Abstract
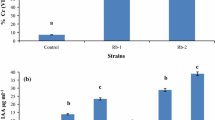
In this work, we isolated four Cd-tolerant endophytic bacteria from Typha latifolia roots that grow at a Cd-contaminated site. Bacterial isolates GRC065, GRC066, GRC093, and GRC140 were identified as Pseudomonas rhodesiae. These bacterial isolates tolerate cadmium and have abilities for phosphate solubilization, siderophore production, indole acetic acid (IAA) synthesis, and ACC deaminase activity, suggesting that they are plant growth-promoting rhizobacteria. Bacterial inoculation in Arabidopsis thaliana seedlings showed that P. rhodesiae strains increase total fresh weight and number of lateral roots concerning non-inoculated plants. These results indicated that P. rhodesiae strains promote A. thaliana seedlings growth by modifying the root system. On the other hand, in A. thaliana seedlings exposed to 2.5 mg/l of Cd, P. rhodesiae strains increased the number and density of lateral roots concerning non-inoculated plants, indicating that they modify the root architecture of A. thaliana seedlings exposed to cadmium. The results showed that P. rhodesiae strains promote the development of lateral roots in A. thaliana seedlings cultivated in both conditions, with and without cadmium. These results suggest that P. rhodesiae strains could exert a similar role inside the roots of T. latifolia that grow in the Cd-contaminated environment.







Similar content being viewed by others
References
Smith SG (1987) Typha: its taxonomy and the ecological significance of hybrids. Archiv fü Hydrobiologie 27:129–138
Baldwin B, Cannon A (2007) Typha review. Utah State University, Logan
Jeke NN, Zvomuya F, Cicek N, Ross L, Badiou P (2015) Biomass, nutrient, and trace element accumulation and partitioning in cattail (Typha latifolia L.) during wetland phytoremediation of municipal biosolids. J Environ Qual 44:1541–1549. https://doi.org/10.2134/jeq2015.02.0064
Bansal S, Lishawa SC, Newman S, Tangen BA, Wilcox D, Albert D, Anteau MJ, Chimney MJ, Cressey RL, DeKeyser E, Elgersma KJ, Finkelstein SA, Freeland J, Grosshans R, Klug PE, Larkin DJ, Lawrence BA, Linz G, Marburger J, Noe G, Otto C, Reo N, Richards J, Richardson C, Rodgers LR, Schrank AJ, Svedarsky D, Travis S, Tuchman N, Windham-Myers L (2019) Typha (cattail) invasion in North American wetlands: biology, regional problems, impacts, ecosystem services, and management. Wetlands 39(4):645–684. https://doi.org/10.1007/s13157-019-01174-7
Manoj SR, Karthik C, Kadirvelu K, Arulselvi PI, Shanmugasundaram T, Bruno B, Rajkumar M (2020) Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: a review. J Environ Manag 254:109779. https://doi.org/10.1016/j.jenvman.2019.109779
Santoyo G, Moreno-Hagelsieb G, del Carmen O-MM, Glick BR (2016) Plant growth-promoting bacterial endophytes. Microbiol Res 183:92–99. https://doi.org/10.1016/j.micres.2015.11.008
Kong Z, Glick BR (2017) The role of plant growth-promoting bacteria in metal phytoremediation. In: Poole RK (ed) Adv Microb Physiol, vol 71. Academic Press, pp 97-132. doi:https://doi.org/10.1016/bs.ampbs.2017.04.001
Paredes-Páliz KI, Caviedes MA, Doukkali B, Mateos-Naranjo E, Rodríguez-Llorente ID, Pajuelo E (2016) Screening beneficial rhizobacteria from Spartina maritima for phytoremediation of metal polluted salt marshes: comparison of Gram-positive and Gram-negative strains. Environ Sci Pollut Res Int 23(19):19825–19837. https://doi.org/10.1007/s11356-016-7184-1
Paredes-Páliz KI, Mateos-Naranjo E, Doukkali B, Caviedes MA, Redondo-Gómez S, Rodríguez-Llorente ID, Pajuelo E (2017) Modulation of Spartina densiflora plant growth and metal accumulation upon selective inoculation treatments: a comparison of Gram negative and Gram positive rhizobacteria. Mar Pollut Bull 125(1):77–85. https://doi.org/10.1016/j.marpolbul.2017.07.072
Chiboub M, Saadani O, Fatnassi IC, Abdelkrim S, Abid G, Jebara M, Jebara SH (2016) Characterization of efficient plant-growth-promoting bacteria isolated from Sulla coronaria resistant to cadmium and to other heavy metals. Comptes Rendus Biol 339(9):391–398. https://doi.org/10.1016/j.crvi.2016.04.015
