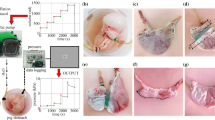
Abstract
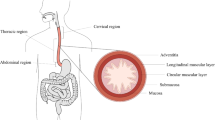
Bariatric surgery is performed on obese people aiming at reducing the capacity of the stomach and/or the absorbing capability of the gastrointestinal tract. A more reliable and effective approach to bariatric surgery may integrate different expertise, in the areas of surgery, physiology and biomechanics, availing of a strong cooperation between clinicians and engineers. This work aimed at developing a computational model of the stomach, as a computational tool for the physio-mechanical investigation of stomach functionality and the planning of bariatric procedures. In this sense, coupled experimental and numerical activities were developed. Experimental investigations on pig and piglet stomachs aimed at providing information about stomach geometrical configuration and structural behavior. The computational model was defined starting from the analysis of data from histo-morphometric investigations and mechanical tests. A fiber-reinforced visco-hyperelastic constitutive model was developed to interpret the mechanical response of stomach tissues; constitutive parameters were identified considering mechanical tests at both tissue and structure levels. Computational analyses were performed to investigate the pressure–volume behavior of the stomach. The developed model satisfactorily interpreted results from experimental activities, suggesting its reliability. Furthermore, the model was exploited to investigate stress and strain fields within gastric tissues, as the stimuli for mechanoreceptors that interact with the central nervous system leading to the feeling of satiety. In this respect, the developed computational model may be employed to evaluate the influence of bariatric intervention on the stimulation of mechanoreceptors, and the following meal induced satiety.







Similar content being viewed by others
References
Anderson, A. E., C. L. Peters, B. D. Tuttle, and J. A. Weiss. Subject-specific finite element model of the pelvis: development, validation and sensitivity studies. J. Biomech. Eng. 127:364–373, 2005.
Aydin, R. C., S. Brandstaeter, F. A. Braeu, M. Steigenberger, R. P. Marcus, K. Nikolaou, M. Notohamiprodjo, and C. J. Cyron. Experimental characterization of the biaxial mechanical properties of porcine gastric tissue. J. Mech. Behav. Biomed. Mater. 74:499–506, 2017.
Bellini, C., P. Glass, M. Sitti, and E. S. Di Martino. Biaxial mechanical modeling of the small intestine. J. Mech. Behav. Biomed. 4:1727–1740, 2011.
Berenson, G. S. Health Consequences of Obesity. Pediatr. Blood Cancer 58:117–121, 2012.
Burton, P. R., W. A. Brown, C. Laurie, M. Richards, G. Hebbard, and P. E. O’Brien. Effects of gastric band adjustments on intraluminal pressure. Obes. Surg. 19:1508–1514, 2009.
Carmagnola, S., P. Cantù, and R. Penagini. Mechanoreceptors of the Proximal Stomach and Perception of Gastric Distension. Am. J. Gastroenterol. 100:1704–1710, 2005.
Carniel, E. L., C. G. Fontanella, L. Polese, S. Merigliano, and A. N. Natali. Computational tools for the analysis of mechanical functionality of gastrointestinal structures. Technol. Health Care 21:271–283, 2013.
Carniel, E. L., C. G. Fontanella, C. Stefanini, and A. N. Natali. A procedure for the computational investigation of stress-relaxation phenomena. Mech. Time-Depend. Mater. 17:25–38, 2013.
Carniel, E. L., A. Frigo, C. G. Fontanella, G. M. De Benedictis, A. Rubini, L. Barp, G. Pluchino, B. Sabbadini, and L. Polese. A biomechanical approach to the analysis of methods and procedures of bariatric surgery. J. Biomech. 56:32–41, 2017.
Carniel, E. L., V. Gramigna, C. G. Fontanella, C. Stefanini, and A. N. Natali. Constitutive formulations for the mechanical investigation of colonic tissues. J. Biomed. Mater. Res. A 102:1243–1254, 2014.
