the overwhelming choice for studying the organ both in vivo and ex vivo, as can be seen in Fig. 15, with one author stating that this decision originated from “the similarities between the porcine and the human digestive systems” (Salmaso et al. 2020). Only 22% of the ex vivo experimentation was performed on human tissue (Fig. 15a), while no human tissue was studied in vivo (Fig. 15b).
4.3 Small intestine
Of all the GI organs, the majority of ex vivo experimentation was conducted on the small intestine (Fig. 13). The summary of ex vivo experiments on the small intestine can be found in Table 7. Conditions affecting the small intestine were studied, including diabetes in rats (Jørgensen et al. 2001; Zhao et al. 2002, 2003a, b, 2013a, 2017), intestinal oedema in rats (Radhakrishnan et al. 2005, 2006), and Chinese medicines, namely Kaiyu Qingwei Jianji (Sha et al. 2006) and Tangweian Jianji (Liu et al. 2012), were investigated in rats regarding their ability to treat the GI symptoms associated with diabetes. In addition, Zhao et al. (2013b) investigated the active mechanical properties of the small intestine from rats with diabetes and rats with a condition that mimics human irritable bowel syndrome (IBS) (Zhao et al. 2019). The effects of clinical interventions on the mechanical properties of the small intestine were also studied, including irradiation as a treatment for jejunal fibrosis in mice (Peck and Gibbs 1987), chronic coeliac ganglionectomy in rats (Ouyang et al. 1996), small intestinal resection in rats (Dou et al. 2002a) and distraction enterogenesis in pigs (Hosseini and Dunn 2020).
The influence of growth on the mechanical behaviour of the small intestine was evaluated naturally, i.e. during physiological growth, in rats (Lu et al. 2005) and using EGF (Vinter-Jensen et al. 1996; Zhao et al. 2002; Liao et al. 2003; Yang et al. 2003). In addition, the effects of partial obstruction of the organ on its mechanical properties were studied in rodents (Liao et al. 2010; Sun et al. 2017), and how these properties changed as a function of obstruction time were also investigated (Zhao et al. 2010; Sun et al. 2018). The effect of partial obstruction on the active behaviour of the small intestine was studied in guinea pigs (Zhao et al. 2011a, b), while Zhao and Gregersen (2015) studied the effect of ageing on the passive material response of the organ in rats.
Several studies investigated the effects of diet on the small intestine: how starvation (Dou et al. 2002b) and re-feeding affects the mechanical properties of the small intestine was evaluated in rats by Dou et al. (2001), how varying amounts of dietary protein affects minks by Chen et al. (2009), the effects of a low-residue (Liu et al. 2017b) and low-fibre (Liu et al. 2017a) diet in rabbits, and the influence of a low-fibre diet on the active mechanical properties in rabbits (Liu et al. 2019). The active properties of the small intestine were considered ex vivo in rabbits (Elbrønd et al. 1991; Liu et al. 2019), rats (Ouyang et al. 1996; Zhao et al. 2008, 2013b, 2019), guinea pigs (Zhao et al. 2011a, b), mice (Lu et al. 2012) and pigs (Terry et al. 2011), while no active studies were conducted using human tissue ex vivo.
There were a number of studies that looked at the properties of the small intestine in vivo, a summary of which can be found in Table 8. Of these, Pedersen et al. (2003), Gregersen et al. (2007) and Gao et al. (2009) evaluated the effect (disease compared to healthy controls) of systemic sclerosis on both the passive and active mechanical properties of the small intestine in humans, and Frøkjær et al. (2007) investigated the active response of the small intestine in patients with type-1 diabetes and compared the observed behaviour to that of healthy controls. Moreover, the active properties of healthy humans and mice were studied in vivo by Gao et al. (2003) and Lu et al. (2012), respectively. Figure 16 shows the proportion of each type of tissue used for both the ex vivo experimentation (Fig. 16a) and the in vivo experimentation (Fig. 16b). The majority of ex vivo experiments were conducted using rats, with only 4% on human tissue, while the main proportion of in vivo experiments were carried out on humans (42%) closely followed by pigs (34%).
4.4 Large intestine
Approximately 20% of all ex vivo articles collected in the review conducted experimentation on the large intestine (Fig. 13); a summary of these experiments can be found in Table 9. Notably, the effects of a number of diseases on the mechanical behaviour of the large intestine were investigated, including chronic obstruction of the colon in mice which mimics human Hirschsprung’s disease (Hillemeier and Biancani 1990), colitis in rodents (Stidham et al. 2011; Gong et al. 2017; Nair et al. 2018, 2019) and human growth hormone as a potential treatment for this in rats (Christensen et al. 1993), ulcerative colitis in mice (Yang et al. 2009), diabetes in rats (Zhao et al. 2009), Crohn’s disease in humans (Stidham et al. 2011), IBS in rats (Zhao et al. 2019), and cancer in humans (Deptuła et al. 2020). Conditions such as hypertension were also studied in rats (Stewart et al. 2016), and the active response of large intestinal muscle to inflammatory mediators was investigated in both humans and rabbits (Percy et al. 1990). Additionally, the effect of coeliac ganglionectomy on the mechanical properties of the large intestine was evaluated in rats (Ouyang et al. 1996). Yang et al. (2003) looked at the result of EGF treatment over varying periods of time on the mechanical properties of the rat large intestine. Watters et al. (1985) and Massalou et al. (2019a) considered the effects of age and sex on the material behaviour of the large intestine in rats and humans, respectively, and in another study, Watters et al. (1985) looked at the influence of ethnic origin in humans. In terms of the effect of food-intake on the mechanical properties of the intestines, Liu et al. (2017a) investigated the consequence of a long-term low-fibre diet in rabbits.
As can be seen in Fig. 17a, experiments on rodents, specifically mice and rats, accounted for 51% of the ex vivo experimentation on the large intestine, with only 18% conducted using human tissue. Contrarily, half of all in vivo experimentation regarding the large intestine was carried out on humans, as shown in Fig. 17b. A summary of the in vivo experiments conducted on the colon can be found in Table 10. Of these experiments, Petersen et al. (2001) assessed the relationship between pain during distension of the large intestine and its stress–strain response in healthy human subjects, while Drewes et al. (2001) studied the difference in pain during large intestinal distension, and its associated biomechanical parameters, between patients with IBS and healthy human controls.
In terms of the active properties of the large intestine, ex vivo experimentation was carried out on rabbit (Pescatori et al. 1979; Percy et al. 1990), human (Gill et al. 1986; Percy et al. 1990), cat (Merlo and Cohen 1988) and rat (Ouyang et al. 1996; Zhao et al. 2019) tissue, and in vivo experimentation was conducted on humans (Bharucha et al. 2001). For further understanding of the active behaviour of the large intestine from a mechanical perspective, readers are referred to the literature review of Bhattarai et al. (2022a).
4.5 Rectum
The rectum had the least amount of ex vivo mechanical experimentation compared to the other GI organs (Fig. 13), a summary of which can be found in Table 11. Notable studies included those by Watters et al. (1985) who looked at the influence of sex and age on the material behaviour of the rectum in rats; Glavind et al. (1993) who conducted experimentation in regard to the active properties of the human rectum’s muscle layer; Gregersen et al. (2002) who studied how the rectum of mice was affected by irradiation; Yang et al. (2003) who evaluated the change in mechanical properties experienced when growth of the rat rectum was induced by EGF; and Brunenieks et al. (2017) who investigated the effect of obstructed defecation syndrome on the biomechanical properties of the human rectal wall, comparing the abnormal tissue extracted from surgical resection to tissue excised from healthy humans post-mortem. Figure 18a shows that most ex vivo experimentation on the rectum was carried out using rodent tissue (specifically, mice and rats), comprising 61% of the total number of experiments conducted.
It can be seen in Fig. 18b that the vast majority of in vivo experimentation of the rectum was conducted on humans. Of these in vivo experiments, of which a summary can be found in Table 12, a few investigated the effects of different conditions. For instance, Arhan et al. (1978) studied the difference in viscoelastic behaviour of the rectal wall between patients with Hirschsprung’s disease and healthy, age-matched controls; Lundby et al. (1999) looked at the effect of age on the mechanical properties of the rectum in mice; and Petersen et al. (2001) conducted experimentation to assess the biomechanical behaviour of the human rectum, studying how the pain felt by the volunteer during distension was associated with the tissue’s stress–strain response. The same group then went on to look at how the mechanical response and pain differed during distension before and after smooth muscle relaxation (Petersen et al. 2003). Furthermore, Drewes et al. (2001) investigated the difference in rectal mechanical parameters and levels of pain between patients with IBS and healthy human controls, and in another study evaluated again the relation between pain and biomechanical properties of the rectum but this time in patients with ulcerative colitis (Drewes et al. 2006), comparing their results against healthy controls.
4.6 Experimental particulars
In this section of the review findings, we will focus on the particulars of the experiments including which experiments involved investigation of the tissue’s time-dependent behaviour (Sect. 4.6.1), whether preconditioning of the sample was performed prior to data collection (Sect. 4.6.2), if, for the ex vivo experimentation, the tests were carried out in a saline solution bath (Sect. 4.6.3), and whether the studies conducted histological analysis alongside their mechanical experimentation to provide information on the microstructural components of the tissue and how they might influence its stress–strain behaviour (Sect. 4.6.4).
4.6.1 Time-dependent behaviour
Soft tissues often present as viscoelastic materials (Righi and Balbi 2021); this means that relaxation and creep can be seen in their material response, and, thus, that their mechanical behaviour is time-dependent. Some of the studies included in this review investigated the time-dependent behaviour of the GI organs, a summary of which can be found in Table 13. The proportion of experiments for each organ in which their material response was considered as a function of time is illustrated in Fig. 19.
4.6.2 Preconditioning
Preconditioning is the process of “conditioning” a sample before collecting data in regard to its material response and involves loading and unloading the sample successively for a predetermined number of cycles. The process came about through the study of polymers, which behave in a similar way to soft tissues in that they are highly elastic, usually possess viscous qualities and can exhibit history-dependent behaviour. Preconditioning of polymers is to remove the Mullins effect: a purely history-dependent softening of the material that depends on the previous maximum strain that it has been subjected to (Dorfmann and Ogden 2004). With soft tissues, the equivalent term is stress-softening. It was once thought that preconditioning reduced the influence of soft tissues’ time-dependent, i.e. viscous, properties, however, through research of the myocardium by Emery et al. (1997), it was established that it has mainly an effect on their history-dependent response. This was confirmed as well for the guinea pig small intestine by Gregersen et al. (1998). Therefore, the preconditioning process for soft tissues results in reducing history-dependent effects on their behaviour, as well as some time-dependent effects, which tend to plateau after a minimum of three repeated cycles. Figure 20 shows the proportion of studies evaluated in this review that preconditioned the tissue before collecting their results, for both in vivo and ex vivo experiments.