Li YH, Liu QF, Liu Y, Zhu JN, Zhang Q (2011) Endophytic bacterial diversity in roots of Typha angustifolia L. in the constructed Beijing Cuihu Wetland (China). Res Microbiol 162(2):124–131. https://doi.org/10.1016/j.resmic.2010.09.021
Ghosh UD, Saha C, Maiti M, Lahiri S, Ghosh S, Seal A, Mitra Ghosh M (2014) Root associated iron oxidizing bacteria increase phosphate nutrition and influence root to shoot partitioning of iron in tolerant plant Typha angustifolia. Plant Soil 381(1):279–295. https://doi.org/10.1007/s11104-014-2085-x
Saha C, Mukherjee G, Agarwal-Banka P, Seal A (2016) A consortium of non-rhizobial endophytic microbes from Typha angustifolia functions as probiotic in rice and improves nitrogen metabolism. Plant Biol J 18(6):938–946. https://doi.org/10.1111/plb.12485
Diazbarriga F, Santos MA, Mejia JD, Batres L, Yanez L, Carrizales L, Vera E, Delrazo LM, Cebrian ME (1993) Arsenic and cadmium exposure in children living near a smelter complex in San Luis Potosı́, Mexico. Environ Res 62(2):242–250. https://doi.org/10.1006/enrs.1993.1109
Carranza-Álvarez C, Alonso-Castro AJ, Alfaro-De La Torre MC, García-De La Cruz RF (2008) Accumulation and distribution of heavy metals in Scirpus americanus and Typha latifolia from an artificial lagoon in San Luis Potosí, México. Water Air Soil Pollut 188(1–4):297–309. https://doi.org/10.1007/s11270-007-9545-3
Sheng X-F, Xia J-J (2006) Improvement of rape (Brassica napus) plant growth and cadmium uptake by cadmium-resistant bacteria. Chemosphere 64:1036–1042
Ndeddy Aka RJ, Babalola OO (2017) Identification and characterization of Cr-, Cd-, and Ni-tolerant bacteria isolated from mine tailings. Bioremediat J 21(1):1–19. https://doi.org/10.1080/10889868.2017.1282933
Chen W-P, Kuo T-T (1993) A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res 21(9):2260–2260. https://doi.org/10.1093/nar/21.9.2260
Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ (2008) Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol 74(8):2461–2470. https://doi.org/10.1128/AEM.02272-07
Zeng X, Tang J, Liu X, Jiang P (2012) Response of P. aeruginosa E1 gene expression to cadmium stress. Curr Microbiol 65(6):799–804. https://doi.org/10.1007/s00284-012-0224-2
Shehata HR, Dumigan C, Watts S, Raizada MN (2017) An endophytic microbe from an unusual volcanic swamp corn seeks and inhabits root hair cells to extract rock phosphate. Sci Rep 7(1):13479. https://doi.org/10.1038/s41598-017-14080-x
King J (1932) The colorimetric determination of phosphorus. Biochem J 26:292–297. https://doi.org/10.1042/bj0260292
Tank N, Rajendran N, Patel B, Saraf M (2012) Evaluation and biochemical characterization of a distinctive pyoverdin from a Pseudomonas isolated from chickpea rhizosphere. Braz J Microbiol 43:639–648. https://doi.org/10.1590/S1517-83822012000200028
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils 12:39–45. https://doi.org/10.1007/BF00369386
Grunennvaldt RL, Degenhardt-Goldbach J, de Cássia TJ, Davila Dos Santos G, Aparecida Vicente V, Deschamps C (2018) Bacillus megaterium: an endophytic bacteria from callus of Ilex paraguariensis with growth promotion activities. Biotecnología Vegetal 18(1):3–13
Dworkin M, Foster J (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75:592–601
Hontzeas N, Richardson AO, Belimov A, Safronova V, Abu-Omar MM, Glick BR (2005) Evidence for horizontal transfer of 1-aminocyclopropane-1-carboxylate deaminase genes. Appl Environ Microbiol 71(11):7556–7558. https://doi.org/10.1128/AEM.71.11.7556-7558.2005
Remy E, Duque P (2016) Assessing tolerance to heavy-metal stress in Arabidopsis thaliana seedlings. In: Duque P (ed) Environmental responses in plants: methods and protocols. Springer New York, New York, pp 197–208. https://doi.org/10.1007/978-1-4939-3356-3_16
Bonanno G, Cirelli GL (2017) Comparative analysis of element concentrations and translocation in three wetland congener plants: Typha domingensis, Typha latifolia and Typha angustifolia. Ecotoxicol Environ Saf 143:92–101. https://doi.org/10.1016/j.ecoenv.2017.05.021