Carniel, E. L., M. Mencattelli, G. Bonsignori, C. G. Fontanella, A. Frigo, A. Rubini, C. Stefanini, and A. N. Natali. Analysis of the structural behaviour of colonic segments by inflation tests: experimental activity and physio-mechanical model. Proc. Inst. Mech. Eng. H 229:794–803, 2015.
Carniel, E. L., A. Rubini, A. Frigo, and A. N. Natali. Analysis of the biomechanical behaviour of gastrointestinal regions adopting an experimental and computational approach. Comput. Methods Programs Biomed. 113:338–345, 2014.
Chang, S. H., C. R. Stoll, J. Song, J. E. Varela, C. J. Eagon, and G. A. Colditz. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 149:275–287, 2014.
Ciarletta, P., P. Dario, F. Tendick, and S. Micera. Hyperelastic model of anisotropic fiber reinforcements within intestinal walls for applications in medical robotics. Int. J. Robot. Res. 28:1279–1288, 2009.
Colville, T. P., and J. M. Bassert. Clinical Anatomy and Physiology for Veterinary Technicians (3rd ed.). St. Louis: Elsevier, 2015.
Dario, P., P. Ciarletta, A. Menciassi, and B. Kim. Modelling and experimental validation of the locomotion of endoscopic robots in the colon. Int. J. Robot. Res. 23:549–556, 2004.
Donahue, T. L., M. L. Hull, M. M. Rashid, and C. R. Jacobs. A finite element model of the human knee joint for the study of tibio-femoral contact. J. Biomech. Eng. 124:273–280, 2002.
Egorov, V. I., I. V. Schastlivtsev, E. V. Prut, A. O. Baranov, and R. A. Turusov. Mechanical properties of the human gastrointestinal tract. J. Biomech. 35:1417–1425, 2002.
Fallah, A., M. T. Ahmadian, K. Firozbakhsh, and M. M. Aghdam. Micromechanical modeling of rate-dependent behavior of connective tissues. J. Theor. Biol. 416:119–128, 2017.
Ferrua, M. J., and R. P. Singh. Modeling the fluid dynamics in a human stomach to gain insight of food digestion. J. Food Sci. 75:R151–R162, 2010.
Fung, Y. C. Biomechanics. New York: Springer, 1993.
Furness, J. B., B. P. Callaghan, L. R. Rivera, and H. J. Cho. The enteric nervous system and gastrointestinal innervation: integrated local and central control. In: Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease. Advances in Experimental Medicine and Biology, Vol. 817, edited by M. Lyte, and J. F. Cryan. New York: Springer, 2014.
Gao, F., D. Liao, J. Zhao, A. M. Drewes, and H. Gregersen. Numerical analysis of pouch filling and emptying after laparoscopic gastric banding surgery. Obes. Surg. 18:243–250, 2008.
Gloy, V. L., M. Briel, D. L. Bhatt, S. R. Kashyap, P. R. Schauer, G. Mingrone, H. C. Bucher, and A. J. Nordmann. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 347:f5934, 2013.
Gravetter, F. J., and L. B. Wallnau. Statistic for the Behavioral Sciences (8th ed.). Belmont, CA: Wadsworth/Cenage Learning, 2009.
Gregersen, H., J. L. Emery, and A. D. McCulloch. History-dependent mechanical behavior of guinea-pig small intestine. Ann. Biomed. Eng. 26:850–858, 1998.
Holzapfel, G. A. Nonlinear Solid Mechanics: A Continuum Approach for Engineering. Chichester: Wiley, 2000.
Janssen, P., S. Verschueren, H. G. Ly, R. Vos, L. Van Oudenhove, and J. Tack. Intragastric pressure during food intake: a physiological and minimally invasive method to assess gastric accommodation. Neurogastroenterol. Motil. 23:316–322, 2011.
Jia, Z. G., W. Li, and Z. R. Zhou. Mechanical characterization of stomach tissue under uniaxial tensile action. J. Biomech. 48:651–658, 2015.