4.6.3 Saline solution bath
As previously mentioned in Sect. 3.1, for ex vivo mechanical experimentation, measures are often taken to simulate a physiological environment. The main method for achieving this is by conducting experiments on samples immersed in a chamber (or bath) filled with a salt solution. This is done to prevent dehydration of the soft tissue, which has been found to cause alteration to their mechanical properties (Nicolle and Palierne 2010). Sometimes these chambers are thermoregulated so that the temperature of the tissue can be maintained at internal body temperature (37°C) throughout testing. As can be seen in Fig. 21, the majority of ex vivo experiments considered in this review were performed using a saline solution bath, the organ with the highest proportion being the oesophagus with 78%. Almost all ex vivo studies stored their tissue specimens in some variety of salt solution between tests; however, Fig. 21 only shows the percentage of those which performed their tests in a solution bath. The other studies, e.g. the remaining 28% of the oesophageal experiments, often kept the samples moist by alternative means such as spraying the samples during testing; however, Nicolle and Palierne (2010) concluded that the best method to prevent dehydration of soft tissue samples is by conducting the tests in a saline bath. The types of salt solutions that were used in the experimental studies on the GI tissues included physiological saline, phosphate-buffered saline (PBS) and Krebs solution, which were sometimes aerated with oxygen and carbon dioxide.
4.6.4 Histology
As previously briefly discussed in Sect. 1, the microstructural components of soft tissues influence their macrostructural behaviour. Histological analysis provides a well-established means to investigate the various microstructural features of tissues, the images from which can be used to establish the prevalence (fraction) and orientation of their collagen, elastin and muscle fibres (Fan et al. 2005). The analysis is carried out by removing a very thin slice of a tissue sample, putting the slices on a slide and then using different stains to highlight different microstructural features (Durcan et al. 2022a; Girard et al. 2019). Finally, images are taken which can then be post-processed and analysed to establish the fraction and orientation of the aforementioned fibres. This information can help to discuss reasons for the experimentally observed behaviour and potentially deduce their more specific affect (for example, by artificially increasing or decreasing the fraction of fibres and using the histological images to quantify the change), and inform micromechanical constitutive modelling (Masri et al. 2018). Figure 22 shows the proportion of experiments that conducted histological inspection alongside their biomechanical investigation. Histological analysis was considered here because it is the most prevalent and traditional means of assessing the microstructure of soft tissues; however, for an outline of more modern techniques such as second-harmonic generation (SHG) microscopy and optical-based analysis, readers are referred to Siri et al. (2020) and Goth et al. (2016), respectively.
5 Discussion
The review findings showed that the GI tissues of a number of different species were tested using an array of experimental approaches. Here, some of the experimental aspects will be discussed in more detail.
5.1 In vivo vs. ex vivo
The main drive of mechanically testing human soft tissues is to establish their material behaviour in the context of their natural environment, i.e. for the GI tract as digestive organs inside the body, for which in vivo studies provide more realistic behaviour being that the tissue is still alive and perfused with blood (Lim et al. 2009). Other aspects such as the internal temperature, moisture levels and structural integrity of the organ are also maintained during in vivo testing (Dargar et al. 2016). The use of a thermoregulated saline bath can be used for ex vivo experimentation in an attempt to control the temperature and moisture variables; however, the tissue is still deceased and will not have exactly the same mechanical properties as it would in vivo due to phenomena such as rigour mortis and the relaxation of residual stresses (Dargar et al. 2016). The structural integrity can be maintained during ex vivo experiments such as distension and inflation–extension tests; however, the organ being tested has still been detached from its natural position and the connective tissue holding the organ in place has been cut; therefore, aspects such as its interaction with surrounding organs or structures are not included in its characterisation (Marie et al. 2019). Despite this, the in vivo experimentation carried out on the GI organs were mostly distension tests where the behaviour was characterised in only one direction and a homogeneous tissue wall was assumed, while testing ex vivo allows for a wider variety of experimental tests and the more complex behaviour of the organ to be investigated. Furthermore, the force–displacement measurements obtained during ex vivo experimentation can be much more accurate compared to those from in vivo experiments, for which measurements are often obtained from relatively low-resolution imaging techniques, while also potentially being disrupted by movement and breathing of the subject (Dargar et al. 2016), thus increasing the error associated with the mechanical properties determined.
In addition, the deformation of the tissue in supraphysiological loading domains, such as is the case in surgery (Lim et al. 2009) or road traffic accidents (Massalou et al. 2016), cannot be carried out in vivo as this may cause irreversible damage to a subject that is still living, whereas ex vivo experimentations allow for the rupture points and dynamic properties of the tissues to be established because the organ is no longer required (Durcan et al. 2022b; Massalou et al. 2019b). The ethical constraints associated with in vivo testing for both animals and humans are much greater than for ex vivo experimentation due to the pain, discomfort and damage the tests might cause to a living subject. Furthermore, data that are collected from a living subject often have more noise associated with it compared to ex vivo testing due to the movement caused by the beating heart and respiration (Rosen et al. 2008). In terms of layer-dependent properties of a GI tissue, these are usually more easily established ex vivo by separating the layers, normally the two main layers (mucosa-submucosa and muscular layer), and testing them individually. However, recently, Dargar et al. (2019) used compression elastography to determine the layer-dependent properties of porcine stomach tissue in situ, while the animal was still alive and was able to characterise the submucosa, mucosa and muscular layers individually up to a strain of 20%, i.e. beyond the linear elastic regime. This provides hope for the development of an experimental technique that allows for a similar characterisation completely in vivo. Moreover, residual strains within the GI organs are traditionally established ex vivo. However, methods to quantify them in vivo are being developed for arterial tissue (Donmazov et al. 2015; Aggarwal et al. 2016), which can be applied to the GI organs due to their similar anatomical structure.
There are benefits and limitations to both in vivo and ex vivo experimentation; however, in vivo testing provides a more realistic understanding of the behaviour of soft tissues in the conditions we are interested in. Therefore, effort should be made to further develop in vivo mechanical characterisation techniques, such as ultrasound elastography (Dargar et al. 2019), that allow for the layer-dependent properties to be established in a direction-dependent manner, as well as the organ’s internal residual stresses and strains. Ex vivo experimental characterisation should still be carried out for the higher end of large strain deformations, i.e. supraphysiological loading, of human tissues.
5.2 Organs tested
Out of all the experimental articles, the oesophagus was the organ investigated the most, with a total of 82 articles collected in this review (Table 2). The tissue studied the least was the rectum for which 21 articles were found in regard to its biomechanical characterisation, closely followed by the stomach with 28 articles. While the small intestine had a greater number of ex vivo articles than the oesophagus, the oesophagus had, overwhelmingly, the highest number of in vivo articles which contributed towards the organ having the most experimental articles overall. The high number of in vivo tests compared with the other organs could be due to the anatomical position of the oesophagus in that it is easily accessible for biomechanical measurements using a probe inserted through the mouth. The same can be said for the rectum, where the number of in vivo articles is almost the same as the ex vivo articles, a relationship not seen for any of the other organs, for which the number of ex vivo articles is much higher than the in vivo studies, particularly for the stomach, small intestine and large intestine. This is also thought be due to the more accessible position of the rectum where in which a probe can be simply positioned through the anus.
5.3 Species tested
The findings show that animal tissue was used far more prevalently than human tissue for mechanical testing of the GI tissues: out of the articles considered, human tissue was investigated in 20% of the studies on the oesophagus, 21% of the studies on the stomach, 8% of the studies on the small intestine, 21% of the studies on the large intestine and in 41% of the studies on the rectum. This could be due to the fact that animals/animal tissues are a lot more accessible and are associated with fewer ethical constraints compared to testing with humans/human tissues. As mentioned in Sect. 5.2, the greater proportion of human studies on the rectum is thought to be due to it being a more easily accessible GI organ (along with the oesophagus) when conducting studies on live humans (in vivo).
For applications within medicine where the material properties of the tissue will be used quantitatively, such as to provide force feedback to a surgeon using a haptic simulator (Chakravarthy et al. 2014), biomechanical data from human tissue should be used. However, there are benefits to using animal tissue, particularly for the investigation of diseased states, and discussing this data qualitatively in regard to the human organ. The greatest benefit may be demonstrated through the use of mice or rat models. These animals are able to be grown in a very controlled environment, where their age, diet, living conditions, etc., can be decided and closely monitored. This allows for the environmental factors that influence the mechanical behaviour of the tissue, and which contribute to variability in the data, to be controlled and recorded, producing more reproducible data than say between different human specimens. Additionally, there are many rat and mice models that exist to simulate different human diseases, such as type-1 and type-2 diabetes, IBS, and Hirschsprung’s disease. Therefore, testing of these animals allows for a highly controlled investigation of the effects of disease on the mechanical properties of the organs. However, quantitatively, the mechanical results of experiments conducted on animals tissues will not be the same as for human tissues as aspects such as size, tissue structure and digestive demands differ, and so these results should not be used to determine the material parameters for models that will be used in medicine unless no human experimental data are available. Porcine tissue is often chosen due to porcine having a digestive system close to that of humans (Salmaso et al. 2020), however, when comparing between human and porcine data, there are still significant differences between their mechanical properties and so, ideally, data from porcine tissue should not be used directly for applications within medicine (Christensen et al. 2015).
5.4 Sample size
In addition to providing a better control of experimental design than with human specimens, animal specimens often offer the possibility to test a larger sample size, making the final results more robust. Either it is difficult to obtain human volunteers for in vivo tests, especially for the GI tract which can bring, compared to testing organs such as the skin, more discomfort, or there is a limited availability of human cadavers for ex vivo testing. For both in vivo and ex vivo testing with humans, there are ethical constraints that must be considered. For in vivo mechanical testing, informed consent must be given and the study protocol must ensure that no unnecessary harm is caused to the patient or volunteer. For ex vivo experimentation, the tissue obtained from the human cadavers must not be wasted and should only be completed when a clear experimental methodology is established: knowing the purpose of each test and its aims. With animals, these ethical constraints are still present but are more relaxed than with humans. High-quality in silico models could reduce the need for animal and human experimentation, which is always preferable from an ethical perspective.
5.5 Anisotropy
Approximately half of the ex vivo experiments and almost all of the in vivo experiments referenced in this review studied the mechanical properties of their respective tissues in only one direction, usually the circumferential direction. However, from the work of Brasseur et al. (2007), it can be seen how the behaviour in the longitudinal direction affects the efficiency of peristalsis within the GI tract and thus the function of the organs. In addition, many studies have found a discrepancy between the longitudinal and circumferential directions in terms of material response, commonly attributed to the arrangement of fibres such as collagen and elastin in the tissue walls (Siri et al. 2020). Therefore, direction-dependent behaviour should be considered in future experimental investigations, particularly for in vivo studies for which anisotropic studies are lacking (Tables 4, 6, 8, 10, 12).
5.6 Layer-dependency
Those who studied the intact wall of the GI organs assumed the mechanical properties in the radial direction to be homogeneous. However, layer-dependent studies show this not to be the case, with the varying amount of microstructural components, namely collagen and elastin, being the main hypothesis as to why the material behaviour of the layers differ (Siri et al. 2020). It can be seen that the oesophagus has a higher proportion of layer-dependent studies compared to the other tissues. This is due to the oesophagus being the only visceral organ, which can be relatively easily separated into its two main layers (the mucosa-submucosa and the muscularis propria) after explantation (Payan and Ohayon 2017). This can be seen in Fig. 23, which shows that the connective tissue attaching the two main layers of the human oesophagus together is loose, making the layers straightforward to dissect (Durcan et al. 2022a). For the small intestine, it was found in the study by Sokolis (2017) that “preliminary attempts to dissect the layers were not successful”. Some have been successful using micro-dissection; however, since the layers of the GI organs apart from the oesophagus are tightly bound, it may be hard to ensure that no damage has been incurred to any of the layers.
