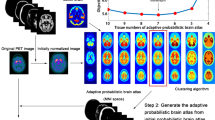
Abstract
Quantification approaches of positron emission tomography (PET) imaging provide user-independent evaluation of pathophysiological processes in living brains, which have been strongly recommended in clinical diagnosis of neurological disorders. Most PET quantification approaches depend on spatial normalization of PET images to brain template; however, the spatial normalization and quantification approaches have not been comprehensively reviewed. In this review, we introduced and compared PET template-based and magnetic resonance imaging (MRI)-aided spatial normalization approaches. Tracer-specific and age-specific PET brain templates were surveyed between 1999 and 2021 for 18F-FDG, 11C-PIB, 18F-Florbetapir, 18F-THK5317, and etc., as well as adaptive PET template methods. Spatial normalization-based PET quantification approaches were reviewed, including region-of-interest (ROI)-based and voxel-wise quantitative methods. Spatial normalization-based ROI segmentation approaches were introduced, including manual delineation on template, atlas-based segmentation, and multi-atlas approach. Voxel-wise quantification approaches were reviewed, including voxel-wise statistics and principal component analysis. Certain concerns and representative examples of clinical applications were provided for both ROI-based and voxel-wise quantification approaches. At last, a recipe for PET spatial normalization and quantification approaches was concluded to improve diagnosis accuracy of neurological disorders in clinical practice.






Similar content being viewed by others
References
Tian M, He X, Jin C, He X, Wu S, Zhou R, et al. Transpathology : molecular imaging-based pathology. Eur J Nucl Med Mol Imaging. 2021;48:2338–50. https://doi.org/10.1007/s00259-021-05234-1.
Kreisl WC, Kim MJ, Coughlin JM, Henter ID, Owen DR, Innis RB. PET imaging of neuroinflammation in neurological disorders. Lancet Neurol. 2020;19:940–50. https://doi.org/10.1016/S1474-4422(20)30346-X.
Van Bogaert P, Massager N, Tugendhaft P, Wikler D, Damhaut P, Levivier M, et al. Statistical parametric mapping of regional glucose metabolism in mesial temporal lobe epilepsy. Neuroimage. 2000;12:129–38. https://doi.org/10.1006/nimg.2000.0606.
Archambaud F, Bouilleret V, Hertz-Pannier L, Chaumet-Riffaud P, Rodrigo S, Dulac O, et al. Optimizing statistical parametric mapping analysis of 18F-FDG PET in children. EJNMMI Res. 2013;3:1–10. https://doi.org/10.1186/2191-219X-3-2.
Morbelli S, Garibotto V, Van De Giessen E, Arbizu J, Chételat G, Drezgza A, et al. A Cochrane review on brain [18F]FDG PET in dementia: limitations and future perspectives. Eur J Nucl Med Mol Imaging. 2015;42:1487–91. https://doi.org/10.1007/s00259-015-3098-2.
Waxman AD, Herholz K, Lewis DH, Herscovitch P, Minoshima S, Ichise M, et al. Society of Nuclear Medicine Procedure Guideline for FDG PET Brain Imaging. 2009;1–12.
Chandra PS, Vaghania G, Bal CS, Tripathi M, Kuruwale N, Arora A, et al. Role of concordance between ictal-subtracted SPECT and PET in predicting long-term outcomes after epilepsy surgery. Epilepsy Res. 2014;108:1782–9. https://doi.org/10.1016/j.eplepsyres.2014.09.024.
Brodbeck V, Spinelli L, Lascano AM, Wissmeier M, Vargas MI, Vulliemoz S, et al. Electroencephalographic source imaging: a prospective study of 152 operated epileptic patients. Brain. 2011;134:2887–97. https://doi.org/10.1093/brain/awr243.
Van’t Klooster MA, Huiskamp G, Zijlmans M, Debets RMC, Comans EFI, Bouvard S, et al. Can we increase the yield of FDG-PET in the preoperative work-up for epilepsy surgery? Epilepsy Res. 2014;108:1095–105. https://doi.org/10.1016/j.eplepsyres.2014.04.011.
Mayoral M, Marti-Fuster B, Carreño M, Carrasco JL, Bargalló N, Donaire A, et al. Seizure-onset zone localization by statistical parametric mapping in visually normal 18F-FDG PET studies. Epilepsia. 2016;57:1236–44. https://doi.org/10.1111/epi.13427.
Zhang T, Li Y, Zhao S, Xu Y, Zhang X, Wu S, et al. High-resolution pediatric age–specific 18F-FDG PET template: a pilot study in epileptogenic focus localization. Eur J Nucl Med Mol Imaging. 2022;49:1560–73. https://doi.org/10.1007/s00259-021-05611-w.
