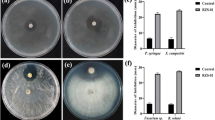
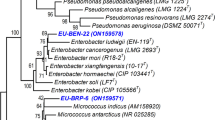
Abstract
Soil contamination by heavy metals is one of the major problems that adversely decrease plant growth and biomass production. Inoculation with the plant growth-promoting rhizobacteria (PGPR) can attenuate the toxicity of heavy metals and enhancing the plant growth. In this study, we evaluated the potential of a novel extremotolerant strain (IS-2 T) isolated from date palm rhizosphere to improve barley seedling growth under heavy metal stress. The species-level identification was carried out using morphological and biochemical methods combined with whole genome sequencing. The bacterial strain was then used in vitro for inoculating Hordeum vulgare L. exposed to three different Cr, Zn, and Ni concentrations (0.5, 1, and 2 mM) in petri dishes and different morphological parameters were assessed. The strain was identified as Bacillus glycinifermentans species. This strain showed high tolerance to pH (6–11), salt stress (0.2–2 M), and heavy metals. Indeed, the minimum inhibitory concentrations at which bacterium was unable to grow were 4 mM for nickel, 3 mM for zinc, more than 8 mM for copper, and 40 mM for chromium, respectively. It was observed that inoculation of Hordeum vulgare L. under metal stress conditions with Bacillus glycinifermentans IS-2 T stain improved considerably the growth parameters. The capacity of the IS-2 T strain to withstand a range of abiotic stresses and improve barley seedling development under lab conditions makes it a promising candidate for use as a PGPR in zinc, nickel, copper, and chromium bioremediation.








Similar content being viewed by others
References
Jiang J, Pan C, Xiao A et al (2017) Isolation, identification, and environmental adaptability of heavy-metal-resistant bacteria from ramie rhizosphere soil around mine refinery. 3 Biotech 7(1):1–6. https://doi.org/10.1007/s13205-017-0603-2
Khan AR, Ullah I, Khan AL et al (2015) Improvement in phytoremediation potential of Solanum nigrum under cadmium contamination through endophytic-assisted Serratia sp. RSC-14 inoculation. Environ Sci Pollut Res 22(18):14032–14042. https://doi.org/10.1007/s11356-015-4647-8
Kerbab S, Silini A, Chenari Bouket A et al (2021) Mitigation of NaCl stress in wheat by rhizosphere engineering using salt habitat adapted PGPR halotolerant bacteria. Appl Sci 11(3):1034. https://doi.org/10.3390/app11031034
Madline A, Benidire L, Boularbah A (2021) Alleviation of salinity and metal stress using plant growth-promoting rhizobacteria isolated from semiarid Moroccan copper-mine soils. Environ Sci Pollut Res 28(47):67185–67202. https://doi.org/10.1007/s11356-021-15168-8
Jinal HN, Gopi K, Kumar K et al (2021) Effect of zinc-resistant Lysinibacillus species inoculation on growth, physiological properties, and zinc uptake in maize (Zea mays L.). Environ Sci Pollut Res 28(6):6540–6548. https://doi.org/10.1007/s11356-020-10998-4
Kotoky R, Nath S, Kumar Maheshwari D et al (2019) Cadmium resistant plant growth promoting rhizobacteria Serratia marcescens S2I7 associated with the growth promotion of rice plant. Environ Sustain 2(2):135–144. https://doi.org/10.1007/s42398-019-00055-3
Rathi M, Nandabalan YK (2017) Copper-tolerant rhizosphere bacteria—characterization and assessment of plant growth promoting factors. Environ Sci Pollut Res 24(10):9723–9733. https://doi.org/10.1007/s11356-017-8624-2
Etesami H, Maheshwari DK (2018) Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: action mechanisms and future prospects. Ecotoxicol Environ Saf 156:225–246. https://doi.org/10.1016/j.ecoenv.2018.03.013
Meena KK, Sorty AM, Bitla UM et al (2017) Abiotic stress responses and microbe-mediated mitigation in plants: the omics strategies. Front Plant Sci 8:172. https://doi.org/10.3389/fpls.2017.00172
Carlos MHJ, Stefani PVY, Janette AM et al (2016) Assessing the effects of heavy metals in ACC deaminase and IAA production on plant growth-promoting bacteria. Microbiol Res 188:53–61. https://doi.org/10.1016/j.micres.2016.05.001