Whether the layer-dependent properties of the organ are considered depends on the application of the experimental work. For instance, for FE modelling of the interaction of a GI organ and a stent, a layer-dependent model will help to understand how the pressure exerted by the stent is supported by each of the layers. However, if the aim is to study the properties of the organ wall under dynamic loading for use in FE models that investigate the impact of blunt trauma, for instance during road traffic collisions, the layer-dependent properties may provide too much detail for the application (Massalou et al. 2016, 2019b, a; Ruan et al. 2003). Nevertheless, many studies show large differences between mechanical behaviour of the different layers, and their influence on the overall function of the organ should be considered to provide a more complete biomechanical understanding of the GI tract (Yang et al. 2007a).
The two main layers of the human oesophagus attached by relatively loose connective tissue. Figure modified from Durcan et al. (2022a)
5.7 Preconditioning
Preconditioning is a technique first employed in the characterisation of polymers to reduce the influence of history-dependent, and some time-dependent, effects on the recorded behaviour of a material, making the results more stable and repeatable (Gregersen et al. 1998; Rosen et al. 2008). Within biomechanics, preconditioning is used both in vivo and ex vivo. Its use, though, is controversial. On the one hand, it makes the behaviour of soft tissues more consistent so the observed material response between different samples and subjects is less variable, however, on the other hand, in many of the applications in which the biomechanics of soft tissues are of interest, their behaviour during the first cycle is the one of most importance, for instance, during normal physiological loading (Bauer et al. 2020), surgery (Rosen et al. 2008), blunt trauma (Johnson et al. 2020) and endoscopy (Carniel et al. 2014b). In these situations, for example, the tissue is not preconditioned before the stomach wall is passively stretched by its contents, or surgical tools manipulate and cut the large intestine. It has even been found that with the rat oesophagus, the material properties of the wall return to what they were before stretching once muscle activation has occurred, i.e. the stress-softening of the wall is reversed during peristalsis (Jiang et al. 2014, 2017, 2019), therefore suggesting that the first-cycle behaviour is the one most often of main interest. In future experimentation, it may be best to quantify both the initial material response and the behaviour after preconditioning as this provides experimental data to be used in the aforementioned applications, as well as information on the history- and time-dependent behaviour of the GI organs (Weizel et al. 2022).
5.8 Limitations of the review
In this review, sphincters of the GI tract have not be included. For comprehensive characterisation of the GI tract, these sphincters would have to be considered and also modelled in silico if the application requires. Additionally, an experimental aspect that has not been extensively discussed here is the different methods for strain measurement used in the characterisation of the GI tissues. These can include digital image correlation (DIC) (Khoo et al. 2016), image analysis (Huang et al. 2019), and extensometers within the testing machine (Durcan et al. 2022b). Another aspect that has not been highlighted in the review is the investigation of the plasticity and damage mechanisms of the GI tissues. To increase the complexity of a constitutive model and for specific applications, such as modelling the perforation of a tissue, these irreversible processes should be considered. Moreover, only one database was used, and although particular care was taken to add any articles known by the authors not found in the PubMed search, some experimental studies may have been missed and therefore may not be included in this review.
In the interest of brevity, not all the articles presented in the tables were mentioned in the text, with only those that studied something more than the passive behaviour of normal tissue being highlighted. Furthermore, a comparison of the numerical values of mechanical properties presented in each article has not been carried out. This was due to the large number of articles collected in the review, and the complex nature of comparing numerical values obtained from experiments carried out in different loading modes and with different protocols (such as strain rate and sample dimensions). Instead, the aim of this review was to provide the reader with an overview of the experiments that have been performed on their organ of interest, from which they may either obtain experimental data for a specific loading mode, or perform their own more in-depth analysis and comparison of the current understanding of the organ’s mechanical properties.
6 Conclusion
This review was written with the aim to consolidate the mechanical experimentation that has been conducted on the GI tract, to highlight what is missing in the literature in terms of the characterisation of the GI organs, and to be used by readers to inform their own experimental choices or to provide a reference of experimental data for their own analysis and/or constitutive and FE modelling. For the latter application, experimental data can be retrieved for a certain GI organ and type of test, with the test condition (in vivo or ex vivo), direction-dependency (isotropic or anisotropic) and layer-dependency (intact wall or layer-dependent) also being specified.
In terms of ex vivo experimentation, there are little data regarding the human oesophagus and small intestine, with no ex vivo active studies being conducted on the small intestine from humans. For in vivo mechanical characterisation, no studies included in this review involved experimentation of the human stomach, with only three studies being carried out in total on the stomach in vivo. Furthermore, very few in vivo characterisations involved determination of the layer-dependent properties of the GI tract. Overall, there is a lack of time-dependent studies on the GI organs, particularly for human tissue with only 4% of all the ex vivo articles considering the tissues’ viscoelastic properties and 2% investigating the time-dependent behaviour of human tissue in vivo. Moreover, very few studies investigated the shear properties of the tissues, and there were no studies that considered the GI tract’s residual stresses and strains in vivo. Compared to the other organs, there was considerably less experimentation conducted on the rectum. Therefore, a focus should be applied to characterising the more complex aspects of the GI organs’ mechanical behaviour using human tissue, ideally in vivo, including their layer-dependent, anisotropic, viscoelastic, shear and active properties, as well as their residual stresses and strains. Experimentation should be particularly focused on the stomach and rectum, for which data are lacking overall.
Abbreviations
- GI:
-
Gastrointestinal
- H&E:
-
Haematoxylin and eosin
- GK:
-
Goto-Kakizaki
- FE:
-
Finite element
- CFD:
-
Computational fluid dynamics
- FSI:
-
Fluid–structure interactions
- CSA:
-
Cross-sectional area
- EGF:
-
Epidermal growth factor
- FCP:
-
Functional chest pain
- IBS:
-
Irritable bowel syndrome
- PBS:
-
Phosphate-buffered saline
- SHG:
-
Second-harmonic generation
- DIC:
-
Digital image correlation.
References
Aggarwal A, Pouch AM, Lai E, Lesicko J, Yushkevich PA, Gorman JH III, Gorman RC, Sacks MS (2016) In-vivo heterogeneous functional and residual strains in human aortic valve leaflets. J Biomech 49(12):2481–2490
Aho JM, Nenadic IZ, Aristizabal S, Wigle DA, Tschumperlin DJ, Urban MW (2016) Use of shear wave ultrasound vibrometry for detection of simulated esophageal malignancy in ex vivo porcine esophagi. Biomed Phys Eng Exp 2(6):065002
Aho JM, Qiang B, Wigle DA, Tschumperlin DJ, Urban MW (2016) Nondestructive measurement of esophageal biaxial mechanical properties utilizing sonometry. Phys Med Biol 61(13):4781
Arhan P, Devroede G, Danis K, Dornic C, Faverdin C, Persoz B, Pellerin D et al (1978) Viscoelastic properties of the rectal wall in Hirschsprung’s disease. J Clin Investig 62(1):82–87
Assentoft J, Gregersen H, O’Brien W Jr (2001) Propagation speed of sound assessment in the layers of the guinea-pig esophagus in vitro by means of acoustic microscopy. Ultrasonics 39(4):263–268
Assentoft J, Gregersen H, O’brien W (2000) Determination of biomechanical properties in guinea pig esophagus by means of high frequency ultrasound and impedance planimetry. Dig Dis Sci 45(7):1260–1266
ASTM International (2016) E8/E8M-16a: standard test methods for tension testing of metallic materials. ASTM International
Aydin R, Brandstaeter S, Braeu F, Steigenberger M, Marcus R, Nikolaou K, Notohamiprodjo M, Cyron C (2017) Experimental characterization of the biaxial mechanical properties of porcine gastric tissue. J Mech Behav Biomed Mater 74:499–506
Badash I, Burtt K, Solorzano CA, Carey JN (2016) Innovations in surgery simulation: a review of past, current and future techniques. Ann Transl Med 4(23):693
Badylak SF, Vorp DA, Spievack AR, Simmons-Byrd A, Hanke J, Freytes DO, Thapa A, Gilbert TW, Nieponice A (2005) Esophageal reconstruction with ecm and muscle tissue in a dog model. J Surg Res 128(1):87–97
Baidoo N, Crawley E, Knowles CH, Sanger GJ, Belai A (2022) Total collagen content and distribution is increased in human colon during advancing age. PLoS ONE 17(6):e0269689
Barlow JD, Gregersen H, Thompson DG (2002) Identification of the biomechanical factors associated with the perception of distension in the human esophagus. Am J Physiol-Gastrointest Liver Physiol 282(4):G683–G689
Bauer M, Morales-Orcajo E, Klemm L, Seydewitz R, Fiebach V, Siebert T, Böl M (2020) Biomechanical and microstructural characterisation of the porcine stomach wall: location-and layer-dependent investigations. Acta Biomater 102:83–99
Bellini C, Glass P, Sitti M, Di Martino ES (2011) Biaxial mechanical modeling of the small intestine. J Mech Behav Biomed Mater 4(8):1727–1740