Smailagic N, Vacante M, Hyde C, Martin S, Ukoumunne O, Sachpekidis C. 18F-FDG PET for the early diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2015;103–11. https://doi.org/10.1002/14651858.CD010632.pub2.www.cochranelibrary.com.
Presotto L, Ballarini T, Caminiti SP, Bettinardi V, Gianolli L, Perani D. Validation of 18F–FDG-PET single-subject optimized SPM procedure with different PET scanners. Neuroinformatics. 2017;15:151–63. https://doi.org/10.1007/s12021-016-9322-9.
Perani D, Cerami C, Caminiti SP, Santangelo R, Coppi E, Ferrari L, et al. Cross-validation of biomarkers for the early differential diagnosis and prognosis of dementia in a clinical setting. Eur J Nucl Med Mol Imaging. 2016;43:499–508. https://doi.org/10.1007/s00259-015-3170-y.
Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54:313–27. https://doi.org/10.1016/j.neuroimage.2010.07.033.
Zhao T, Liao X, Fonov VS, Wang Q, Men W, Wang Y, et al. Unbiased age-specific structural brain atlases for Chinese pediatric population. Neuroimage. 2019;189:55–70. https://doi.org/10.1016/j.neuroimage.2019.01.006.
Gispert JD, Pascau J, Reig S, Martínez-Lázaro R, Molina V, García-Barreno P, et al. Influence of the normalization template on the outcome of statistical parametric mapping of PET scans. Neuroimage. 2003;19:601–12. https://doi.org/10.1016/S1053-8119(03)00072-7.
Della Rosa PA, Cerami C, Gallivanone F, Prestia A, Caroli A, Castiglioni I, et al. A standardized 18F-FDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics. 2014;12:575–93. https://doi.org/10.1007/s12021-014-9235-4.
Ishii K, Willoch F, Minoshima S, Drzezga A, Ficaro EP, Cross DJ, et al. Statistical brain mapping of 18F-FDG PET in Alzheimer’s disease: validation of anatomic standardization for atrophied brains. J Nucl Med. 2001;42:548–57.
Steele JD, Lawrie SM. Neuroimaging. Companion Psychiatr Stud. 2010; 77–94. https://doi.org/10.1016/B978-0-7020-3137-3.00004-8.
Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56. https://doi.org/10.1016/S1361-8415(01)00036-6.
Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–21. https://doi.org/10.1109/42.796284.
Friston KJ, Ashburner J, Frith CD, Poline J -B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995;3:165–89. https://doi.org/10.1002/hbm.460030303.
Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12:26–41. https://doi.org/10.1016/j.media.2007.06.004.
Meyer JH, Gunn RN, Myers R, Grasby PM. Assessment of spatial normalization of PET ligand images using ligand- specific templates. Neuroimage. 1999;9:545–53. https://doi.org/10.1006/nimg.1999.0431.
Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL Neuroimage. 2012;62:782–90. https://doi.org/10.1016/j.neuroimage.2011.09.015.
Hammers A, Koepp MJ, Free SL, Brett M, Richardson MP, Labbé C, et al. Implementation and application of a brain template for multiple volumes of interest. Hum Brain Mapp. 2002;15:165–74. https://doi.org/10.1002/hbm.10016.
Svarer C, Madsen K, Hasselbalch SG, Pinborg LH, Haugbøl S, Frøkjær VG, et al. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage. 2005;24:969–79. https://doi.org/10.1016/j.neuroimage.2004.10.017.
Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res Neuroimaging. 2006;147:79–89. https://doi.org/10.1016/j.pscychresns.2006.01.011.
Rabinovici GD, Furst AJ, O’Neil JP, Racine CA, Mormino EC, Baker SL, et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007;68:1205–12. https://doi.org/10.1212/01.wnl.0000259035.98480.ed.
Morbelli S, Piccardo A, Villavecchia G, Dessi B, Brugnolo A, Piccini A, et al. Mapping brain morphological and functional conversion patterns in amnestic MCI: a voxel-based MRI and FDG-PET study. Eur J Nucl Med Mol Imaging. 2010;37:36–45. https://doi.org/10.1007/s00259-009-1218-6.
Ashburner J, Friston K. Multimodal image coregistration and partitioning - a unified framework. Neuroimage. 1997;6:209–17. https://doi.org/10.1006/nimg.1997.0290.
Kiebel SJ, Ashburner J, Poline JB, Friston KJ. MRI and PET coregistration - a cross validation of statistical parametric mapping and automated image registration. Neuroimage. 1997;5:271–9. https://doi.org/10.1006/nimg.1997.0265.
Edison P, Carter SF, Rinne JO, Gelosa G, Herholz K, Nordberg A, et al. Comparison of MRI based and PET template based approaches in the quantitative analysis of amyloid imaging with PIB-PET. Neuroimage. 2013;70:423–33. https://doi.org/10.1016/j.neuroimage.2012.12.014.
