Abstract
Background
Esophageal biomechanical studies are important to understand structural changes resulting from stretches during repair of esophageal atresias as well as to obtain values to compare with the biomechanics of tissue-engineered esophagus in the future. This study aimed to investigate light microscopic changes after uniaxial stretching of the ovine esophagus.
Methods
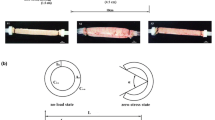
In vitro uniaxial stretching was performed on esophagi (n = 20) of 1-month-old lambs within 4–6 h post-mortem. Esophagi were divided into 5 groups: control and stretched (1.1, 1.2, 1.3 and 1.4). Force and lengthening were measured with 5 cycles performed on every specimen using a PBS organ bath at 37 °C. Histological studies were performed on the 5 groups.
Results
Low forces of ~ 2 N (N) were sufficient for a 1.2–1.25 stretch in the 1st cycle, whereas a three times higher force (~ 6 N) was needed for a stretch of 1.3. In the 2nd to 5th cycle, the tissue weakened and a force of ~ 3 N was sufficient for a stretch of 1.3. Histologically, in the 1.3–1.4 stretch groups, rupture of muscle fibers and capillaries were observed, respectively. Changes in mucosa and collagen fibers could not be observed.
Conclusions
These results offer norm values from the native esophagus to compare with the biomechanics of future tissue-engineered esophagus. Esophageal stretching > 1.3 leads to tears in muscle fibers and to rupture of capillaries. These findings can explain the decrease in microcirculation and scarring in mobilized tissue and possibly offer clues to impaired motility in esophagus atresias repaired under excessive tension.






Similar content being viewed by others
References
Clark DC. Esophageal atresia and tracheoesophageal fistula. Am Fam Phycisian. 1999;59:910–6.
Haight C, Towsley H. Congenital atresia of the esophagus with tracheoesophageal fistula: extrapleural ligation of fistula and end-to-end anastomosis of esophageal segments. Surg Gynecol Obstet. 1943;76:672–88.
Spitz L. Oesophageal atresia. Orphanet J Rare Dis. 2007;2:24.
Biller JA, Allen JL, Schuster SR, Treves ST, Winter HS. Long-term evaluation of esophageal and pulmonary function in patients with repaired esophageal atresia and tracheoesophageal fistula. Dig Dis Sci. 1987;32:985–90.
Dutta HK, Rajani M, Bhatnagar V. Cineradiographic evaluation of postoperative patients with esophageal atresia and tracheoesophageal fistula. Pediatr Surg Int. 2000;16:322–5.
Lemoine C, Aspirot A, Le Henaff G, Piloquet H, Lévesque D, Faure C. Characterization of esophageal motility following esophageal atresia repair using high-resolution esophageal manometry. J Pediatr Gastroenterol Nutr. 2013;56:609–14.
Rintala RJ, Pakarinen MP. Long-term outcome of esophageal anastomosis. Eur J Pediatr Surg. 2013;23:219–25.
Spitz L, Ruangtrakool R. Esophageal substitution. Semin Pediatr Surg. 1998;7:130–3.
Cusick EL, Batchelor AA, Spicer RD. Development of a technique for jejunal interposition in long-gap esophageal atresia. J Pediatr Surg. 1993;28:990–4.
Raffensperger JG, Kuck SR, Reynolds M, et al. Intestinal bypass of the esophagus. J Pediatr Surg. 1996;31:38–46.
Spitz L. Esophageal atresia- lessons I have learned in a 40-year experience. J Pediatr Surg. 2006;41:1635–40.
Arul GS, Parikh D. Oesophageal replacement in children. Ann R Coll Surg Engl. 2008;90:7–12.
Atug O, Dobrucali A, Orlando RC. Critical pH level of lye (NaOH) for esophageal injury. Dig Dis Sci. 2009;54:980–7.
Bronstein AC, Spyker DA, Cantilena LR Jr, et al. 2008 Annual report of the American association of poison control centers’ national poison data system (NPDS): 26th annual report. Clin Toxicol (Phila). 2009;47:911–1084.
Saxena AK, Ainoedhofer H, Höllwarth ME. Culture of ovine epithelial cells and in vitro esophagus tissue engineering. Tissue Eng Part C Methods. 2010;16:109–14.