Bharucha AE, Hubmayr RD, Ferber IJ, Zinsmeister AR (2001) Viscoelastic properties of the human colon. Am J Physiol Gastrointest Liver Physiol 281(2):G459–G466
Bhattarai A, Horbach AJ, Staat M, Kowalczyk W, Tran TN (2022) Virgin passive colon biomechanics and a literature review of active contraction constitutive models. Biomechanics 2(2):138–157
Bhattarai A, May CA, Staat M, Kowalczyk W, Tran TN (2022) Layer-specific damage modeling of porcine large intestine under biaxial tension. Bioengineering 9(10):528
Borsdorf M, Böl M, Siebert T (2021) Influence of layer separation on the determination of stomach smooth muscle properties. Pflügers Archiv-Eur J Physiol 473(6):911–920
Bourgouin S, Bège T, Masson C, Arnoux P, Mancini J, Garcia S, Brunet C, Berdah S (2012) Biomechanical characterisation of fresh and cadaverous human small intestine: applications for abdominal trauma. Med Biol Eng Comput 50(12):1279–1288
Brasseur J, Nicosia M, Pal A, Miller L (2007) Function of longitudinal vs circular muscle fibers in esophageal peristalsis, deduced with mathematical modeling. World J Gastroenterol 13(9):1335–1346
Brunenieks I, Pekarska K, Kasyanov V, Groma V (2017) Biomechanical and morphological peculiarities of the rectum in patients with obstructed defecation syndrome. Romanian J Morphol Embryol Revue Roumaine de Morphologie et Embryologie 58(4):1193–1200
Carniel EL, Albanese A, Fontanella CG, Pavan PG, Prevedello L, Salmaso C, Todros S, Toniolo I, Foletto M (2020) Biomechanics of stomach tissues and structure in patients with obesity. J Mech Behav Biomed Mater 110:103883
Carniel E, Gramigna V, Fontanella C, Frigo A, Stefanini C, Rubini A, Natali A (2014) Characterization of the anisotropic mechanical behaviour of colonic tissues: experimental activity and constitutive formulation. Exp Physiol 99(5):759–771
Carniel EL, Gramigna V, Fontanella CG, Stefanini C, Natali AN (2014) Constitutive formulations for the mechanical investigation of colonic tissues. J Biomed Mater Res Part A 102(5):1243–1254
Carniel EL, Rubini A, Frigo A, Natali AN (2014) Analysis of the biomechanical behaviour of gastrointestinal regions adopting an experimental and computational approach. Comput Methods Programs Biomed 113(1):338–345
Carvalho MR, Ferreira JP, Oliveira DA, Parente MP, Natal Jorge RM (2022) Biomechanical characterization of the small intestine to simulate gastrointestinal tract chyme propulsion. Int J Numer Methods Biomed Eng 38(5):e3588
Chagnon G, Rebouah M, Favier D (2015) Hyperelastic energy densities for soft biological tissues: a review. J Elast 120(2):129–160
Chakravarthy S, Rao A, Ananthasuresh G (2014) A haptic simulator for upper gastrointestinal endoscopy. In: The Hamlyn Symposium on Medical Robotics, p 16
Chen X, Zhao J, Gregersen H (2008) The villi contribute to the mechanics in the guinea pig small intestine. J Biomech 41(4):806–812
Chen P, Zhao J, Nielsen VH, Clausen T, Gregersen H (2009) Intestinal remodelling in mink fed with reduced protein content. J Biomech 42(4):443–448
Christensen H, Flyvbjerg A, Ørskov H, Laurberg S (1993) Effect of growth hormone on the inflammatory activity of experimental colitis in rats. Scand J Gastroenterol 28(6):503–511
Christensen MB, Oberg K, Wolchok JC (2015) Tensile properties of the rectal and sigmoid colon: a comparative analysis of human and porcine tissue. Springerplus 4(1):1–10
Ciarletta P, Dario P, Tendick F, Micera S (2009) Hyperelastic model of anisotropic fiber reinforcements within intestinal walls for applications in medical robotics. Int J Robot Res 28(10):1279–1288
Cleveland Clinic (2023) Stomach: Anatomy, function, diagram, parts of, structure. URL https://my.clevelandclinic.org/health/body/21758-stomach
Dall F, Jørgensen C, Djurhuus J, Gregersen H (1991) Biomechanical wall properties of the porcine rectum: a study using impedance planimetry. Dig Dis 9(6):347–353
Dall F, Jørgensen C, Houe D, Gregersen H, Djurhuus J (1993) Biomechanical wall properties of the human rectum: a study with impedance planimetry. Gut 34(11):1581–1586
Dargar S, Akyildiz AC, De S (2016) In situ mechanical characterization of multilayer soft tissue using ultrasound imaging. IEEE Trans Biomed Eng 64(11):2595–2606
Dargar S, Rahul R, Kruger U, De S (2019) In vivo layer-specific mechanical characterization of porcine stomach tissue using a customized ultrasound elastography system. J Biomech Eng 141(10):1010041–10100410
Davis NF, Mulvihill JJ, Mulay S, Cunnane EM, Bolton DM, Walsh MT (2018) Urinary bladder vs gastrointestinal tissue: a comparative study of their biomechanical properties for urinary tract reconstruction. Urology 113:235–240
Deptuła P, Łysik D, Pogoda K, Cieśluk M, Namiot A, Mystkowska J, Król G, Głuszek S, Janmey PA, Bucki R (2020) Tissue rheology as a possible complementary procedure to advance histological diagnosis of colon cancer. ACS Biomater Sci Eng 6(10):5620–5631
Donmazov S, Piskin S, Pekkan K (2015) Noninvasive in vivo determination of residual strains and stresses. J Biomech Eng 137(6):061011
Dorfmann A, Ogden RW (2004) A constitutive model for the mullins effect with permanent set in particle-reinforced rubber. Int J Solids Struct 41(7):1855–1878
Dou Y, Fan Y, Zhao J, Gregersen H (2006) Longitudinal residual strain and stress-strain relationship in rat small intestine. Biomed Eng Online 5(1):1–9
Dou Y, Gregersen S, Zhao J, Zhuang F, Gregersen H (2001) Effect of re-feeding after starvation on biomechanical properties in rat small intestine. Med Eng Phys 23(8):557–566
Dou Y, Gregersen S, Zhao J, Zhuang F, Gregersen H (2002) Morphometric and biomechanical intestinal remodeling induced by fasting in rats. Dig Dis Sci 47(5):1158–1168
Dou Y, Lu X, Zhao J, Gregersen H (2002) Morphometric and biomechanical remodelling in the intestine after small bowel resection in the rat. Neurogastroenterol Motili 14(1):43–53
Dou Y, Zhao J, Gregersen H (2003) Morphology and stress-strain properties along the small intestine in the rat. J Biomech Eng 125(2):266–273
Drewes A, Pedersen J, Liu W, Arendt-Nielsen L, Gregersen H (2003) Controlled mechanical distension of the human oesophagus: sensory and biomechanical findings. Scand J Gastroenterol 38(1):27–35
Drewes A, Pedersen J, Reddy H, Rasmussen K, Funch-Jensen P, Arendt-Nielsen L, Gregersen H (2006) Central sensitization in patients with non-cardiac chest pain: a clinical experimental study. Scand J Gastroenterol 41(6):640–649
Drewes AM, Petersen P, Rössel P, Gao C, Hansen J, Arendt-Nielsen L (2001) Sensitivity and distensibility of the rectum and sigmoid colon in patients with irritable bowel syndrome. Scand J Gastroenterol 36(8):827–832
Drewes AM, Reddy H, Staahl C, Pedersen J, Funch-Jensen P, Arendt-Nielsen L, Gregersen H (2005) Sensory-motor responses to mechanical stimulation of the esophagus after sensitization with acid. World J Gastroenterol WJG 11(28):4367
Drewes AM, Schipper K-P, Dimcevski G, Petersen P, Andersen OK, Gregersen H, Arendt-Nielsen L (2002) Multimodal assessment of pain in the esophagus: a new experimental model. Am J Physiol Gastroint Liver Physiol 283(1):G95–G103
Drewes AM, Fr\(\varphi\)kjær JB, Larsen E, Reddy H, Arendt-Nielsen L, Gregersen H (2006) Pain and mechanical properties of the rectum in patients with active ulcerative colitis, Inflamm Bowel Dis 12(4):294–303
Durcan C, Hossain M, Chagnon G, Perić D, Bsiesy L, Karam G, Girard E (2022) Experimental investigations of the human oesophagus: anisotropic properties of the embalmed muscular layer under large deformation. Biomech Model Mechanobiol 21:1169–1186
Durcan C, Hossain M, Chagnon G, Perić D, Karam G, Bsiesy L, Girard E (2022) Experimental investigations of the human oesophagus: anisotropic properties of the embalmed mucosa-submucosa layer under large deformation. Biomech Model Mechanobiol 21:1685–1702
Egorov VI, Schastlivtsev IV, Prut EV, Baranov AO, Turusov RA (2002) Mechanical properties of the human gastrointestinal tract. J Biomech 35(10):1417–1425
Elbrønd H, Tøttrup A, Forman A (1991) Mechanical properties of isolated smooth muscle from rabbit sphincter of oddi and duodenum. Scand J Gastroenterol 26(3):289–294
Emery J, Omens J, McCulloch A (1997) Strain softening in rat left ventricular myocardium. J Biomech Eng 119(1):6–12
Evans A, Whelehan P, Thomson K, McLean D, Brauer K, Purdie C, Jordan L, Baker L, Thompson A (2010) Quantitative shear wave ultrasound elastography: initial experience in solid breast masses. Breast Cancer Res 12(6):1–11
Fan Y, Gregersen H, Kassab GS (2004) A two-layered mechanical model of the rat esophagus: experiment and theory. Biomed Eng Online 3:40
Fan Y, Zhao J, Liao D, Gregersen H (2005) The effect of digestion of collagen and elastin on histomorphometry and the zero-stress state in rat esophagus. Dig Dis Sci 50(8):1497–1505
Ferrua M, Singh R (2010) Modeling the fluid dynamics in a human stomach to gain insight of food digestion. J Food Sci 75(7):R151–R162
Fontanella CG, Salmaso C, Toniolo I, de Cesare N, Rubini A, De Benedictis GM, Carniel EL (2019) Computational models for the mechanical investigation of stomach tissues and structure. Ann Biomed Eng 47(5):1237–1249
Frøkjaer J, Andersen S, Lundbye-christensen S, Funch-Jensen P, Drewes AM, Gregersen H (2006) Sensation and distribution of stress and deformation in the human oesophagus. Neurogastroenterol Motili 18(2):104–114
Frøkjær JB, Andersen SD, Ejskjær N, Funch-Jensen P, Drewes AM, Gregersen H (2007) Impaired contractility and remodeling of the upper gastrointestinal tract in diabetes mellitus type-1. World J Gastroenterol WJG 13(36):4881
Frøkjær JB, Liao D, Bergmann A, McMahon B, Steffensen E, Drewes AM, Gregersen H (2005) Three-dimensional biomechanical properties of the human rectum evaluated with magnetic resonance imaging. Neurogastroenterol Motili 17(4):531–540
Fung Y (1984) What principle governs the stress distribution in living organs? Biomech China 2:1–13
Fung Y, Liu S (1989) Change of residual strains in arteries due to hypertrophy caused by aortic constriction. Circ Res 65(5):1340–1349
Gao C, Arendt-Nielsen L, Liu W, Petersen P, Drewes AM, Gregersen H (2003) Sensory and biomechanical responses to ramp-controlled distension of the human duodenum. Am J Physiol-Gastrointest Liver Physiol 284(3):G461–G471
