Abstract
Introduction
The impact of age is crucial and must be taken into account when applying a voxel-based quantitative analysis on brain images from [18F]-fluorodeoxyglucose Positron Emission Tomography (FDG-PET). This study aimed to determine whether age-related changes in brain FDG-PET images are more accurately assessed when the conventional statistical parametric mapping (SPM) normalization method is used with an adaptive template, obtained from analysed PET images using a Block-Matching (BM) algorithm to fit with the characteristics of these images.
Methods
Age-related changes in FDG-PET images were computed with linear models in 84 neurologically healthy subjects (35 women, 19 to 82-year-old), and compared between results provided by the SPM normalization algorithm applied on its dedicated conventional template or on the adaptive BM template. A threshold P value of 0.05 was used together with a family-wise error correction.
Results
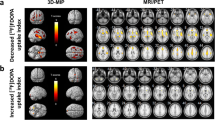
The age-related changes in FDG-PET images were much more apparent when computed with the adaptive template than with the conventional template as evidenced by: (1) stronger correlation coefficients with age for the overall frontal and temporal uptake values (respective R 2 values of 0.20 and 0.07) and (2) larger extents of involved areas (13 and 5 % of whole brain template volume, respectively), leading to reveal several age-dependent areas (especially in dorsolateral prefrontal, inferior temporal/fusiform and primary somatosensory cortices).
Conclusion
Age-related changes in brain FDG uptake may be more accurately determined when applying the SPM method of voxel-based quantitative analysis on a template that best fits the characteristics of the analysed TEP images.



Similar content being viewed by others
References
Mosconi L, Pupi A, De Cristofaro MTR, Fayyaz M, Sorbi S, Herholz K. Functional interactions of the entorhinal cortex: an 18F-FDG PET study on normal aging and Alzheimer’s disease. J Nucl Med. 2004;45:382–92.
Diehl-Schmid J, Grimmer T, Drzezga A, Bornschein S, Riemenschneider M, Förstl H, et al. Decline of cerebral glucose metabolism in frontotemporal dementia: a longitudinal 18F-FDG-PET-study. Neurobiol Aging. 2007;28:42–50.
Fujimoto T, Takeuch K, Matsumoto T, Kamimura K, Hamada R, Nakamura K, et al. Abnormal glucose metabolism in the anterior cingulate cortex in patients with schizophrenia. Psychiatry Res. 2007;154:49–58.
Delvenne V, Goldman S, De Maertelaer V, Lotstra F. Brain glucose metabolism in eating disorders assessed by positron emission tomography. Int J Eat Disord. 1999;25:29–37.
Alavi A, Dann R, Chawluk J, Alavi J, Kushner M, Reivich M. Positron emission tomography imaging of regional cerebral glucose metabolism. Semin Nucl Med. 1986;16:2–34.
Signorini M, Paulesu E, Friston K, Perani D, Colleluori A, Lucignani G, et al. Rapid assessment of regional cerebral metabolic abnormalities in single subjects with quantitative and nonquantitative [18F]FDG PET: a clinical validation of statistical parametric mapping. Neuroimage. 1999;9:63–80.
Friston K, Ashbuner J, Frith C, Poline J, Heather J, Frackowiak RS. Spatial registration and normalization of images. Hum Brain Mapp. 1995;2:165–89.
Dukart J, Mueller K, Horstmann A, Vogt B, Frisch S, Barthel H, et al. Differential effects of global and cerebellar normalization on detection and differentiation of dementia in FDG-PET studies. Neuroimage. 2010;49:1490–5.
Yoshizawa H, Gazes Y, Stern Y, Miyata Y, Uchiyama S. Characterizing the normative profile of 18F-FDG PET brain imaging: sex difference, aging effect, and cognitive reserve. Psychiatry Res. 2014;221:78–85.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89.
Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–9.
Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36.
Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113.
Bieth M, Lombaert H, Reader AJ, Siddiqi K. Atlas construction for dynamic (4D) PET using diffeomorphic transformations. Med Image Comput Comput Assist Interv. 2013;16:35–42.
Martino ME, de Villoria JG, Lacalle-Aurioles M, Olazarán J, Cruz I, Navarro E, et al. Comparison of different methods of spatial normalization of FDG-PET brain images in the voxel-wise analysis of MCI patients and controls. Ann Nucl Med. 2013;27:600–9.
Della Rosa PA, Cerami C, Gallivanone F, Prestia A, Caroli A, Castiglioni I, et al. A standardized [18F]-FDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics. 2014;12:575–93.