Kampe, J., A. Stefanidis, S. H. Lockie, W. A. Brown, J. B. Dixon, A. Odoi, S. J. Spencer, J. Raven, and B. J. Oldfield. Neural and humoral changes associated with the adjustable gastric band: insights from a rodent model. Int. J. Obes. (London) 36:1403–1411, 2012.
Kelly, K. A. Gastric emptying of liquids and solids: roles of proximal and distal stomach. Am. J. Physiol. 239:G71–G76, 1980.
Kitahara, C. M., A. J. Flint, A. Berrington-de-Gonzalez, L. Bernstein, M. Brotzman, R. J. MacInnis, S. C. Moore, K. Robien, P. S. Rosenberg, P. N. Singh, E. Weiderpass, H. O. Adami, H. Anton-Culver, R. Ballard-Barbash, J. E. Buring, D. M. Freedman, G. E. Fraser, L. E. Beane-Freeman, S. M. Gapstur, J. M. Gaziano, G. G. Giles, N. Håkansson, J. A. Hoppin, F. B. Hu, K. Koenig, M. S. Linet, Y. Park, A. V. Patel, M. P. Purdue, C. Schairer, H. D. Sesso, K. Visvanathan, E. White, A. Wolk, A. Zeleniuch-Jacquotte, and P. Hartge. Association between class III obesity (BMI of 40–59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med. 11:1001673, 2014.
Lehnert, T., D. Sonntag, A. Konnopka, S. Riedel-Heller, and H. H. König. Economic costs of overweight and obesity. Best Pract. Res. Clin. Endocrinol. Metab. 27:105–115, 2013.
Marieb, E. N., and K. Hoehn. Human Anatomy and Physiology (7th ed.). San Francisco: Pearson International Edition, 2007.
Miftahof, R. N. Biomechanics of the Human Stomach. Cham: Springer, 2017.
Mingrone, G., S. Panunzi, A. De Gaetano, C. Guidone, A. Iaconelli, G. Nanni, M. Castagneto, S. Bornstein, and F. Rubino. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 386:964–973, 2015.
Natali, A. N., E. L. Carniel, C. G. Fontanella, A. Frigo, S. Todros, A. Rubini, G. M. De Benedictis, M. A. Cerruto, and W. Artibani. Mechanics of the urethral duct: tissue constitutive formulation and structural modeling for the investigation of lumen occlusion. Biomech. Model. Mechanobiol. 16:439–447, 2017.
Natali, A. N., E. L. Carniel, A. Frigo, C. G. Fontanella, A. Rubini, Y. Avital, and G. M. De Benedictis. Experimental investigation of the structural behavior of equine urethra. Comput. Methods Programs Biomed. 141:35–41, 2017.
Natali, A. N., E. L. Carniel, A. Frigo, P. G. Pavan, S. Todros, P. Pachera, C. G. Fontanella, A. Rubini, L. Cavicchioli, Y. Avital, and G. M. De Benedictis. Experimental investigation of the biomechanics of urethral tissues and structures. Exp. Physiol. 101:641–656, 2016.
Natali, A. N., C. G. Fontanella, and E. L. Carniel. Constitutive formulation and numerical analysis of the heel pad region. Comput. Methods Biomech. Biomed. Eng. 15:401–409, 2012.
Palanca, M., G. Tozzi, and L. Cristofolini. The use of digital image correlation in the biomechanical area: a review. Int. Biomech. 3:1–21, 2016.
Phillips, R. J., and T. L. Powley. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res. Brain Res. Rev. 34:1–26, 2000.
Pories, W. J. Bariatric surgery: risks and rewards. J. Clin. Endocrinol. Metab. 93:S89–S96, 2008.
Powley, T. L., C. N. Hudson, J. L. McAdams, E. A. Baronowsky, F. N. Martin, J. K. Mason, and R. J. Phillips. Organization of vagal afferents in pylorus: mechanoreceptors arrayed for high sensitivity and fine spatial resolution? Auton. Neurosci. 183:36–48, 2014.