Etesami H (2018) Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: mechanisms and future prospects. Ecotoxicol Environ Saf 147:175–191. https://doi.org/10.1016/j.ecoenv.2017.08.032
Ghosh PK, Maiti TK, Pramanik K et al (2018) The role of arsenic resistant Bacillus aryabhattai MCC3374 in promotion of rice seedlings growth and alleviation of arsenic phytotoxicity. Chemosphere 211:407–419. https://doi.org/10.1016/j.chemosphere.2018.07.148
Khanna K, Jamwal VL, Gandhi SG et al (2019) Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci Rep 9(1):1–14. https://doi.org/10.1038/s41598-019-41899-3
Zhang M, Jin Z, Zhang X et al (2020) Alleviation of Cd phytotoxicity and enhancement of rape seedling growth by plant growth–promoting bacterium Enterobacter sp. Zm-123. Environ Sci Pollut Res 27(26):33192–33203. https://doi.org/10.1007/s11356-020-09558-7
Orji OU, Awoke JN, Aja PM et al (2021) Halotolerant and metalotolerant bacteria strains with heavy metals biorestoration possibilities isolated from Uburu Salt Lake, Southeastern. Nigeria Heliyon 7(7):e07512. https://doi.org/10.1016/j.heliyon.2021.e07512
Mahdi I, Hafidi M, Allaoui A et al (2021) Halotolerant endophytic bacterium Serratia rubidaea ED1 enhances phosphate solubilization and promotes seed germination. Agriculture 11(3):224. https://doi.org/10.3390/agriculture11030224
Geetha K, Venkatesham E, Hindumathi A et al (2014) Isolation, screening and characterization of plant growth promoting bacteria and their effect on Vigna radita (L.) R. Wilczek. Int J Curr Microbiol Appl Sci 3(6):799–899
Braga LF, Oliveira FAD, Couto EAPD et al (2018) Polyphasic characterization of bacteria obtained from upland rice cultivated in Cerrado soil. Braz J Microbiol 49:20–28. https://doi.org/10.1016/j.bjm.2017.04.004
Guenoun K, Chattaoui M, Bouri M et al (2019) Biological control of growth promoting rhizobacteria against Verticillium wilt of pepper plant. Biologia 74(3):237–250. https://doi.org/10.2478/s11756-018-00169-9
Farzana Y, Radziah O, Kamaruzaman S et al (2009) Characterization of beneficial properties of plant growth-promoting rhizobacteria isolated from sweet potato rhizosphere. Afr J Microbiol Res 3(11):815–821. https://doi.org/10.5897/AJMR.9000151
Shylla L, Barik SK, Joshi SR (2021) Characterization and bioremediation potential of native heavy-metal tolerant bacteria isolated from rat-hole coal mine environment. Arch Microbiol 203(5):2379–2392. https://doi.org/10.1007/s00203-021-02218-5
Sher S, Ghani A, Sultan S et al (2021) Bacterial strains isolated from heavy metals contaminated soil and wastewater with potential to oxidize arsenite. Environ Process 8(1):333–347. https://doi.org/10.1007/s40710-020-00488-7
Xia Y, Farooq MA, Javed MT et al (2020) Multi-stress tolerant PGPR Bacillus xiamenensis PM14 activating sugarcane (Saccharum officinarum L.) red rot disease resistance. Plant Physiol Biochem 151:640–649. https://doi.org/10.1016/j.plaphy.2020.04.016
Wash P, Batool A, Mulk S, Nazir S, Yasmin H, Mumtaz S, Hassan MN (2022) Prevalence of antimicrobial resistance and respective genes among Bacillus spp., a versatile bio-fungicide. Int J Environ Res Public Health 19(22):14997
CLSI C (2015) Performance standards for antimicrobial susceptibility testing: 25th informational supplement. CLSI document M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA
Das A, Belgaonkar P, Raman AS et al (2017) Bioremoval of lead using Pennisetum purpureum augmented with Enterobacter cloacae-VITPASJ1: a pot culture approach. Environ Sci Pollut Res 24(18):15444–15453. https://doi.org/10.1007/s11356-017-8988-3
Islam F, Yasmeen T, Ali Q et al (2016) Copper-resistant bacteria reduces oxidative stress and uptake of copper in lentil plants: potential for bacterial bioremediation. Environ Sci Pollut Res 23(1):220–233. https://doi.org/10.1007/s11356-015-5354-1
Barman D, Dutta I, Jha DK (2022) Heavy metal resistant bacteria from coal dumping site with plant growth promoting potentials. Biologia 77(2):533–545. https://doi.org/10.1007/s11756-021-00963-y
Rahman Z, Thomas L, Singh VP (2019) Biosorption of heavy metals by a lead (Pb) resistant bacterium, Staphylococcus hominis strain AMB-2. J Basic Microbiol 59(5):477–486