Gao C, Gregersen H (2000) Biomechanical and morphological properties in rat large intestine. J Biomech 33(9):1089–1097
Gao M, Hu C, Chen Z, Zhang H, Liu S (2010) Design and fabrication of a magnetic propulsion system for self-propelled capsule endoscope. IEEE Trans Biomed Eng 57(12):2891–2902
Gao F, Liao D, Drewes AM, Gregersen H (2009) Modelling the elastin, collagen and smooth muscle contribution to the duodenal mechanical behaviour in patients with systemic sclerosis. Neurogastroenterol Motili 21(9):914-e68
Gao F, Liao D, Zhao J, Drewes AM, Gregersen H (2008) Numerical analysis of pouch filling and emptying after laparoscopic gastric banding surgery. Obes Surg 18(3):243–250
Gao C, Zhao J, Gregersen H (2000) Histomorphometry and strain distribution in pig duodenum with reference to zero-stress state. Dig Dis Sci 45(8):1500–1508
Gilchrist M, Murphy JG, Parnell W, Pierrat B (2014) Modelling the slight compressibility of anisotropic soft tissue. Int J Solids Struct 51(23–24):3857–3865
Gill R, Cote K, Bowes K, Kingma Y (1986) Human colonic smooth muscle: spontaneous contractile activity and response to stretch. Gut 27(9):1006–1013
Girard E, Chagnon G, Gremen E, Calvez M, Masri C, Boutonnat J, Trilling B, Nottelet B (2019) Biomechanical behaviour of human bile duct wall and impact of cadaveric preservation processes. J Mech Behav Biomed Mater 98:291–300
Glavind E, Forman A, Madsen G, Svane D, Andersson K, Tottrup A (1993) Mechanical properties of isolated smooth muscle from human rectum and internal anal sphincter. Am J Physiol Gastrointest Liver Physiol 265(4):G792–G798
Gong X, Xu X, Lin S, Cheng Y, Tong J, Li Y (2017) Alterations in biomechanical properties and microstructure of colon wall in early-stage experimental colitis. Exp Ther Med 14(2):995–1000
Goth W, Lesicko J, Sacks MS, Tunnell JW (2016) Optical-based analysis of soft tissue structures. Annu Rev Biomed Eng 18:357–385
Goto Y, Kakizaki M (1981) The spontaneous-diabetes rat: a model of noninsulin dependent diabetes mellitus. Proc Japan Acad Ser B 57(10):381–384
Goyal R, Biancani P, Phillips A, Spiro H (1971) Mechanical properties of the esophageal wall. J Clin Investig 50(7):1456–1465
Gregersen H, Emery J, McCulloch A (1998) History-dependent mechanical behavior of guinea-pig small intestine. Ann Biomed Eng 26(5):850–858
Gregersen H, Giversen IM, Rasmussen LM, Tøttrup A (1992) Biomechanical wall properties and collagen content in the partially obstructed opossum esophagus. Gastroenterology 103(5):1547–1551
Gregersen H, Jensen L, Djurhuus J (1988) Changes in oesophageal wall biomechanics after portal vein banding and variceal sclerotherapy measured by a new technique. An experimental study in rabbits. Gut 29(12):1699–1704
Gregersen H, Kassab G (1996) Biomechanics of the gastrointestinal tract. Neurogastroenterol Motili 8(4):277–297
Gregersen H, Kassab G, Fung Y (2000) The zero-stress state of the gastrointestinal tract. Dig Dis Sci 45(12):2271–2281
Gregersen H, Kassab G, Pallencaoe E, Lee C, Chien S, Skalak R, Fung Y (1997) Morphometry and strain distribution in guinea pig duodenum with reference to the zero-stress state. Am J Physiol-Gastrointest Liver Physiol 273(4):G865–G874
Gregersen H, Knudsen L, Eika B, Nerstrøm LS, Rasmussen L, Jensen L (1991) A time-dependent study of passive esophageal wall properties and collagen content in rabbits with esophageal varices. Dig Dis Sci 36(8):1050–1056
Gregersen H, Lee C, Chien S, Skalak R, Fung Y (1999) Strain distribution in the layered wall of the esophagus. J Biomech Eng 121:442–448
Gregersen H, Liao D, Fung Y (2008) Determination of homeostatic elastic moduli in two layers of the esophagus. J Biomech Eng 130(1):011005–011005
Gregersen H, Liao D, Pedersen J, Drewes A (2007) A new method for evaluation of intestinal muscle contraction properties: studies in normal subjects and in patients with systemic sclerosis. Neurogastroenterol Motili 19(1):11–19
Gregersen H, Lu X, Zhao J (2004) Physiological growth is associated with esophageal morphometric and biomechanical changes in rats. Neurogastroenterol Motili 16(4):403–412
Gregersen H, Lundby L, Overgaard J (2002) Early and late effects of irradiation on morphometry and residual strain of mouse rectum. Dig Dis Sci 47(7):1472–1479
Gregersen H, Villadsen GE, Liao D (2011) Mechanical characteristics of distension-evoked peristaltic contractions in the esophagus of systemic sclerosis patients. Dig Dis Sci 56(12):3559
Gregersen H, Vinter-Jensen L, Juhl CO, Dajani EZ (1996) Impedance planimetric characterization of the distal oesophagus in the goettingen minipig. J Biomech 29(1):63–68
Gregersen H, Weis S, McCulloch A (2001) Oesophageal morphometry and residual strain in a mouse model of osteogenesis imperfecta. Neurogastroenterol Motili 13(5):457–464
Guo H, Hu J, Shen Z, Du D, Zheng Y, Peng J (2021) In vitro and in vivo studies of biodegradable zn-li-mn alloy staples designed for gastrointestinal anastomosis. Acta Biomater 121:713–723
Helander HF, Fändriks L (2014) Surface area of the digestive tract-revisited. Scand J Gastroenterol 49(6):681–689
Higa M, Luo Y, Okuyama T, Takagi T, Shiraishi Y, Yambe T (2006) Passive mechanical properties of large intestine under in vivo and in vitro compression. Med Eng Phys 29(8):840–844
Higa M, Luo Y, Okuyama T, Takagi T, Shiraishi Y, Yambe T (2007) Passive mechanical properties of large intestine under in vivo and in vitro compression. Med Eng Phys 29(8):840–844
Hillemeier C, Biancani P (1990) Mechanical properties of obstructed colon in a hirschsprung’s model. Gastroenterology 99(4):995–1000
Holzapfel GA, Ogden RW (eds) (2006) Mechanics of biological tissue. Springer, Berlin
Holzapfel GA, Ogden RW (2020) An arterial constitutive model accounting for collagen content and cross-linking. J Mech Phys Solids 136:103682
Horowitz M, Samsom M (2004) Gastrointestinal function in diabetes mellitus, vol 5. Wiley, London
Hossain M, Vu DK, Steinmann P (2012) Experimental study and numerical modelling of vhb 4910 polymer. Comput Mater Sci 59:65–74
Hosseini HS, Dunn JC (2020) Biomechanical force prediction for lengthening of small intestine during distraction enterogenesis. Bioengineering 7(4):140
Huang L, Korhonen RK, Turunen MJ, Finnilä MA (2019) Experimental mechanical strain measurement of tissues. PeerJ 7:e6545
Itasaka S, Shiratori K, Takahashi T, Ishikawa M, Kaneko K, Suzuki Y (1992) Stimulation of intramural secretory reflex by luminal distension pressure in rat distal colon. Am J Physiol Gastrointest Liver Physiol 263(1):G108–G114
Ivakhov G, Kolygin A, Titkova S, Anurov M, Sazhin A (2020) Development and evaluation of a novel simulation model for transabdominal preperitoneal (tapp) inguinal hernia repair. Hernia 24(1):159–166
Jensen L, Laurberg S, Juhl C, Andreassen T (1987) Esophageal collagen content and mechanical strength after endoscopic sclerotherapy of esophageal varices: an experimental study in rabbits. Scand J Gastroenterol 22(6):743–749
Jia Z, Li W, Zhou Z (2015) Mechanical characterization of stomach tissue under uniaxial tensile action. J Biomech 48(4):651–658
Jiang H, Liao D, Zhao J, Wang G, Gregersen H (2014) Contractions reverse stress softening in rat esophagus. Ann Biomed Eng 8(42):1717–1728
Jiang H, Liao D, Zhao J, Wang G, Gregersen H (2017) Reversible stress softening in layered rat esophagus in vitro after potassium chloride activation. Biomech Model Mechanobiol 16(3):1065–1075
Jiang H, Zhao J, Liao D, Wang G, Gregersen H (2019) Esophageal stress softening recovery is altered in stz-induced diabetic rats. J Biomech 92:126–136
Johnson B, Campbell S, Campbell-Kyureghyan N (2020) Biomechanical properties of abdominal organs under tension with special reference to increasing strain rate. J Biomech 109:109914
Juhl CO, Vinter-Jensen L, Djurhuus J, Gregersen H, Dajani EZ (1994) Biomechanical properties of the oesophagus damaged by endoscopic sclerotherapy: an impedance planimetric study in minipigs. Scand J Gastroenterol 29(10):867–873
Julie FS, Strøm HT, Mette P, Hans G, Vinge NJ (2022) Dynamic viscoelastic properties of porcine gastric tissue: effects of loading frequency, region and direction. J Biomech 143:111302
Jørgensen CS, Ahrensberg JM, Gregersen H, Flyvberg A (2001) Tension-strain relations and morphometry of rat small intestine in experimental diabetes. Dig Dis Sci 46(5):960–967
Jørgensen C, Dall F, Jensen S, Gregersen H (1995) A new combined high-frequency ultrasound-impedance planimetry measuring system for the quantification of organ wall biomechanics in vivo. J Biomech 28(7):863–867
Jørgensen C, Dall F, Storkholm J, Jensen S, Gregersen H (1991) Elastic properties of the isolated perfused porcine duodenum. Dig Dis 9(6):401–407
Kennedy BF, Kennedy KM, Sampson DD (2013) A review of optical coherence elastography: fundamentals, techniques and prospects. IEEE J Sel Top Quantum Electron 20(2):272–288
Kennedy BF, Wijesinghe P, Sampson DD (2017) The emergence of optical elastography in biomedicine. Nat Photon 11(4):215–221
Khan AS, Liu H (2012) Strain rate and temperature dependent fracture criteria for isotropic and anisotropic metals. Int J Plast 37:1–15
Khoo S-W, Karuppanan S, Tan C-S (2016) A review of surface deformation and strain measurement using two-dimensional digital image correlation. Metrol Measur Syst 23(3):461–480
Kim J, Sung I, Kim Y, Kim D, Jang Y (2007) Analytical model development for the prediction of the frictional resistance of a capsule endoscope inside an intestine. Proc Inst Mech Eng [H] 221(8):837–845
Klemm L, Seydewitz R, Borsdorf M, Siebert T, Böl M (2020) On a coupled electro-chemomechanical model of gastric smooth muscle contraction. Acta Biomater 109:163–181
Kunkel M, Moral A, Westphal R, Rode D, Rilk M, Wahl F (2008) Using robotic systems in order to determine biomechanical properties of soft tissues. Stud Health Technol Inform 133:156–165
Lefloch S, Cañadas P, Ambard D, Dusfour G (2021) Residual strains estimation in the annulus fibrosus through digital image correlation. J Theor Comput Appl Mech 2:6971
Li G-Y, Cao Y (2017) Mechanics of ultrasound elastography. Proc R Soc A Math Phys Eng Sci 473(2199):20160841
Li J, Zhao J, Liao D, Gregersen H (2008) Effect of smooth muscle tone on morphometry and residual strain in rat duodenum, jejunum and ileum. J Biomech 41(12):2667–2672