Rolls, B. J., V. H. Castellanos, J. C. Halford, A. Kilara, D. Panyam, C. L. Pelkman, G. P. Smith, and M. L. Thorwart. Volume of food consumed affects satiety in men. Am. J. Clin. Nutr. 67:1170–1177, 1998.
Schauer, P. R., S. R. Kashyap, K. Wolski, S. A. Brethauer, J. P. Kirwan, C. E. Pothier, S. Thomas, B. Abood, S. E. Nissen, and D. L. Bhatt. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N. Engl. J. Med. 366:1567–1576, 2012.
Screen, H. R., S. Toorani, and J. C. Shelton. Microstructural stress relaxation mechanics in functionally different tendons. Med. Eng. Phys. 35:96–102, 2013.
Simo, J. C., and T. J. R. Hughes. Computational Inelasticity. New York: Springer, 1998.
Sunyer, F. X. P. Health implications of obesity. Am. J. Clin. Nutr. 53:1595S–1603S, 1991.
Wang, F. B., and T. L. Powley. Topographic inventories of vagal afferents in gastrointestinal muscle. J. Comp. Neurol. 421:302–324, 2000.
Wang, G. J., D. Tomasi, W. Backus, R. Wang, F. Telang, A. Geliebter, J. Korner, A. Bauman, J. S. Fowler, K. Panayotis, P. K. Thanos, and N. D. Volkow. Gastric distention activates satiety circuitry in the human brain. NeuroImage 39:1824–1831, 2008.
Weiss, J. A., B. N. Makerc, and S. Govindjeed. Finite element implementation of incompressible, transversely isotropic hyperelasticity. Comput. Methods. Appl. Mech. Eng. 135:107–128, 1996.
Woods, S. C. Gastrointestinal satiety signals I. An overview of gastrointestinal signals that influence food intake. Am. J. Physiol. Gastrointest. Liver Physiol. 286:7–13, 2004.
Yang, W., T. C. Fung, K. S. Chian, and C. K. Chong. Viscoelasticity of esophageal tissue and application of a QLV model. J. Biomech. Eng. 128:909–916, 2006.
Zagorodnyuk, V. P., B. N. Chen, and S. J. H. Brookes. Intraganglionic laminar endings are mechanotransduction sites of vagal tension receptors in the guinea-pig stomach. J. Physiol. 534:255–268, 2001.
Zhao, J., D. Liao, P. Chen, P. Kunwald, and H. Gregersen. Stomach stress and strain depend on location, direction and the layered structure. J. Biomech. 41:3441–3447, 2008.
Zienkiewicz, O. C., and R. L. Taylor. The Finite Element Method (5th ed.). Oxford: Butterworth Heinemann, 2000.
Acknowledgments
The authors warrant that the article is the authors’ original work, hasn’t received prior publication and isn’t under consideration for publication elsewhere. No specifying funding was received to support the reported research activities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix A
Appendix A
Constitutive parameters were identified by the inverse analysis of experimental activities. Aiming at investigating the complex distribution of stomach tissues, experimentations performed by Zhao et al.56 were analysed. Tensile tests were developed on tissue specimens harvested from pig stomachs, considering the influence of location, as fundus, corpus and antrum, wall layer, as connective layer and muscular layers, and direction, as circumferential and longitudinal ones. Specimens loading occurred at low strain rate, leading to almost equilibrium response. Mathematical procedures led to the following model formulation that allowed interpreting results from tensile tests:
where \(\gamma^{\infty } = 1 - \sum\limits_{i = 1}^{n} {\gamma^{i} }\) is a parameter that specifies the equilibrium relative stiffness of the tissue, while parameters \(C_{r}^{\infty } = \gamma^{\infty } C_{r}\) (r = 1,4,6) specify equilibrium initial stiffness terms. The specific formulation of the deformation gradient F was derived by considering the orientation of the specific tensile test along longitudinal or circumferential direction, the boundary conditions, as null values of normal stress components along lateral directions, and the incompressibility constraint.10
The comparison between model results and experimental data was performed by a cost function, which specified a measure of relative error, rather than an absolute one to provide a better approximation for both low and high result values:
where ω is the set of constitutive parameters, u the number of experimental data, \(\lambda_{j}^{\exp }\) the jth experimentally imposed input data (in terms of strain), \(P_{j}^{\exp }\) the jth experimentally measured output data (in terms of stress), \(P_{j}^{\bmod }\) the model output data evaluated by assuming constitutive parameters ω and input condition \(\lambda_{j}^{\exp }\). With regard to each stomach region and each wall layer, the cost function was evaluated considering results from tensile tests developed along both longitudinal and circumferential directions. Optimization techniques were adopted to minimize the cost function,7 leading to the constitutive parameters \(C_{r}^{\infty } ,\alpha_{r} \;(r = 1, 4, 6)\) for connective layer and muscular layers from fundus, corpus and antrum. Data from tensile tests along circumferential and longitudinal directions only were at disposal. Aiming at the almost univocal identification of constitutive parameters,7 all the gastric tissues were assumed to have the same mechanical contribution from the isotropic ground matrix. It follows that the same values of C1 and α1 parameters were assumed for connective layer and muscular layers from fundus, corpus and antrum.