Andrews, S (2010) FastQC: a quality control tool for high throughput sequence data [Online]. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J 17(1):10–12. https://doi.org/10.14806/ej.17.1.200. (ISSN 2226-6089)
Wick RR, Judd LM, Gorrie CL, Holt KE (2017) Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 13(6):e1005595. https://doi.org/10.1371/journal.pcbi.1005595
Brettin T, Davis JJ, Disz T, Edwards RA, Gerdes S, Olsen GJ, Olson R, Overbeek R, Parrello B, Pusch GD et al (2015) RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci Rep 5:8365. https://doi.org/10.1038/srep08365
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30(4):772–780. https://doi.org/10.1093/molbev/mst010
Wattam AR, Davis JJ, Assaf R, Boisvert S, Brettin T, Bun C, Conrad N, Dietrich EM, Disz T, Gabbard JL et al (2017) Improvements to PATRIC, the all-bacterial Bioinformatics Database and Analysis Resource Center. Nucleic Acids Res 45:535–542. https://doi.org/10.1093/nar/gkw1017
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 57:758–767. https://doi.org/10.1080/10635150802429642
Arkin AP, Cottingham RW, Henry CS, Harris NL, Stevens RL, Maslov S et al (2018) KBase: the United States department of energy systems biology knowledgebase. Nat Biotechnol 36:566. https://doi.org/10.1038/nbt.4163
Ondov BD, Treangen TJ, Melsted P, Mallonee AB, Bergman NH, Koren S, Phillippy AM (2016) Mash: fast genome and metagenome distance estimation using MinHash. Genome Biol 17:132. https://doi.org/10.1186/s13059-016-0997-x
Praburaman L, Park SH, Cho M et al (2017) Significance of diazotrophic plant growth-promoting Herbaspirillum sp. GW103 on phytoextraction of Pband Zn by Zea mays L. Environ Sci Pollut Res 24(3):3172–3180. https://doi.org/10.1007/s11356-016-8066-2
Akhtar N, Ilyas N, Yasmin H et al (2021) Role of Bacillus cereus in improving the growth and phytoextractability of Brassica nigra (L.) K. Koch in chromium contaminated soil. Molecules 26(6):1569. https://doi.org/10.3390/molecules26061569
Ramadoss D, Lakkineni VK, Bose P et al (2013) Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. Springer Plus 2(1):1–7. https://doi.org/10.1186/2193-1801-2-6
Begum MF, Rahman MA, Alam MF (2010) Biological control of Alternaria fruit rot of chili by Trichoderma species under field conditions. Mycobiology 38(2):113–117. https://doi.org/10.4489/MYCO.2010.38.2.113
Raza S, Saleem MF, Khan IH et al (2012) Evaluating the drought stress tolerance efficiency of wheat (Triticum aestivum L.) cultivars. Rjoas 12(12):41–46. https://doi.org/10.18551/rjoas.2012-12.04
Sethy SK, Ghosh S (2013) Effect of heavy metals on germination of seeds. J Nat Sci Biol Med 4(2):272. https://doi.org/10.4103/0976-9668.116964
Andrades-Moreno L, Del Castillo I, Parra R et al (2014) Prospecting metal-resistant plant-growth promoting rhizobacteria for rhizoremediation of metal contaminated estuaries using Spartina densiflora. Environ Sci Pollut Res 21(5):3713–3721. https://doi.org/10.1007/s11356-013-2364-8
Bokhari A, Essack M, Lafi FF et al (2019) Bioprospecting desert plant Bacillus endophytic strains for their potential to enhance plant stress tolerance. Sci Rep 9(1):1–13. https://doi.org/10.1038/s41598-019-54685-y
Mishra RK, Pandey S, Rathore US et al (2023) Characterization of plant growth-promoting, antifungal, and enzymatic properties of beneficial bacterial strains associated with pulses rhizosphere from Bundelkhand region of India. Braz J Microbiol 54(3):2349–2360. https://doi.org/10.1007/s42770-023-01051-w
Shi P, Zhang J, Li X et al (2021) Multiple metabolic phenotypes as screening criteria are correlated with the plant growth-promoting ability of rhizobacterial isolates. Front Microbiol 12:747982. https://doi.org/10.3389/fmicb.2021.747982
Meyer T, Vigouroux A, Aumont-Nicaise M et al (2018) The plant defense signal galactinol is specifically used as a nutrient by the bacterial pathogen Agrobacterium fabrum. JBC 293(21):7930–7941. https://doi.org/10.1074/jbc.RA118.001856