Nordberg A, Carter SF, Rinne J, Drzezga A, Brooks DJ, Vandenberghe R, et al. A European multicentre PET study of fibrillar amyloid in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2013;40:104–14. https://doi.org/10.1007/s00259-012-2237-2.
Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos Trans R Soc B Biol Sci. 2001;356:1293–322. https://doi.org/10.1098/rstb.2001.0915.
Fillmore PT, Phillips-Meek MC, Richards JE. Age-specific MRI brain and head templates for healthy adults from 20 through 89 years of age. Front Aging Neurosci. 2015;7:1–14. https://doi.org/10.3389/fnagi.2015.00044.
Richards JE, Sanchez C, Phillips-Meek M, Xie W. A database of age-appropriate average MRI templates. Neuroimage. 2016;124:1254–9. https://doi.org/10.1016/j.neuroimage.2015.04.055.
Ridwan AR, Niaz MR, Wu Y, Qi X, Zhang S, Kontzialis M, et al. Development and evaluation of a high performance T1-weighted brain template for use in studies on older adults. Hum Brain Mapp. 2021;42:1758–76. https://doi.org/10.1002/hbm.25327.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002. https://doi.org/10.1006/nimg.2001.0978.
Rolls ET, Huang CC, Lin CP, Feng J, Joliot M. Automated anatomical labelling atlas 3. Neuroimage. 2020;206: 116189. https://doi.org/10.1016/j.neuroimage.2019.116189.
Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016;26:3508–26. https://doi.org/10.1093/cercor/bhw157.
Evans AC, Janke AL, Collins DL, Baillet S. Brain templates and atlases. Neuroimage. 2012;62:911–22. https://doi.org/10.1016/j.neuroimage.2012.01.024.
Sun X, Liang S, Fu L, Zhang X, Feng T, Li P, et al. A human brain tau PET template in MNI space for the voxel-wise analysis of Alzheimer’s disease. J Neurosci Methods. 2019;328: 108438. https://doi.org/10.1016/j.jneumeth.2019.108438.
Akamatsu G, Ikari Y, Ohnishi A, Nishida H, Aita K, Sasaki M, et al. Automated PET-only quantification of amyloid deposition with adaptive template and empirically pre-defined ROI. Phys Med Biol. 2016;61:5768–80. https://doi.org/10.1088/0031-9155/61/15/5768.
Imabayashi E, Matsuda H, Tabira T, Arima K, Araki N, Ishii K, et al. Comparison between brain CT and MRI for voxel-based morphometry of Alzheimer’s disease. Brain Behav. 2013;3:487–93. https://doi.org/10.1002/brb3.146.
Saint-Aubert L, Nemmi F, Péran P, Barbeau EJ, Payoux P, Chollet F, et al. Comparison between PET template-based method and MRI-based method for cortical quantification of florbetapir (AV-45) uptake in vivo. Eur J Nucl Med Mol Imaging. 2014;41:836–43. https://doi.org/10.1007/s00259-013-2656-8.
Saint-Aubert L, Barbeau EJ, Péran P, Nemmi F, Vervueren C, Mirabel H, et al. Cortical florbetapir-PET amyloid load in prodromal Alzheimer’s disease patients. EJNMMI Res. 2013;3:1–22. https://doi.org/10.1186/2191-219X-3-43.
Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. https://doi.org/10.1016/j.neuroimage.2009.06.060.
Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. https://doi.org/10.1016/j.neuroimage.2008.12.037.
Hosaka K, Ishii K, Sakamoto S, Sadato N, Fukuda H, Kato T, et al. Validation of anatomical standardization of FDG PET images of normal brain: comparison of SPM and NEUROSTAT. Eur J Nucl Med Mol Imaging. 2005;32:92–7. https://doi.org/10.1007/s00259-004-1576-z.
Gispert JD, Pascau J, Reig S, Martinez R, Molina V, Desco M. Effect of the normalization template in statistical parametric mapping of PET scans. Proc - Int Symp Biomed Imaging. 2002;2002-Janua:851–4. https://doi.org/10.1109/ISBI.2002.1029393.
Li Y, Rinne JO, Mosconi L, Pirraglia E, Rusinek H, Desanti S, et al. Regional analysis of FDG and PIB-PET images in normal aging, mild cognitive impairment, and Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2008;35:2169–81. https://doi.org/10.1007/s00259-008-0833-y.
Hsiao IT, Huang CC, Hsieh CJ, Wey SP, Kung MP, Yen TC, et al. Perfusion-like template and standardized normalization-based brain image analysis using 18F-florbetapir (AV-45/Amyvid) PET. Eur J Nucl Med Mol Imaging. 2013;40:908–20. https://doi.org/10.1007/s00259-013-2350-x.