Viscous parameters τi and γi were identified by analyzing relaxation data from structural tests developed on piglet stomachs. The following model formulation was assumed to interpret exponential decay of the normalized pressure Pnorm with time8,39:
Again, parameters identification was performed by minimizing the discrepancy between experimental and model results. Two viscous branches were assumed to contemporarily minimize the number of parameters and correctly interpret the trend of experimental data.12 The same viscous parameters were assumed for all the stomach tissues. Subsequently, the instantaneous initial stiffness terms \(C_{r} = C_{r}^{\infty } /\gamma^{\infty } (r = 1,4,6)\) were calculated.
The developed computational model of the stomach was exploited to evaluate and enhance its capability in interpreting the stomach mechanical behavior. Inflation tests were analyzed to compare computational results and data from experimental tests. Directly assuming the identified constitutive parameters led to imprecise results. In detail, the pressure–volume curves from computational and experimental analyses showed similar stiffness values (as the curve slope in the quasi-linear region), but at different inflation conditions. The situation is typical in the field of soft tissue mechanics, because of unsuitable post-processing of data from mechanical tests at tissue level. In detail, the tensile response of soft biological tissues shows an initial toe region and a subsequent quasi-linear tract. Experimental results are usually post-processed by low-pass filtering procedures, aiming to remove force data that are below the load cell sensitivity. The operation fundamentally concerns data from the toe region, leading to move the zero stress condition of the specimen to an actually strained specimen length. It follows a reduction of the strain amplitude of the toe region and the subsequent shift of the high stiffness quasi-linear region to improper low strain conditions.
Aiming to actually interpret the mechanical response of the stomach, constitutive parameters have been updated. Different sets of parameters were evaluated by defining a grid around the basic set, according to a variational process by using multipliers mC and mα of groups of parameters, as initial stiffness Cr and non linearity αr parameters (r = 1,4,6), respectively. Each set of parameters was evaluated assuming the same multipliers for tissues from the different stomach regions and the different layers. With regard to each set, tensile loading conditions were simulated aiming to evaluate tissues tensile stiffness values in the quasi-linear region. Among all the sets of parameters, a sub-domain of multipliers mC and mα was identified, which provided stiffness values in the quasi-linear regions matching the experimental quasi-linear tracts. On the other side, the sets of the sub-domain provided different strain amplitudes of the toe regions. Computational analyses of stomach inflation were performed considering sets of parameters from the sub-domain. The final set of parameters (Table 1) was identified by comparing computational results with experimental data from inflation tests (Fig. 4c).
Rights and permissions
About this article
Cite this article
Fontanella, C.G., Salmaso, C., Toniolo, I. et al. Computational Models for the Mechanical Investigation of Stomach Tissues and Structure. Ann Biomed Eng 47, 1237–1249 (2019). https://doi.org/10.1007/s10439-019-02229-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02229-w