Rameshkumar N, Lang E, Tanaka N (2016) Description of Vogesella oryzae sp. nov., isolated from the rhizosphere of saline tolerant pokkali rice. Syst Appl Microbiol 39(1):20–24. https://doi.org/10.1016/j.syapm.2015.10.003
Wang R, He C, Dong K et al (2020) Delineation of the crucial evolutionary amino acid sites in trehalose-6-phosphate synthase from higher plants. Evol Bioinform 16:1176934320910145. https://doi.org/10.1177/11769343209101
Lebeis SL, Paredes SH, Lundberg DS et al (2015) Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 349(6250):860–864. https://doi.org/10.1126/science.aaa8764
Ahmed M, Khan M, Alam M et al (2020) Comparative molecular studies of halophilic bacteria from saline water and soil in the Saudi environment. Biosci J(Online) 36(3):1024–1031. https://doi.org/10.14393/BJ-v36n3a2020-49988
Karthik C, Elangovan N, Kumar TS et al (2017) Characterization of multifarious plant growth promoting traits of rhizobacterial strain AR6 under chromium (VI) stress. Microbiol Res 204:65–71. https://doi.org/10.1016/j.micres.2017.07.008
Wu X, Fan Y, Wang R et al (2022) Bacillus halotolerans KKD1 induces physiological, metabolic and molecular reprogramming in wheat under saline condition. Front Plant Sci 13:978066. https://doi.org/10.3389/fpls.2022.978066
Pandey S, Ghosh PK, Ghosh S et al (2013) Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J Microbiol 51(1):11–17. https://doi.org/10.1007/s12275-013-2330-7
Maksimov IV, Veselova SV, Nuzhnaya TV et al (2015) Plant growth-promoting bacteria in regulation of plant resistance to stress factors. Russ J Plant Physiol 62(6):715–726. https://doi.org/10.1134/S1021443715060114
Aktuganov GE, Galimzyanova NF, Melent’Ev AI et al (2007) Extracellular hydrolases of strain Bacillus sp. 739 and their involvement in the lysis of micromycete cell walls. Microbiology 76(4):413–420. https://doi.org/10.1134/S002626170704005
Kumari M, Thakur IS (2018) Biochemical and proteomic characterization of Paenibacillus sp. ISTP10 for its role in plant growth promotion and in rhizostabilization of cadmium. Bioresour Technol Reports 3:59–66. https://doi.org/10.1016/j.biteb.2018.06.001
Hansda A, Kumar V (2017) Cu-resistant Kocuria sp. CRB15: a potential PGPR isolated from the dry tailing of Rakha copper mine. 3 Biotech 7(2):1–11. https://doi.org/10.1007/s13205-017-0757-y
Biswas A, Sharma Y, Kundu N et al (2019) Isolation and molecular characterization of heavy metal resistant bacteria from barrages of Yamuna River, New Delhi. India Plant Cell Biotechnol Mol Biol 20(5–6):212–221
Shahid M, Zeyad MT, Syed A et al (2022) Stress-tolerant endophytic isolate Priestia aryabhattai BPR-9 modulates physio-biochemical mechanisms in wheat (Triticum aestivum L.) for enhanced salt tolerance. IJERPH 19(17):10883. https://doi.org/10.3390/ijerph191710883
Majeed A, Muhammad Z, Siyar S (2019) Assessment of heavy metal induced stress responses in pea (Pisum sativum L.). Acta Ecol Sin 39(4):284–288. https://doi.org/10.1016/j.chnaes.2018.12.002
El Rasafi T, Bouda S, Nouri M et al (2020) Assessment of metals (Cu, Ni) and metalloids (As) induced stress responses in Barley (Hordeum vulgare) and wheat (Triticum aestivum). J Mater Environ Sci 11: 795–807. http://www.jmaterenvironsci.com
Seneviratne M, Gunaratne S, Bandara T et al (2016) Plant growth promotion by Bradyrhizobium japonicum under heavy metal stress. S Afr J Bot 105:19–24. https://doi.org/10.1016/j.sajb.2016.02.206
Jan R, Khan MA, Asaf S et al (2019) Metal resistant endophytic bacteria reduces cadmium, nickel toxicity, and enhances expression of metal stress related genes with improved growth of Oryza sativa, via regulating its antioxidant machinery and endogenous hormones. Plants 8(10):363. https://doi.org/10.3390/plants8100363
Yahaghi Z, Shirvani M, Nourbakhsh F et al (2019) Uptake and effects of lead and zinc on alfalfa (Medicago sativa L.) seed germination and seedling growth: role of plant growth promoting bacteria. S Afr J Bot 124:573–582. https://doi.org/10.1016/j.sajb.2019.01.006