Kofler K, Ainoedhofer H, Tausendschön J, Höllwarth ME, Saxena AK. Esophageal smooth muscle cells dedifferentiate with loss of a-smooth muscle actin expression after 8 weeks of explant expansion in vitro culture: Implications on esophagus tissue engineering. Eur Surg. 2011;43:168–73.
Kofler K, Ainoedhofer H, Höllwarth ME, Saxena AK. Fluorescence-activated cell sorting of PCK-26 antigen-positive cells enables selection of ovine esophageal epithelial cells with improved viability on scaffolds for esophagus tissue engineering. Pediatr Surg Int. 2010;26:97–104.
Malvasio V, Ainoedhofer H, Ackbar R, Höllwarth ME, Saxena AK. Effects of sodium hydroxide exposure on esophageal epithelial cells in an in vitro ovine model: implications for esophagus tissue engineering. J Pediatr Surg. 2012;47:874–80.
Saxena AK, Baumgart H, Komann C, Ainoedhofer H, Soltysiak P, Kofler K, Höllwarth ME. Esophagus tissue engineering: in situ generation of rudimentary tubular vascularized esophageal conduit using the ovine model. J Pediatr Surg. 2010;45:859–64.
Holzapfel GA, Sommer G, Regitnig P. Anisotropic mechanical properties of tissue components in human atherosclerotic plaques. J Biomech Eng. 2004;126:657–65.
Sommer G, Gasser TC, Regitnig P, Auer M, Holzapfel GA. Dissection properties of the human aortic media: an experimental study. J Biomech Eng. 2008;130:021007.
Mullins L. Effect of stretching on the properties of rubber. J Rubber Res. 1947;16:275–89.
Saxena AK. Congenital anomalies of soft tissues: birth defects depending on tissue engineering solutions and present advances in regenerative medicine. Tissue Eng Part B Rev. 2010;16:455–66.
Saxena AK. Tissue engineering and regenerative medicine research perspectives for pediatric surgery. Pediatr Surg Int. 2010;26:557–73.
Aspirot A, Faure C. Esophageal dysmotility: characterization and pathophysiology. Dis Esophagus. 2013;26:405–59.
Shono T, Suita S, Arima T, et al. Motility function of the esophagus before primary anastomosis in esophageal atresia. J Pediatr Surg. 1993;28:673–6.
Soltysiak P, Höllwarth ME, Saxena AK. Comparisson of suture techniques in the formation of collagen scaffold tubes for composite tubular organ tissue engineering. Biomed Mater Eng. 2010;20:1–11.
Macheiner T, Ackbar R, Saxena AK. Isolation, identification and culture of myenteric plexus cells from ovine esophagus. Esophagus. 2013;10:144–8.
Ackbar R, Malvasio V, Holzer P, Saxena AK. In vitro effect of bethanechol and suberyldicholine on regions of guinea pig esophagus. J Surg Res. 2012;174:56–61.
Sommer G, Schriefl A, Zeindlinger G, Katzensteiner A, Ainödhofer H, Saxena AK, Holzapfel GA. Multiaxial mechanical response and constitutive modeling of esophageal tissues: impact on esophageal tissue engineering. Acta Biomater. 2013;9:9379–91.
Acknowledgements
This research was funded by an European Union Grant within the 6th Framework Program (EuroSTEC; LSHC-CT-2006-037409). The authors would like to thank Mrs. Anna Kuess from the Unit of Experimental Fetal Surgery and Tissue Engineering, Medical University of Graz, Austria for her valuable support, and Mr. Georg Zeindlinger from the Institute of Biomechanics, Graz University of Technology, Austria for his support on the experimental tests.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statement
All experiments were performed according to the local and national research guidelines approved for the EuroSTEC project.
Conflict of interest
All authors declare no conflict of interest.
Informed consent
For this type of study, no formal consent is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saxena, A.K., Biro, E., Sommer, G. et al. Esophagus stretch tests: Biomechanics for tissue engineering and possible implications on the outcome of esophageal atresia repairs performed under excessive tension. Esophagus 18, 346–352 (2021). https://doi.org/10.1007/s10388-020-00769-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-020-00769-y