Hsieh T-C, Lin W-Y, Ding H-J, Sun S-S, Wu Y-C, Yen K-Y, et al. Sex- and age-related differences in brain FDG metabolism of healthy adults: an SPM analysis. J Neuroimaging. 2012;22:21–7.
Kim I-J, Kim S-J, Kim Y-K. Age- and sex-associated changes in cerebral glucose metabolism in normal healthy subjects: statistical parametric mapping analysis of F-18 fluorodeoxyglucose brain positron emission tomography. Acta Radiol. 2009;50:1169–74.
Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–30.
Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, et al. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10:464–72.
Tisserand DJ, van Boxtel MPJ, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cereb Cortex. 2004;14:966–73.
Yanase D, Matsunari I, Yajima K, Chen W, Fujikawa A, Nishimura S, et al. Brain FDG PET study of normal aging in Japanese: effect of atrophy correction. Eur J Nucl Med Mol Imaging. 2005;32:794–805.
Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, et al. Age-related differences in regional brain volumes: a comparison of optimized voxel-based morphometry to manual volumetry. Neurobiol Aging. 2009;30:1657–76.
Pieperhoff P, Hömke L, Schneider F, Habel U, Shah NJ, Zilles K, et al. Deformation field morphometry reveals age-related structural differences between the brains of adults up to 51 years. J Neurosci. 2008;28:828–42.
Gokdemir S, Halac M, Albayram S, Oz B, Yeni N, Uzan M, et al. Contribution of fdg-pet in epilepsy surgery: consistency and postoperative results compared with magnetic resonance imaging and electroencephalography. Turk Neurosurg. 2015;25:53–7.
Silverman DH, Small GW, Chang CY, Lu CS, Kung De Aburto MA, Chen W, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA. 2001;286:2120–7.
Jeong Y, Cho SS, Park JM, Kang SJ, Lee JS, Kang E, et al. 18F-FDG PET findings in frontotemporal dementia: an SPM analysis of 29 patients. J Nucl Med. 2005;46:233–9.
Shinto AS, Kamaleshwaran KK, Srinivasan D, Paranthaman S, Selvaraj K, Pranesh MB, et al. “Hyperfrontality” as seen on FDG PET in unmedicated schizophrenia patients with positive symptoms. Clin Nucl Med. 2014;39:694–7.
Chiaravalloti A, Pagani M, Di Pietro B, Danieli R, Tavolozza M, Travascio L, et al. Is cerebral glucose metabolism affected by chemotherapy in patients with Hodgkin’s lymphoma? Nucl Med Commun. 2013;34:57–63.
Nonokuma M, Kuwabara Y, Takano K, Tamura K, Ishitsuka K, Yoshimitsu K. Evaluation of regional cerebral glucose metabolism in patients with malignant lymphoma of the body using statistical image analysis. Ann Nucl Med. 2014;28:950–60.
Sorokin J, Saboury B, Ahn JA, Moghbel M, Basu S, Alavi A. Adverse functional effects of chemotherapy on whole-brain metabolism: a PET/CT quantitative analysis of FDG metabolic pattern of the “chemo-brain”. Clin Nucl Med. 2014;39:e35–9.
Baudino B, D’agata F, Caroppo P, Castellano G, Cauda S, Manfredi M, et al. The chemotherapy long-term effect on cognitive functions and brain metabolism in lymphoma patients. Q J Nucl Med Mol Imaging. 2012;56:559–68.
D’Agata F, Costa T, Caroppo P, Baudino B, Cauda F, Manfredi M, et al. Multivariate analysis of brain metabolism reveals chemotherapy effects on prefrontal cerebellar system when related to dorsal attention network. EJNMMI Res. 2013;3:22.
Acknowledgments
The authors thank Pierre Pothier, for critical review of the manuscript, and the Nancyclotep experimental imaging platform, for organizational support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no potential conflicts of interest to report.
Ethical standards
The procedure followed was in accordance with the ethical standards and guidelines of the responsible committee on human experimentation.
Additional information
A. Van Der Gucht and A. Verger contributed equally to this work.
Rights and permissions
About this article
Cite this article
Van Der Gucht, A., Verger, A., Guedj, E. et al. Age-related changes in FDG brain uptake are more accurately assessed when applying an adaptive template to the SPM method of voxel-based quantitative analysis. Ann Nucl Med 29, 921–928 (2015). https://doi.org/10.1007/s12149-015-1022-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-015-1022-2