Landau SM, Breault C, Joshi AD, Pontecorvo M, Mathis CA, Jagust WJ, et al. Amyloid-β imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J Nucl Med. 2013;54:70–7. https://doi.org/10.2967/jnumed.112.109009.
Jeong E, Oh SY, Pahk K, Lee CN, Park KW, Lee JS, et al. Feasibility of PET template-based analysis on F-18 FP-CIT PET in patients with de novo Parkinson’s disease. Nucl Med Mol Imaging. 2010;2013(47):73–80. https://doi.org/10.1007/s13139-013-0196-6.
Kuhn FP, Warnock GI, Burger C, Ledermann K, Martin-Soelch C, Buck A. Comparison of PET template-based and MRI-based image processing in the quantitative analysis of C11-raclopride PET. EJNMMI Res. 2014;4:1–7. https://doi.org/10.1186/2191-219X-4-7.
Landau SM, Thomas BA, Thurfjell L, Schmidt M, Margolin R, Mintun M, et al. Amyloid PET imaging in Alzheimer’s disease: a comparison of three radiotracers. Eur J Nucl Med Mol Imaging. 2014;41:1398–407. https://doi.org/10.1007/s00259-014-2753-3.
Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid depositionin elderly subjects. Brain. 2008;132:1310–23. https://doi.org/10.1093/brain/awn320.
Frisoni GB, Lorenzi M, Caroli A, Kemppainen N, Någren K, Rinne JO. In vivo mapping of amyloid toxicity in Alzheimer disease. Neurology. 2009;72:1504–1501. https://doi.org/10.1212/WNL.0b013e3181a2e896.
Lundqvist R, Lilja J, Thomas BA, Lötjönen J, Villemagne VL, Rowe CC, et al. Implementation and validation of an adaptive template registration method for 18F-flutemetamol imaging data. J Nucl Med. 2013;54:1472–8. https://doi.org/10.2967/jnumed.112.115006.
Guimond A, Meunier J, Thirion JP. Average brain models: a convergence study. Comput Vis Image Underst. 2000;77:192–210. https://doi.org/10.1006/cviu.1999.0815.
Signorini M, Paulesu E, Friston K, Perani D, Colleluori A, Lucignani G, et al. Rapid assessment of regional cerebral metabolic abnormalities in single subjects with quantitative and nonquantitative [18F]FDG PET: a clinical validation of statistical parametric mapping. Neuroimage. 1999;9:63–80. https://doi.org/10.1006/nimg.1998.0381.
Aalto S, Scheinin NM, Kemppainen NM, Någren K, Kailajärvi M, Leinonen M, et al. Reproducibility of automated simplified voxel-based analysis of PET amyloid ligand [11C]PIB uptake using 30-min scanning data. Eur J Nucl Med Mol Imaging. 2009;36:1651–60. https://doi.org/10.1007/s00259-009-1174-1.
Van Der Gucht A, Verger A, Guedj E, Malandain G, Hossu G, Yagdigul Y, et al. Age-related changes in FDG brain uptake are more accurately assessed when applying an adaptive template to the SPM method of voxel-based quantitative analysis. Ann Nucl Med. 2015;29:921–8. https://doi.org/10.1007/s12149-015-1022-2.
Jung Lung H, Weng Y-H, Wen M-C, Hsiao I-T, Lin K-J. Quantitative study of 18F-(+)DTBZ image: comparison of PET template-based and MRI based image analysis. Sci Rep. 2018;8:16027. https://doi.org/10.1038/s41598-018-34388-6.
De Blasi B, Barnes A, Galazzo IB, Hua CH, Shulkin B, Koepp M, et al. Age-specific 18F-FDG image processing pipelines and analysis are essential for individual mapping of seizure foci in pediatric patients with intractable epilepsy. J Nucl Med. 2018;59:1590–6. https://doi.org/10.2967/jnumed.117.203950.
Wang H, Tian Y, Liu Y, Chen Z, Zhai H, Zhuang M. Population-specific brain [18F]-FDG PET templates of Chinese subjects for statistical parametric mapping. Sci Data. 2021;8:305. https://doi.org/10.1038/s41597-021-01089-1.
Liang P, Shi L, Chen N, Luo Y, Wang X, Liu K, et al. Construction of brain atlases based on a multi-center MRI dataset of 2020 Chinese adults. Sci Rep. 2016;5:18216. https://doi.org/10.1038/srep18216.
Jae SL, Dong SL, Kim J, Yu KK, Kang E, Kang H, et al. Development of Korean standard brain templates. J Korean Med Sci. 2005;20:483–8. https://doi.org/10.3346/jkms.2005.20.3.483.