Liao D, Cassin J, Zhao J, Gregersen H (2006) The geometric configuration and morphometry of the rabbit oesophagus during luminal pressure loading. Physiol Meas 27(8):703
Liao D, Fan Y, Zeng Y, Gregersen H (2003) Stress distribution in the layered wall of the rat oesophagus. Med Eng Phys 25(9):731–738
Liao D, Frøkjær JB, Yang J, Zhao J, Drewes AM, Gilja OH, Gregersen H (2006) Three-dimensional surface model analysis in the gastrointestinal tract. World J Gastroenterol WJG 12(18):2870
Liao D, Lu X, Kirkup AJ, Jiang W, Grundy D, Gregersen H (2012) Interdependency of stress relaxation and afferent nerve discharge in rat small intestine. J Biomech 45(9):1574–1579
Liao D, Villadsen G, Gregersen H (2013) Distension-evoked motility analysis in human esophagus. Neurogastroenterol Motili 25(5):407
Liao D, Yang J, Zhao J, Zeng Y, Vinter-Jensen L, Gregersen H (2003) The effect of epidermal growth factor on the incremental young’s moduli in the rat small intestine. Med Eng Phys 25(5):413–418
Liao D, Zhao J, Gregersen H (2004) Regional surface geometry of the rat stomach based on three-dimensional curvature analysis. Phys Med Biol 50(2):231
Liao D, Zhao J, Gregersen H (2010) 3d mechanical properties of the partially obstructed guinea pig small intestine. J Biomech 43(11):2079–2086
Liao D, Zhao J, Kunwald P, Gregersen H (2009) Tissue softening of guinea pig oesophagus tested by the tri-axial test machine. J Biomech 42(7):804–810
Lim Y-J, Deo D, Singh TP, Jones DB, De S (2009) In situ measurement and modeling of biomechanical response of human cadaveric soft tissues for physics-based surgical simulation. Surg Endosc 6(23):1298–1307
Lin C, Li W, Deng H, Li K, Zhou Z (2019) Friction behavior of esophageal mucosa under axial and circumferential extension. Tribol Lett 67(1):1–14
Lin C, Ren P, Li W, Deng H, Zhou Z (2020) Finite-element modelling of frictional behaviour between oesophagus and endoscope. Biosurface Biotribol 6(3):75–81
Lin C, Yu Q, Wang J, Ji W, Li W, Zhou Z (2017) Friction behavior between endoscopy and esophageal internal surface. Wear 376:272–280
Liu Y, Zhao J, Liao D, Bao L, Gregersen H (2017) Low-residue diet fed to rabbits induces histomorphological and biomechanical remodeling of small intestine. Neurogastroenterol Motili 29(4):e12983
Liu Y, Zhao J, Liao D, Wang G, Gregersen H (2017) Intestinal mechanomorphological remodeling induced by long-term low-fiber diet in rabbits. Ann Biomed Eng 45(12):2867–2878
Liu Y, Zhao J, Liao D, Wang G, Gregersen H (2019) Stress-strain analysis of duodenal contractility in response to flow and ramp distension in rabbits fed low-fiber diet. Neurogastroenterol Motili 31(1):e13476
Liu G-F, Zhao J-B, Zhen Z, Sha H, Chen P-M, Li M, Zhang J-C, Yuan M-Z, Gao W, Gregersen H et al (2012) Effect of tangweian jianji on upper gastrointestinal remodeling in streptozotocin-induced diabetic rats. World J Gastroenterol WJG 18(35):4875
Low G, Kruse SA, Lomas DJ (2016) General review of magnetic resonance elastography. World J Radiol 8(1):59–72
Lu X, Gregersen H (2001) Regional distribution of axial strain and circumferential residual strain in the layered rabbit oesophagus. J Biomech 34(2):225–233
Lu X, Zhang Z, Choy J, Kassab G (2012) Role of distension on duodenal and colonic contractility in mice: a novel myograph for intestines. Neurogastroenterol Motili 24(5):487–493
Lu X, Zhao J, Gregersen H (2005) Small intestinal morphometric and biomechanical changes during physiological growth in rats. J Biomech 38(3):417–426
Lundby L, Dall F, Gregersen H, Overgaard J, Laurberg S (1999) Distensibility of the mouse rectum: application of impedance planimetry for studying age-related changes. Colorectal Dis 1:34–41
Lyons M, Winter D, Simms C (2012) Extrusion properties of porcine intestines and surrogate materials for ventral hernia modelling. J Mech Behav Biomed Mater 18:57–66
Marie L, Masson C, Gaborit B, Berdah SV, Bège T (2019) An experimental study of intraluminal hyperpressure reproducing a gastric leak following a sleeve gastrectomy. Obes Surg 29(9):2773–2780
Masri C, Chagnon G, Favier D, Sartelet H, Girard E (2018) Experimental characterization and constitutive modeling of the biomechanical behavior of male human urethral tissues validated by histological observations. Biomech Model Mechanobiol 17(4):939–950
Massalou D, Masson C, Afquir S, Baque P, Arnoux P-J, Bège T (2019) Influence of gender, age, shelf-life, and conservation method on the biomechanical behavior of colon tissue under dynamic solicitation. Clin Biomech 65:34–40
Massalou D, Masson C, Afquir S, Baqué P, Arnoux P-J, Bège T (2019) Mechanical effects of load speed on the human colon. J Biomech 91:102–108
Massalou D, Masson C, Foti P, Afquir S, Baqué P, Berdah S-V, Bège T (2016) Dynamic biomechanical characterization of colon tissue according to anatomical factors. J Biomech 49(16):3861–3867
Mehnert M, Steinmann P (2019) On the influence of the compliant electrodes on the mechanical behavior of vhb 4905. Comput Mater Sci 160:287–294
Merlo A, Cohen S (1988) Neuropeptide responses and mechanics of the proximal and distal feline colon in vitro. Am J Physiol Gastrointest Liver Physiol 255(6):G787–G793
Merlo A, Cohen S (1989) Mechanics and neuropeptide responses of feline pylorus and gastric muscle in vitro. Am J Physiol-Gastrointest Liver Physiol 256(5):G862–G867
Mishra M, Rao AR (2005) Peristaltic transport in a channel with a porous peripheral layer: model of a flow in gastrointestinal tract. J Biomech 38(4):779–789
Mojoli F, Iotti GA, Torriglia F, Pozzi M, Volta CA, Bianzina S, Braschi A, Brochard L (2016) In vivo calibration of esophageal pressure in the mechanically ventilated patient makes measurements reliable. Crit Care 20(1):98
Moreira D, Nunes L (2013) Comparison of simple and pure shear for an incompressible isotropic hyperelastic material under large deformation. Polym Testing 32(2):240–248
Mujica VR, Mudipalli RS, Rao SS (2001) Pathophysiology of chest pain in patients with nutcracker esophagus. Am J Gastroenterol 96(5):1371–1377
Mulvihill JJ, Walsh MT (2013) On the mechanical behaviour of carotid artery plaques: the influence of curve-fitting experimental data on numerical model results. Biomech Model Mechanobiol 12(5):975–985
Nagaraja S, Leichsenring K, Ambati M, De Lorenzis L, Böl M (2021) On a phase-field approach to model fracture of small intestine walls. Acta Biomater 130:317–331
Nair A, Liu CH, Singh M, Das S, Le T, Du Y, Soomro S, Aglyamov S, Mohan C, Larin KV (2019) Assessing colitis ex vivo using optical coherence elastography in a murine model. Quant Imaging Med Surg 9(8):1429
Nair A, Liu CH, Das S, Ho T, Du Y, Soomro S, Mohan C, Larin KV (2018) Detecting murine inflammatory bowel disease using optical coherence elastography. In: 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), IEEE, pp 830–833
Nasr I, Attaluri A, Hashmi S, Gregersen H, Rao SS (2010) Investigation of esophageal sensation and biomechanical properties in functional chest pain. Neurogastroenterol Motili 22(5):520
Natali AN, Carniel EL, Gregersen H (2009) Biomechanical behaviour of oesophageal tissues: material and structural configuration, experimental data and constitutive analysis. Med Eng Phys 31(9):1056–1062
Ngwangwa H, Pandelani T, Msibi M, Mabuda I, Semakane L, Nemavhola F (2022) Biomechanical analysis of sheep oesophagus subjected to biaxial testing including hyperelastic constitutive model fitting. Heliyon 8(5):e09312
Nicolle S, Palierne J-F (2010) Dehydration effect on the mechanical behaviour of biological soft tissues: observations on kidney tissues. J Mech Behav Biomed Mater 3(8):630–635
Nolan D, McGarry J (2016) On the compressibility of arterial tissue. Ann Biomed Eng 44(4):993–1007
Nováček V, Trân T, Klinge U, Tolba R, Staat M, Bronson D, Miesse A, Whiffen J, Turquier F (2012) Finite element modelling of stapled colorectal end-to-end anastomosis: advantages of variable height stapler design. J Biomech 45(15):2693–2697
Ogobuiro I, Gonzales J, Tuma F (2021) Physiology, gastrointestinal. In: StatPearls [Internet], StatPearls Publishing
Orvar KB, Gregersen H, Christensen J (1993) Biomechanical characteristics of the human esophagus. Dig Dis Sci 38(2):197–205
Ouyang A, Zimmerman K, Wong K-L, Sharp D, Reynolds JC (1996) Effect of celiac ganglionectomy on tachykinin innervation, receptor distribution and intestinal responses in the rat. J Auton Nerv Syst 61(3):292–300
Palmada N, Hosseini S, Avci R, Cater JE, Suresh V, Cheng LK (2023) A systematic review of computational fluid dynamics models in the stomach and small intestine. Appl Sci 13(10):6092
Panda SK, Buist ML (2019) A finite element approach for gastrointestinal tissue mechanics. Int J Numer Methods Biomed Eng 35(12):e3269
Patel B, Chen H, Ahuja A, Krieger JF, Noblet J, Chambers S, Kassab GS (2018) Constitutive modeling of the passive inflation-extension behavior of the swine colon. J Mech Behav Biomed Mater 77:176–186
Patel B, Gizzi A, Hashemi J, Awakeem Y, Gregersen H, Kassab G (2022) Biomechanical constitutive modeling of the gastrointestinal tissues: a systematic review. Mater Des 6:110576
Patel RS, Rao SS (1998) Biomechanical and sensory parameters of the human esophagus at four levels. Am J Physiol-Gastrointest Liver Physiol 275(2):G187–G191
Payan Y, Ohayon J (2017) Biomechanics of living organs: hyperelastic constitutive laws for finite element modeling. Academic Press, London
Peck JW, Gibbs FA Jr (1987) Assay of premorbid murine jejunal fibrosis based on mechanical changes after x irradiation and hyperthermia. Radiat Res 112(3):525–543
Pedersen J, Gao C, Egekvist H, Bjerring P, Arendt-Nielsen L, Gregersen H, Drewes AM (2003) Pain and biomechanical responses to distention of the duodenum in patients with systemic sclerosis. Gastroenterology 124(5):1230–1239
Pedro S-D, Moreno-Sanz C, Morandeira-Rivas A, Tenías-Burillo JM, Alhambra-Rodríguez De Guzmán C et al (2014) Colorectal anastomosis facilitated by the use of the ligasure® sealing device: comparative study in an animal model. Surg Endosc 28(2):508–514
Peirlinck M, Debusschere N, Iannaccone F, Siersema PD, Verhegghe B, Segers P, De Beule M (2018) An in silico biomechanical analysis of the stent-esophagus interaction. Biomech Model Mechanobiol 17(1):111–131