Ali J, Ali F, Ahmad I et al (2021) Mechanistic elucidation of germination potential and growth of Sesbania sesban seedlings with Bacillus anthracis PM21 under heavy metals stress: An in vitro study. Ecotoxicol Environ Saf 208:111769. https://doi.org/10.1016/j.ecoenv.2020.111769
Rolón-Cárdenas GA, Arvizu-Gómez JL, Pacheco-Aguilar JR, Vázquez-Martínez J, Hernández-Morales A (2021) Cadmium-tolerant endophytic Pseudomonas rhodesiae strains isolated from Typha latifolia modify the root architecture of Arabidopsis thaliana Col-0 in presence and absence of Cd. Braz J Microbiol 52:349–361. https://doi.org/10.1007/s42770-020-00408-9
Ndeddy Aka RJ, Babalola OO (2016) Effect of bacterial inoculation of strains of Pseudomonas aeruginosa, Alcaligenes feacalis and Bacillus subtilis on germination, growth and heavy metal (Cd, Cr, and Ni) uptake of Brassica juncea. Int J Phytoremediation 18(2):200–209. https://doi.org/10.1080/15226514.2015.1073671
De Nunes PSO, de Medeiros FH, de Oliveira TS, de Almeida Zago JR, Bettiol W (2023) Bacillus subtilis and Bacillus licheniformis promote tomato growth. Braz J Microbiol 54(1):397–406. https://doi.org/10.1007/s42770-022-00874-3
Liaquat F, Munis MFH, Arif S, Haroon U, Shengquan C, Qunlu L (2020) Cd-tolerant SY-2 strain of Stenotrophomonas maltophilia: a potential PGPR, isolated from the Nanjing mining area in China. 3 Biotech 10:1–10. https://doi.org/10.1007/s13205-020-02524-7
Shreya D, Jinal H, Kartik VP et al (2020) Amelioration effect of chromium-tolerant bacteria on growth, physiological properties and chromium mobilization in chickpea (Cicer arietinum) under chromium stress. Arch Microbiol 202(4):887–894. https://doi.org/10.1007/s00203-019-01801-1
Islam F, Yasmeen T, Ali Q et al (2014) Influence of Pseudomonas aeruginosa as PGPR on oxidative stress tolerance in wheat under Zn stress. Ecotoxicol Environ Saf 104:285–293. https://doi.org/10.1016/j.ecoenv.2014.03.008
Khan WU, Ahmad SR, Yasin NA et al (2017) Application of Bacillus megaterium MCR-8 improved phytoextraction and stress alleviation of nickel in Vinca rosea. Int J Phytoremediation 19(9):813–824. https://doi.org/10.1080/15226514.2017.1290580
Madhaiyan M, Poonguzhali S, Sa T (2007) Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 69(2):220–228. https://doi.org/10.1016/j.chemosphere.2007.04.017
Adediran GA, Ngwenya BT, Mosselmans JFW et al (2016) Bacteria–zinc co-localization implicates enhanced synthesis of cysteine-rich peptides in zinc detoxification when Brassica juncea is inoculated with Rhizobium leguminosarum. New Phytol 209(1):280–293. https://doi.org/10.1111/nph.13588
Pramanik K, Mitra S, Sarkar A et al (2017) Characterization of cadmium-resistant Klebsiella pneumoniae MCC 3091 promoted rice seedling growth by alleviating phytotoxicity of cadmium. Environ Sci Pollut Res 24(31):24419–24437. https://doi.org/10.1007/s11356-017-0033-z
Ghosh A, Pramanik K, Bhattacharya S et al (2021) Abatement of arsenic-induced phytotoxic effects in rice seedlings by an arsenic-resistant Pantoea dispersa strain. Environ Sci Pollut Res 28(17):21633–21649. https://doi.org/10.1007/s11356-020-11816-7
Funding
This research was supported by PRIMA program (BENEFIT-Med project).
Author information
Authors and Affiliations
Contributions
MB and BK: conceptualization, validation, investigation, writing—original draft, writing—review and editing. AF: conceptualization, validation, resources, data curation, writing—original draft preparation, writing—reviewing and editing. HEA: formal analysis, data curation, visualization. ZC: supervision, resources, methodology. FB: investigation and writing the genomic characterization section. AE: resources, review and editing, funding acquisition. IF: supervision, writing—reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Jerri Zilli
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
belhassan, M., Farhat, A., Abed, H.E. et al. Isolation and identification of a new Bacillus glycinifermentans strain from date palm rhizosphere and its effect on barley seeds under heavy metal stress. Braz J Microbiol 55, 843–854 (2024). https://doi.org/10.1007/s42770-024-01263-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-024-01263-8