Pai PP, Mandal PK, Punjabi K, Shukla D, Goel A, Joon S, et al. BRAHMA: population specific T1, T2, and FLAIR weighted brain templates and their impact in structural and functional imaging studies. Magn Reson Imaging. 2020;70:5–21. https://doi.org/10.1016/j.mri.2019.12.009.
Yoon U, Fonov VS, Perusse D, Evans AC. The effect of template choice on morphometric analysis of pediatric brain data. Neuroimage. 2009;45:769–77. https://doi.org/10.1016/J.NEUROIMAGE.2008.12.046.
Bourgeat P, Villemagne VL, Dore V, Brown B, Macaulay SL, Martins R, et al. Comparison of MR-less PiB SUVR quantification methods. Neurobiol Aging. 2015;36:S159–66. https://doi.org/10.1016/j.neurobiolaging.2014.04.033.
Zhu Y, Feng J, Wu S, Hou H, Ji J, Zhang K, et al. Glucose metabolic profile by visual assessment combined with statistical parametric mapping analysis in pediatric patients with epilepsy. J Nucl Med. 2017;58:1293–9. https://doi.org/10.2967/jnumed.116.187492.
Li Y, Feng J, Zhang T, Shi K, Ding Y, Zhang X, et al. Brain metabolic characteristics distinguishing typical and atypical benign epilepsy with centro-temporal spikes. Eur Radiol. 2021;8. https://doi.org/10.1007/s00330-021-08051-0.
Lin TW, Kung De Aburto MA, Dahlbom M, Huang LL, Marvi MM, Tang M, et al. Predicting seizure-free status for temporal lobe epilepsy patients undergoing surgery: prognostic value of quantifying maximal metabolic asymmetry extending over a specified proportion of the temporal lobe. J Nucl Med. 2007;48:776–82. https://doi.org/10.2967/jnumed.106.034249.
Kong XZ, Mathias SR, Guadalupe T, Abé C, Agartz I, Akudjedu TN, et al. Mapping cortical brain asymmetry in 17,141 healthy individuals worldwide via the ENIGMA consortium. Proc Natl Acad Sci U S A. 2018;115:E5154–63. https://doi.org/10.1073/pnas.1718418115.
Didelot A, Mauguière F, Redouté J, Bouvard S, Lothe A, Reilhac A, et al. Voxel-based analysis of asymmetry index maps increases the specificity of 18F-MPPF PET abnormalities for localizing the epileptogenic zone in temporal lobe epilepsies. J Nucl Med. 2010;51:1732–9. https://doi.org/10.2967/jnumed.109.070938.
Fripp J, Bourgeat P, Raniga P, Acosta O, Villemagne V, Jones G, et al. MR-less high dimensional spatial normalization of 11C PiB PET images on a population of elderly, mild cognitive impaired and alzheimer disease patients. Lect Notes Comput Sci (including Subser Lect Notes Artif Intell Lect Notes Bioinformatics). 2008;5241 LNCS:442–9. https://doi.org/10.1007/978-3-540-85988-8_53.
Chae SY, Kim HO, Oh M, Lee DY, Jin S, Oh SJ, et al. Evaluation of selective positron emission tomography template method for spatial normalization of amyloid imaging with 11 C-pittsburgh compound B. J Comput Assist Tomogr. 2014;38:924–9. https://doi.org/10.1097/RCT.0000000000000123.
Lilja J, Leuzy A, Chiotis K, Savitcheva I, Sörensen J, Nordberg A. Spatial normalization of [18F]flutemetamol PET images utilizing an adaptive principal components template. J Nucl Med. 2018;60:285–91. https://doi.org/10.2967/jnumed.118.207811.
Hu X, Sun X, Hu F, Liu F, Ruan W, Wu T, et al. Multivariate radiomics models based on 18F-FDG hybrid PET/MRI for distinguishing between Parkinson’s disease and multiple system atrophy. Eur J Nucl Med Mol Imaging. 2021;48:3469–81.
Tuszynski T, Rullmann M, Luthardt J, Butzke D, Tiepolt S, Gertz HJ, et al. Evaluation of software tools for automated identification of neuroanatomical structures in quantitative β-amyloid PET imaging to diagnose Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2016;43:1077–87. https://doi.org/10.1007/s00259-015-3300-6.
Sun FT, Schriber RA, Greenia JM, He J, Gitcho A, Jagust WJ. Automated template-based PET region of interest analyses in the aging brain. Neuroimage. 2007;34:608–17. https://doi.org/10.1016/j.neuroimage.2006.09.022.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. https://doi.org/10.1016/S1053-8119(03)00169-1.
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. https://doi.org/10.1016/j.neuroimage.2006.01.021.
Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53:1–15. https://doi.org/10.1016/j.neuroimage.2010.06.010.
Hammers A, Chen CH, Lemieux L, Allom R, Vossos S, Free SL, et al. Statistical neuroanatomy of the human inferior frontal gyrus and probabilistic atlas in a standard stereotaxic space. Hum Brain Mapp. 2007;28:34–48. https://doi.org/10.1002/hbm.20254.
Landau SM, Harvey D, Madison CM, Reiman EM, Trojanowski JQ. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–8.
Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–86. https://doi.org/10.1002/ana.23650.
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–62. https://doi.org/10.1038/nrclinonc.2017.141.
Mayerhoefer ME, Materka A, Langs G, Ida H, Szczypi P, Gibbs P, et al. Introduction to radiomics. J Nucl Med. 2020;61:488–95. https://doi.org/10.2967/jnumed.118.222893.
Jiang J, Wang M, Alberts I, Sun X, Li T, Rominger A, et al. Using radiomics-based modelling to predict individual progression from mild cognitive impairment to Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2022. https://doi.org/10.1007/s00259-022-05687-y.
Chen SD, Lu JY, Li HQ, Yang YX, Jiang JH, Cui M, et al. Staging tau pathology with tau PET in Alzheimer’s disease: a longitudinal study. Transl Psychiatry. 2021;11:1–12. https://doi.org/10.1038/s41398-021-01602-5.
Hanseeuw BJ, Betensky RA, Jacobs HIL, Schultz AP, Sepulcre J, Becker JA, et al. Association of amyloid and tau with cognition in preclinical Alzheimer disease a longitudinal study. JAMA Neurol. 2019;76:915–24. https://doi.org/10.1001/jamaneurol.2019.1424.
Bozoki AC, Korolev IO, Davis NC, Hoisington LA, Berger KL. Disruption of limbic white matter pathways in mild cognitive impairment and Alzheimer’s disease: a DTI/FDG-PET study. Hum Brain Mapp. 2012;33:1792–802. https://doi.org/10.1002/hbm.21320.
Marchitelli R, Aiello M, Cachia A, Quarantelli M, Cavaliere C, Postiglione A, et al. Simultaneous resting-state FDG-PET/fMRI in Alzheimer disease: relationship between glucose metabolism and intrinsic activity. Neuroimage. 2018;176:246–58. https://doi.org/10.1016/j.neuroimage.2018.04.048.
Chung SJ, Lee HS, Yoo HS, Lee YH, Lee PH, Sohn YH. Patterns of striatal dopamine depletion in early Parkinson disease. Neurology. 2020;95:e280–90. https://doi.org/10.1212/WNL.0000000000009878.
Shang S, Li D, Tian Y, Li R, Zhao H, Zheng L, et al. Hybrid PET-MRI for early detection of dopaminergic dysfunction and microstructral degradation involved in Parkinson’s disease. Commun Biol. 2021;4:1–9. https://doi.org/10.1038/s42003-021-02705-x.
Kamm J, Boles Ponto LL, Manzel K, Gaasedelen OJ, Nagahama Y, Abel T, et al. Temporal lobe asymmetry in FDG-PET uptake predicts neuropsychological and seizure outcomes after temporal lobectomy. Epilepsy Behav. 2018;78:62–7. https://doi.org/10.1016/j.yebeh.2017.10.006.
Zhang Q, Liao Y, Wang X, Zhang T, Feng J, Deng J, et al. A deep learning framework for 18F-FDG PET imaging diagnosis in pediatric patients with temporal lobe epilepsy. Eur J Nucl Med Mol Imaging. 2021;48:2476–85. https://doi.org/10.1007/s00259-020-05108-y.
Li Y, Zhang T, Feng J, Qian S, Wu S, Zhou R, et al. Processing speed dysfunction is associated with functional corticostriatal circuit alterations in childhood epilepsy with centrotemporal spikes: a PET and fMRI study. Eur J Nucl Med Mol Imaging. 2022. https://doi.org/10.1007/s00259-022-05740-w.
Perani D, Della Rosa PA, Cerami C, Gallivanone F, Fallanca F, Vanoli EG, et al. Validation of an optimized SPM procedure for FDG-PET in dementia diagnosis in a clinical setting. NeuroImage Clin. 2014;6:445–54. https://doi.org/10.1016/j.nicl.2014.10.009.
Caminiti SP, Sala A, Iaccarino L, Beretta L, Pilotto A, Gianolli L, et al. Brain glucose metabolism in Lewy body dementia: implications for diagnostic criteria. Alzheimer’s Res Ther. 2019;11:1–14. https://doi.org/10.1186/s13195-019-0473-4.