Percy W, Burton M, Fallick F, Burakoff R (1990) A comparison in vitro of human and rabbit distal colonic muscle responses to inflammatory mediators. Gastroenterology 99(5):1324–1332
Pescatori M, Grassetti F, Ronzoni G, Mancinelli R, Bertuzzi A, Salinari S (1979) Peristalsis in distal colon of the rabbit: an analysis of mechanical events. Am J Physiol-Endocrinol Metabol 236(4):E464
Petersen P, Gao C, Arendt-Nielsen L, Gregersen H, Drewes AM (2003) Pain intensity and biomechanical responses during ramp-controlled distension of the human rectum. Dig Dis Sci 48(7):1310–1316
Petersen P, Gao C, Rössel P, Qvist P, Arendt-Nielsen L, Gregersen H, Drewes AM (2001) Sensory and biomechanical responses to distension of the normal human rectum and sigmoid colon. Digestion 64(3):191–199
Puértolas S, Peña E, Herrera A, Ibarz E, Gracia L (2020) A comparative study of hyperelastic constitutive models for colonic tissue fitted to multiaxial experimental testing. J Mech Behav Biomed Mater 102:103507
Qiao Y, Pan E, Chakravarthula S, Han F, Liang J, Gudlavalleti S (2005) Measurement of mechanical properties of rectal wall. J Mater Sci Mater Med 16(2):183–188
Radhakrishnan RS, Xue H, Moore-Olufemi SD, Weisbrodt NW, Moore FA, Allen SJ, Laine GA, Cox CS Jr (2006) Hypertonic saline resuscitation prevents hydrostatically induced intestinal edema and ileus. Crit Care Med 34(6):1713–1718
Radhakrishnan RS, Xue H, Weisbrodt N, Moore FA, Allen SJ, Laine GA, Cox CS Jr (2005) Resuscitation-induced intestinal edema decreases the stiffness and residual stress of the intestine. Shock 24(2):165–170
Rao SSC, Hayek B, Mudipalli R, Gregersen H (2002) Does esophageal function vary at the striated and smooth muscle segments in functional chest pain? Am J Gastroenterol 97(9):2201–2207
Rao S, Hayek B, Summers R (2001) Functional chest pain of esophageal origin: hyperalgesia or motor dysfunction. Am J Gastroenterol 96(9):2584–2589
Remes-Troche JM, Attaluri A, Chahal P, Rao SSC (2012) Barostat or dynamic balloon distention test: which technique is best suited for esophageal sensory testing? Dis Esophagus 25(7):584–589
Ren P, Deng X, Li K, Li G, Li W (2021) 3d biomechanical properties of the layered esophagus: fung-type sef and new constitutive model. Biomech Model Mechanobiol 20(5):1775–1788
Righi M, Balbi V (2021) Foundations of viscoelasticity and application to soft tissue mechanics. In: Modeling Biomaterials, Springer, pp 71–103
Rosen J, Brown J, De S, Sinanan M, Hannaford B (2008) Biomechanical properties of abdominal organs in vivo and postmortem under compression loads. J Biomech Eng 130(2):021020–021020
Ruan J, El-Jawahri R, Chai L, Barbat S, Prasad P (2003) Prediction and analysis of human thoracic impact responses and injuries in cadaver impacts using a full human body finite element model. Stapp Car Crash J 47:299–321
Rubod C, Brieu M, Cosson M, Rivaux G, Clay J-C, de Landsheere L, Gabriel B (2012) Biomechanical properties of human pelvic organs. Urology 79(4):968-e17
Salmaso C, Toniolo I, Fontanella C, Da Roit P, Albanese A, Polese L, Stefanini C, Foletto M, Carniel E (2020) Computational tools for the reliability assessment and the engineering design of procedures and devices in bariatric surgery. Ann Biomed Eng 48(10):2466–2483
Sanchez-Molina D, Velazquez-Ameijide J, Arregui-Dalmases C, Rodríguez D, Quintana V, Shafieian M, Crandall J (2014) A microcontinuum model for mechanical properties of esophageal tissue: experimental methodology and constitutive analysis. Ann Biomed Eng 42(1):62–72
Saraf H, Ramesh K, Lennon A, Merkle A, Roberts J (2007) Mechanical properties of soft human tissues under dynamic loading. J Biomech 40(9):1960–1967
Saxena AK, Biro E, Sommer G, Holzapfel GA (2021) Esophagus stretch tests: biomechanics for tissue engineering and possible implications on the outcome of esophageal atresia repairs performed under excessive tension. Esophagus 18(2):346–352
Sha H, Zhao J-B, Zhang Z-Y, Zhou S-P, Tong X-L, Zhuang F-Y, Gregersen H (2006) Effect of Kaiyu Qingwei Jianji on the morphometry and residual strain distribution of small intestine in experimental diabetic rats. World J Gastroenterol 12(44):7149–7154
Shafik A (1998) Effect of duodenal distension on the pyloric sphincter and antrum and the gastric corpus: duodenopyloric reflex. World J Surg 22(10):1061–1064
Shanahan C, Tofail SA, Tiernan P (2017) Viscoelastic braided stent: finite element modelling and validation of crimping behaviour. Mater Des 121:143–153
Sigaeva T, Destrade M, Di Martino ES (2019) Multi-sector approximation method for arteries: the residual stresses of circumferential rings with non-trivial openings. J R Soc Interface 16(156):20190023
Siri S, Maier F, Chen L, Santos S, Pierce DM, Feng B (2019) Differential biomechanical properties of mouse distal colon and rectum innervated by the splanchnic and pelvic afferents. Am J Physiol Gastrointest Liver Physiol 316(4):G473–G481
Siri S, Maier F, Santos S, Pierce DM, Feng B (2019) Load-bearing function of the colorectal submucosa and its relevance to visceral nociception elicited by mechanical stretch. Am J Physiol Gastrointest Liver Physiol 317(3):G349–G358
Siri S, Zhao Y, Maier F, Pierce DM, Feng B (2020) The macro-and micro-mechanics of the colon and rectum I: experimental evidence. Bioengineering 7(4):130
Skamniotis C, Edwards CH, Bakalis S, Frost G, Charalambides M (2020) Eulerian-lagrangian finite element modelling of food flow-fracture in the stomach to engineer digestion. Innov Food Sci Emerg Technol 66:102510
Smith JB, Zhao J-B, Dou Y-L, Gregersen H (2005) Time-dependent viscoelastic properties along rat small intestine. World J Gastroenterol 11(32):4974–4978
Sokolis D (2010) Strain-energy function and three-dimensional stress distribution in esophageal biomechanics. J Biomech 43(14):2753–2764
Sokolis DP (2013) Structurally-motivated characterization of the passive pseudo-elastic response of esophagus and its layers. Comput Biol Med 9(43):1273–1285
Sokolis D (2017) Experimental study and biomechanical characterization for the passive small intestine: identification of regional differences. J Mech Behav Biomed Mater 74:93–105
Sokolis DP, Orfanidis IK, Peroulis M (2011) Biomechanical testing and material characterization for the rat large intestine: regional dependence of material parameters. Physiol Meas 32(12):1969
Sokolis DP, Sassani SG (2013) Microstructure-based constitutive modeling for the large intestine validated by histological observations. J Mech Behav Biomed Mater 21:149–166
Sommer G, Schriefl AJ, Zeindlinger G, Katzensteiner A, Ainödhofer H, Saxena A, Holzapfel G (2013) Multiaxial mechanical response and constitutive modeling of esophageal tissues: impact on esophageal tissue engineering. Acta Biomater 9(12):9379–9391
Stavropoulou EA, Dafalias YF, Sokolis DP (2009) Biomechanical and histological characteristics of passive esophagus: experimental investigation and comparative constitutive modeling. J Biomech 16(42):2654–2663
Stavropoulou EA, Dafalias YF, Sokolis DP (2012) Biomechanical behavior and histological organization of the three-layered passive esophagus as a function of topography. Proc Inst Mech Eng [H] 226(6):477–490
Stewart DC, Rubiano A, Santisteban MM, Shenoy V, Qi Y, Pepine CJ, Raizada MK, Simmons CS (2016) Hypertension-linked mechanical changes of rat gut. Acta Biomater 45:296–302
Stidham RW, Xu J, Johnson LA, Kim K, Moons DS, McKenna BJ, Rubin JM, Higgins PD (2011) Ultrasound elasticity imaging for detecting intestinal fibrosis and inflammation in rats and humans with Crohn’s disease. Gastroenterology 141(3):819–826
Storkholm J, Villadsen G, Jensen S, Gregersen H (1995) Passive elastic wall properties in isolated guinea pig small intestine. Dig Dis Sci 40(5):976–982
Sun D, Zhao J, Liao D, Chen P, Gregersen H (2017) Shear modulus of the partially obstructed rat small intestine. Ann Biomed Eng 45(4):1069–1082
Sun D, Zhao J, Liao D, Huang Z, Gregersen H (2018) The turning point for morphomechanical remodeling during complete intestinal obstruction in rats occurs after 12–24 h. Ann Biomed Eng 46(5):705–716
Taira Y, Kamiya K, Shiraishi Y, Miura H, Shiga T, Hashem M, Yamada A, Tsuboko Y, Sano K, Homma D et al (2014) Examination of natural esophageal mechanical properties for the design of an artificial esophagus. Trans Japn Soc Med Biol Eng 52:557–558
Takeda T, Kassab G, Liu J, Nabae T, Mittal RK (2003) Effect of atropine on the biomechanical properties of the oesophageal wall in humans. J Physiol 547(2):621–628
Takeda T, Kassab G, Liu J, Puckett JL, Mittal RR, Mittal RK (2002) A novel ultrasound technique to study the biomechanics of the human esophagus in vivo. Am J Physiol Gastrointest Liver Physiol 282(5):G785–G793
Tay BK, Kim J, Srinivasan MA (2006) In vivo mechanical behavior of intra-abdominal organs. IEEE Trans Biomed Eng 53(11):2129–2138
Terry BS, Lyle AB, Schoen JA, Rentschler ME (2011) Preliminary mechanical characterization of the small bowel for in vivo robotic mobility. J Biomech Eng 133(9):091010
Terry BS, Wang X, Schoen JA, Rentschler ME (2014) A preconditioning protocol and biaxial mechanical measurement of the small intestine. Int J Exper Comput Biomech 2(4):293–309
Tomalka A, Borsdorf M, Böl M, Siebert T (2017) Porcine stomach smooth muscle force depends on history-effects. Front Physiol 8:802
Toniolo I, Berardo A, Foletto M, Fiorillo C, Quero G, Perretta S, Carniel EL (2022) Patient-specific stomach biomechanics before and after laparoscopic sleeve gastrectomy. Surg Endosc 5:1–14
Toniolo I, Berardo A, Gagner M, Foletto M, Carniel EL (2023) Unveiling the effects of key factors in enhancing gastroesophageal reflux: a fluid-structure analysis before and after laparoscopic sleeve gastrectomy. Comput Methods Programs Biomed 231:107409
Toniolo I, Fontanella CG, Foletto M, Carniel EL (2022) Coupled experimental and computational approach to stomach biomechanics: towards a validated characterization of gastric tissues mechanical properties. J Mech Behav Biomed Mater 125:104914
Tøttrup A, Forman A, Uldbjerg N, Funch-Jensen P, Andersson K (1990) Mechanical properties of isolated human esophageal smooth muscle. Am J Physiol 258(3 Pt 1):G338-43