Caminiti SP, Ballarini T, Sala A, Cerami C, Presotto L, Santangelo R, et al. FDG-PET and CSF biomarker accuracy in prediction of conversion to different dementias in a large multicentre MCI cohort. NeuroImage Clin. 2018;18:167–77. https://doi.org/10.1016/j.nicl.2018.01.019.
Tian M, Civelek AC, Carrio I, Watanabe Y, Kang KW, Murakami K, et al. International consensus on the use of tau PET imaging agent 18F-flortaucipir in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2022;49:895–904. https://doi.org/10.1007/s00259-021-05673-w.
Pilotto A, Premi E, Caminiti SP, Presotto L, Turrone R, Alberici A, et al. Single-subject SPM FDG-PET patterns predict risk of dementia progression in Parkinson disease. Neurology. 2018;90:e1029–37. https://doi.org/10.1212/WNL.0000000000005161.
Zhao P, Zhang B, Gao S, Li X. Clinical features, MRI, and 18F-FDG-PET in differential diagnosis of Parkinson disease from multiple system atrophy. Brain Behav. 2020;10:1–9. https://doi.org/10.1002/brb3.1827.
Etminani K, Soliman A, Davidsson A, Chang JR, Martínez-Sanchis B, Byttner S, et al. A 3D deep learning model to predict the diagnosis of dementia with Lewy bodies, Alzheimer’s disease, and mild cognitive impairment using brain 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2021. https://doi.org/10.1007/s00259-021-05483-0.
Choi H, Kim YK, Yoon EJ, Lee JY, Lee DS. Cognitive signature of brain FDG PET based on deep learning: domain transfer from Alzheimer’s disease to Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2020;47:403–12. https://doi.org/10.1007/s00259-019-04538-7.
de Vries BM, Golla SSV, Ebenau J, Verfaillie SCJ, Timmers T, Heeman F, et al. Classification of negative and positive 18F-florbetapir brain PET studies in subjective cognitive decline patients using a convolutional neural network. Eur J Nucl Med Mol Imaging. 2021;48:721–8. https://doi.org/10.1007/s00259-020-05006-3.
Lee R, Choi H, Park KY, Kim JM, Seok JW. Prediction of post-stroke cognitive impairment using brain FDG PET: deep learning-based approach. Eur J Nucl Med Mol Imaging. 2021. https://doi.org/10.1007/s00259-021-05556-0.
Visvikis D, Cheze Le Rest C, Jaouen V, Hatt M. Artificial intelligence, machine (deep) learning and radio(geno)mics: definitions and nuclear medicine imaging applications. Eur J Nucl Med Mol Imaging. 2019;46:2630–7. https://doi.org/10.1007/s00259-019-04373-w.
Shen D, Wu G, Suk H-I. Deep learning in medical image analysis. Annu Rev Biomed Eng. 2017;19:221–48. https://doi.org/10.1146/annurev-bioeng-071516-044442.
Litjens G, Kooi T, Bejnordi BE, Setio AAA, Ciompi F, Ghafoorian M, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60–88. https://doi.org/10.1016/j.media.2017.07.005.
Alexander GE, Moeller JR. Application of the scaled subprofile model to functional imaging in neuropsychiatric disorders: a principal component approach to modeling brain function in disease. Hum Brain Mapp. 1994;2:79–94. https://doi.org/10.1002/hbm.460020108.
Spetsieris PG, Ma Y, Dhawan V, Eidelberg D. Differential diagnosis of parkinsonian syndromes using PCA-based functional imaging features. Neuroimage. 2009;45:1241–52. https://doi.org/10.1016/J.NEUROIMAGE.2008.12.063.
Feigin A, Tang C, Ma Y, Mattis P, Zgaljardic D, Guttman M, et al. Thalamic metabolism and symptom onset in preclinical Huntington’s disease. Brain. 2007;130:2858–67. https://doi.org/10.1093/brain/awm217.
Teune L, Strijkert F, Renken R, Izaks G, Vries J, Segbers M, et al. The Alzheimer’s Disease-related glucose metabolic brain pattern. Curr Alzheimer Res. 2014;11:725–32. https://doi.org/10.2174/156720501108140910114230.
Scarmeas N, Habeck CG, Zarahn E, Anderson KE, Park A, Hilton J, et al. Covariance PET patterns in early Alzheimer’s disease and subjects with cognitive impairment but no dementia: utility in group discrimination and correlations with functional performance. Neuroimage. 2004;23:35–45. https://doi.org/10.1016/j.neuroimage.2004.04.032.
Huang C, Tang C, Feigin A, Lesser M, Ma Y, Pourfar M, et al. Changes in network activity with the progression of Parkinson’s disease. Brain. 2007;130:1834–46. https://doi.org/10.1093/brain/awm086.