Vaishnav RN, Vossoughi J (1983) Estimation of residual strains in aortic segments. In: Biomedical engineering II, Elsevier, pp 330–333
Vanags I, Petersons A, Ose V, Ozolanta I, Kasyanov V, Laizans J, Vjaters E, Gardovskis J, Vanags A (2003) Biomechanical properties of oesophagus wall under loading. J Biomech 36(9):1387–1390
Venkatesh SK, Yin M, Ehman RL (2013) Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging 37(3):544–555
Villadsen GE, Storkholm JH, Hendel L, Vilstrup H, Gregersen H (1997) Impedance planimetric characterization of esophagus in systemic sclerosis patients with severe involvement of esophagus. Dig Dis Sci 42(11):2317–2326
Villadsen G, Storkholm J, Zachariae H, Hendel L, Bendtsen F, Gregersen H (2001) Oesophageal pressure-cross-sectional area distributions and secondary peristalsis in relation to subclassification of systemic sclerosis. Neurogastroenterol Motili 13(3):199–210
Vinter-Jensen L, Duch BU, Petersen JAK, Ryslev A, Gregersen H (1996) Systemic treatment with epidermal growth factor in the rat. Biomechanical properties of the growing small intestine. Regul Peptides 61(2):135–142
Vinter-Jensen L, Juhl CO, Eika B, Gregersen H, Dajani E (1995) Epidermal growth factor attenuates the sclerotherapy-induced biomechanical properties of the oesophagus: an experimental study in minipigs. Scand J Gastroenterol 30(7):614–619
Van de Graaff K (1986) Anatomy and physiology of the gastrointestinal tract. Pediatric Infect Dis 5:11–16
Wanamaker R, Grimm I (2004) Encyclopedia of gastroenterology. Gastroenterology 127(4):1274–1275
Wareham A, Whitmore I (1982) A comparison of the mechanical properties of oesophageal striated muscle with skeletal muscles of the guinea pig. Pflugers Arch 395(4):312–317
Watters D, Smith A, Eastwood M, Anderson K, Elton R (1985) Mechanical properties of the rat colon: the effect of age, sex and different conditions of storage. Quart J Exp Physiol Transl Integr 70(1):151–162
Watters D, Smith A, Eastwood M, Anderson K, Elton R, Mugerwa J (1985) Mechanical properties of the colon: comparison of the features of the African and European colon in vitro. Gut 26(4):384–392
Weizel A, Distler T, Detsch R, Boccaccini A, Bräuer L, Paulsen F, Seitz H, Budday S (2022) Hyperelastic parameter identification of human articular cartilage and substitute materials. J Mech Behav Biomed Mater 133:105292
Yang W, Fung T, Chian K, Chong C (2006) 3d mechanical properties of the layered esophagus: experiment and constitutive model. J Biomech Eng 128(6):899–908
Yang W, Fung T, Chian K, Chong C (2006) Directional, regional, and layer variations of mechanical properties of esophageal tissue and its interpretation using a structure-based constitutive model. J Biomech Eng 128(3):409–418
Yang W, Fung T, Chian K, Chong C (2006) Viscoelasticity of esophageal tissue and application of a QLV model. J Biomech Eng 128(6):909–916
Yang W, Fung T, Chian K, Chong C (2006) Investigations of the viscoelasticity of esophageal tissue using incremental stress-relaxation test and cyclic extension test. J Mech Med Biol 6(03):261–272
Yang W, Fung T, Chian K, Chong C (2007) Three-dimensional finite element model of the two-layered oesophagus, including the effects of residual strains and buckling of mucosa. Proc Inst Mech Eng [H] 221(4):417–426
Yang W, Fung TC, Chian KS, Chong CK (2007) Finite element simulation of food transport through the esophageal body. World J Gastroenterol WJG 13(9):1352
Yang J, Liao D, Zhao J, Gregersen H (2004) Shear modulus of elasticity of the esophagus. Ann Biomed Eng 32(9):1223–1230
Yang J, Zhao J, Jiang W, Nakaguchi T, Kunwald P, Grundy D, Gregersen H (2012) Neurogenic adaptation contributes to the afferent response to mechanical stimulation. Am J Physiol-Gastrointest Liver Physiol 302(9):G1025–G1034
Yang J, Zhao J, Liao D, Gregersen H (2006) Biomechanical properties of the layered oesophagus and its remodelling in experimental type-1 diabetes. J Biomech 39(5):894–904
Yang J, Zhao J, Nakaguchi T, Gregersen H (2009) Biomechanical changes in oxazolone-induced colitis in balb/c mice. J Biomech 42(7):811–817
Yang J, Zhao J-B, Zeng Y-J, Gregersen H (2003) Biomechanical properties of ileum after systemic treatment with epithelial growth factor. World J Gastroenterol 9(10):2278
Yang J, Zhao J, Zeng Y, Gregersen H (2004) Biomechanical properties of the rat oesophagus in experimental type-1 diabetes. Neurogastroenterol Motili 16(2):195–203
Yang J, Zhao J, Zeng Y, Vinter-Jensen L, Gregersen H (2003) Morphological properties of zero-stress state in rat large intestine during systemic egf treatment. Dig Dis Sci 48(3):442–448
Ye D, Zhang F, Yuan S, Song S, Meng MQ-H (2019) Magnetically driven wireless capsule robot with targeting biopsy function. In: 2019 IEEE International Conference on Robotics and Biomimetics (ROBIO), IEEE, pp 1222–1227
Zeng Y, Qiao Y, Yang J, Gregersen H, Zhang E, Xu X, Xu H (2004) Torque properties of a rat oesophagus for physiological and diabetic conditions. Physiol Meas 25(5):1211
Zeng Y-J, Yang J, Zhao J-B, Liao D-H, Zhang E-P, Gregersen H, Xu X-H, Xu H, Xu C-Q (2004) Morphologic and biomechanical changes of rat oesophagus in experimental diabetes. World J Gastroenterol WJG 10(17):2519
Zhang C, Liu H, Tan R, Li H (2014) Interaction model between capsule robot and intestine based on nonlinear viscoelasticity. Proc Inst Mech Eng [H] 228(3):287–296
Zhang Y, VanRiper T, Rague B, Weidman D (2016) Fea modeling and fluid flow simulation of human rectum with inserted bowel catheters. In: 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), IEEE, pp 2652–2655
Zhao J, Chen P, Gregersen H (2013) Morpho-mechanical intestinal remodeling in type 2 diabetic gk rats-is it related to advanced glycation end product formation? J Biomech 46(6):1128–34
Zhao J-B, Chen P-M, Gregersen H (2013) Changes of phasic and tonic smooth muscle function of jejunum in type 2 diabetic goto-kakizaki rats. World J Diabetes 4(6):339
Zhao J, Chen X, Yang J, Liao D, Gregersen H (2007) Opening angle and residual strain in a three-layered model of pig oesophagus. J Biomech 40(14):3187–3192
Zhao J, Gregersen H (2015) Esophageal morphometric and biomechanical changes during aging in rats. Neurogastroenterol Motili 27(11):1638–1647
Zhao J, Gregersen H (2015) Morphometric and biomechanical remodeling of the small intestine during aging in rats. J Biomech 48(16):4271–4278
Zhao J, Gregersen H (2016) Diabetes-induced mechanophysiological changes in the esophagus. Ann N Y Acad Sci 1380(1):139–154
Zhao J, Jørgensen CS, Liao D, Gregersen H (2007) Dimensions and circumferential stress-strain relation in the porcine esophagus in vitro determined by combined impedance planimetry and high-frequency ultrasound. Dig Dis Sci 52(5):1338–1344
Zhao J, Liao D, Chen P, Kunwald P, Gregersen H (2008) Stomach stress and strain depend on location, direction and the layered structure. J Biomech 41:3441–7
Zhao J, Liao D, Gregersen H (2005) Tension and stress in the rat and rabbit stomach are location-and direction-dependent. Neurogastroenterol Motili 17(3):388–398
Zhao J, Liao D, Gregersen H (2007) Biomechanical and histomorphometric esophageal remodeling in type 2 diabetic gk rats. J Diabetes Complications 21(1):34–40
Zhao J, Liao D, Gregersen H (2008) Phasic and tonic stress-strain data obtained in intact intestinal segment in vitro. Dig Dis Sci 53(12):3145–3151
Zhao J, Liao D, Gregersen H (2019) Mechanical analysis of intestinal contractility in a neonatal maternal deprivation irritable bowel syndrome rat model. J Biomech 93:42–51
Zhao J, Liao D, Yang J, Gregersen H (2003) Viscoelastic behavior of small intestine in streptozotocin-induced diabetic rats. Dig Dis Sci 48(12):2271–2277
Zhao J, Liao D, Yang J, Gregersen H (2010) Biomechanical remodelling of obstructed guinea pig jejunum. J Biomech 43(7):1322–1329
Zhao J, Liao D, Yang J, Gregersen H (2011) Phasic and tonic smooth muscle function of the partially obstructed guinea pig intestine. J Biomed Biotechnol 2011(3):489720
Zhao J, Liao D, Yang J, Gregersen H (2011) Stress and strain analysis of contractions during ramp distension in partially obstructed guinea pig jejunal segments. J Biomech 44(11):2077–2082
Zhao J, Nakaguchi T, Gregersen H (2009) Biomechanical and histomorphometric colon remodelling in stz-induced diabetic rats. Dig Dis Sci 54(8):1636–1642
Zhao J, Sha H, Zhou S, Tong X, Zhuang F, Gregersen H (2002) Remodelling of zero-stress state of small intestine in streptozotocin-induced diabetic rats. Effect of gliclazide. Dig Liver Dis 34(10):707–716
Zhao J-B, Sha H, Zhuang F-Y, Gregersen H (2002) Morphological properties and residual strain along the small intestine in rats. World J Gastroenterol 8(2):312–317
Zhao J, Yang J, Gregersen H (2003) Biomechanical and morphometric intestinal remodelling during experimental diabetes in rats. Diabetologia 46(12):1688–1697
Zhao J, Yang J, Liao D, Gregersen H (2017) Interdependency between mechanical parameters and afferent nerve discharge in remodeled diabetic goto-kakizaki rat intestine. Clin Exp Gastroenterol 10:303–314
Zhao J, Yang J, Vinter-Jensen L, Zhuang F, Gregersen H (2002) The morphometry and biomechanical properties of the rat small intestine after systemic treatment with epidermal growth factor. Biorheology 39(6):719–733
Zhou H, Alici G, Than TD, Li W (2014) Experimental investigation into biomechanical and biotribological properties of a real intestine and their significance for design of a spiral-type robotic capsule. Proc Inst Mech Eng [H] 228(3):280–286
Acknowledgements
C. Durcan and M. Hossain are indebted to the Swansea University Strategic Partnerships Research Scholarships (SUSPRS) for funding of the project.
Author information
Authors and Affiliations
Contributions
C.D. did methodology, manuscript preparation, and analysis; M.H., G.C., D.P. did manuscript preparation, supervision, fund acquisition; E.G. contributed to methodology and analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Durcan, C., Hossain, M., Chagnon, G. et al. Mechanical experimentation of the gastrointestinal tract: a systematic review. Biomech Model Mechanobiol 23, 23–59 (2024). https://doi.org/10.1007/s10237-023-01773-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-023-01773-8