Caminiti SP, Sala A, Presotto L, Chincarini A, Sestini S, Perani D, et al. Validation of FDG-PET datasets of normal controls for the extraction of SPM-based brain metabolism maps. Eur J Nucl Med Mol Imaging. 2021;48:2486–99. https://doi.org/10.1007/s00259-020-05175-1.
Mosconi L, Wai HT, Pupi A, De Santi S, Drzezga A, Minoshima S, et al. 18F-FDG PET database of longitudinally confirmed healthy elderly individuals improves detection of mild cognitive impairment and Alzheimer’s disease. J Nucl Med. 2007;48:1129–34. https://doi.org/10.2967/jnumed.107.040675.
Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack C, Jagust W, et al. The Alzheimer’s disease neuroimaging initiative. Neuroimaging Clin. 2005;15:869–77. https://doi.org/10.1016/j.nic.2005.09.008.
Van Hecke W, Leemans A, De Backer S, Jeurissen B, Parizel PM, Sijbers J. Comparing isotropic and anisotropic smoothing for voxel-based DTI analyses: a simulation study. Hum Brain Mapp. 2010;31:98–114. https://doi.org/10.1002/hbm.20848.
Kumar A, Juhász C, Asano E, Sood S, Muzik O, Chugani HT. Objective detection of epileptic foci by 18F-FDG PET in children undergoing epilepsy surgery. J Nucl Med. 2010;51:1901–7. https://doi.org/10.2967/jnumed.110.075390.
Lyoo CH, Ikawa M, Liow JS, Zoghbi SS, Morse CL, Pike VW, et al. Cerebellum can serve as a pseudo-reference region in Alzheimer disease to detect neuroinflammation measured with PET radioligand binding to translocator protein. J Nucl Med. 2015;56:701–6. https://doi.org/10.2967/jnumed.114.146027.
Boscolo Galazzo I, Mattoli MV, Pizzini FB, De Vita E, Barnes A, Duncan JS, et al. Cerebral metabolism and perfusion in MR-negative individuals with refractory focal epilepsy assessed by simultaneous acquisition of 18F-FDG PET and arterial spin labeling. NeuroImage Clin. 2016;11:648–57. https://doi.org/10.1016/j.nicl.2016.04.005.
Verger A, Doyen M, Campion JY, Guedj E. The pons as reference region for intensity normalization in semi-quantitative analysis of brain 18FDG PET: application to metabolic changes related to ageing in conventional and digital control databases. EJNMMI Res. 2021;11. https://doi.org/10.1186/s13550-021-00771-0.
Nugent S, Croteau E, Potvin O, Castellano CA, Dieumegarde L, Cunnane SC, et al. Selection of the optimal intensity normalization region for FDG-PET studies of normal aging and Alzheimer’s disease. Sci Rep. 2020;10:1–8. https://doi.org/10.1038/s41598-020-65957-3.
Lange C, Suppa P, Frings L, Brenner W, Spies L, Buchert R. Optimization of statistical single subject analysis of brain FDG PET for the prognosis of mild cognitive impairment-to-Alzheimer’s disease conversion. J Alzheimer’s Dis. 2016;49:945–59. https://doi.org/10.3233/JAD-150814.
Martí Fuster B, Esteban O, Planes X, Wollny G, Sureda SR, Setoain X, et al. FocusDET, a new toolbox for SISCOM analysis. Evaluation of the registration accuracy using Monte Carlo simulation. Neuroinformatics. 2013;11:77–89. https://doi.org/10.1007/s12021-012-9158-x.
López-González FJ, Silva-Rodríguez J, Paredes-Pacheco J, Niñerola-Baizán A, Efthimiou N, Martín-Martín C, et al. Intensity normalization methods in brain FDG-PET quantification. Neuroimage. 2020;222: 117229. https://doi.org/10.1016/j.neuroimage.2020.117229.
Kim YK, Lee DS, Lee SK, Chung CK, Chung JK, Lee MC. 18F-FDG PET in localization of frontal lobe epilepsy: comparison of visual and SPM analysis. J Nucl Med. 2002;43:1167–74.
Funding
This work was partially sponsored by grants from the National Natural Science Foundation of China (NSFC) (No. 81725009, 82030049, 32027802) and Fundamental Research Funds for the Central Universities and Key R&D Program of Zhejiang (2022C03071).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional review board of the Second Hospital of Zhejiang University School of Medicine (Approval No. 2021–0190) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Exemption from informed consent was approved by the institutional review board.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Rights and permissions
About this article
Cite this article
Zhang, T., Wu, S., Zhang, X. et al. Spatial normalization and quantification approaches of PET imaging for neurological disorders. Eur J Nucl Med Mol Imaging 49, 3809–3829 (2022). https://doi.org/10.1007/s00259-022-05809-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05809-6