Mazhar SH, Herzberg M, Ben Fekih I, Zhang C, Bello SK, Li YP, Su J, Xu J, Feng R, Zhou S, Rensing C (2020) Comparative insights into the complete genome sequence of highly metal resistant Cupriavidus metallidurans strain BS1 isolated from a gold–copper mine. Front Microbiol 11:47. https://doi.org/10.3389/fmicb.2020.00047
Chow LC (2001) Solubility of calcium phosphates. In: Chow LC, Eanes ED (eds) Octacalcium phosphate, vol 18. Karger, Basel, pp 94–111
Alonso-Castro AJ, Carranza-Álvarez C, Alfaro-De la Torre MC, Chávez-Guerrero L, García-De la Cruz RF (2009) Removal and accumulation of cadmium and lead by Typha latifolia exposed to single and mixed metal solutions. Arch Environ Contam Toxicol 57(4):688–696. https://doi.org/10.1007/s00244-009-9351-6
Leura-Vicencio A, Alonso-Castro AJ, Carranza-Álvarez C, Loredo-Portales R, Alfaro-De La Torre MC, García-De La Cruz RF (2013) Removal and accumulation of As, Cd and Cr by Typha latifolia. Bull Environ Contam Toxicol 90(6):650–653. https://doi.org/10.1007/s00128-013-0962-2
Kandasamy S, Loganathan K, Muthuraj R, Duraisamy S, Seetharaman S, Thiruvengadam R, Ponnusamy B, Ramasamy S (2009) Understanding the molecular basis of plant growth promotional effect of Pseudomonas fluorescens on rice through protein profiling. Proteome Sci 7(1):47. https://doi.org/10.1186/1477-5956-7-47
Coroler L, Elomari M, Hoste B, Gillis M, Izard D, Leclerc H (1996) Pseudomonas rhodesiae sp. nov., a new species isolated from natural mineral waters. Syst Appl Microbiol 19(4):600–607. https://doi.org/10.1016/S0723-2020(96)80032-4
Yoon B-J, Lee D-H, Kang Y-S, Oh D-C, Kim S-I, Oh K-H, Kahng H-Y (2002) Evaluation of carbazole degradation by Pseudomonas rhodesiae strain KK1 isolated from soil contaminated with coal tar. J Basic Microbiol 42(6):434–443. https://doi.org/10.1002/1521-4028(200212)42:6<434::Aid-jobm434>3.0.Co;2-c
Rolli E, Marasco R, Saderi S, Corretto E, Mapelli F, Cherif A, Borin S, Valenti L, Sorlini C, Daffonchio D (2017) Root-associated bacteria promote grapevine growth: from the laboratory to the field. Plant Soil 410(1):369–382. https://doi.org/10.1007/s11104-016-3019-6
John-Jimtha C, Radhakrishnan EK (2016) Multipotent plant probiotic rhizobacteria from western ghats and its effect on quantitative enhancement of medicinal natural product biosynthesis. Proc Natl A Sci India B 88(2):755–768. https://doi.org/10.1007/s40011-016-0810-3
Romero FM, Marina M, Pieckenstain FL (2016) Novel components of leaf bacterial communities of field-grown tomato plants and their potential for plant growth promotion and biocontrol of tomato diseases. Res Microbiol 167(3):222–233. https://doi.org/10.1016/j.resmic.2015.11.001
Zhang R, Zhang Q, Huang X, Guo X (2016) Endophytic bacterial diversity in roots of typha and the relationship of water quality factors in reclaimed water replenishment constructed wetland. China Environ Sci 36(3):875–886
Satchanska G, Topalova Y, Ivanov I, Golovinsky E (2006) Xenobiotic biotransformation potential of Pseudomonas rhodesiae KCM-R5 and Bacillus subtilis KCM-RG5, tolerant to heavy metals and phenol derivatives. Biotechnol Biotechnol Equip 20(1):97–102. https://doi.org/10.1080/13102818.2006.10817312
Kim S-I, Kukor JJ, Oh K-H, Kahng H-Y (2006) Evaluating the genetic diversity of dioxygenases for initial catabolism of aromatic hydrocarbons in Pseudomonas rhodesiae KK1. Enzym Microb Technol 40(1):71–78. https://doi.org/10.1016/j.enzmictec.2005.10.041
Phillips LA, Germida JJ, Farrell RE, Greer CW (2008) Hydrocarbon degradation potential and activity of endophytic bacteria associated with prairie plants. Soil Biol Biochem 40(12):3054–3064. https://doi.org/10.1016/j.soilbio.2008.09.006
Poirier I, Jean N, Guary JC, Bertrand M (2008) Responses of the marine bacterium Pseudomonas fluorescens to an excess of heavy metals: physiological and biochemical aspects. Sci Total Environ 406(1–2):76–87
Hu N, Zhao B (2009) Key genes involved in heavy-metal resistance in Pseudomonas putida CD2. FEMS Microbiol Lett 267:17–22
Maynaud G, Brunel B, Mornico D, Durot M, Severac D, Dubois E, Navarro E, Cleyet-Marel J-C, Le Quéré A (2013) Genome-wide transcriptional responses of two metal-tolerant symbiotic Mesorhizobium isolates to zinc and cadmium exposure. BMC Genomics 14(1):292. https://doi.org/10.1186/1471-2164-14-292
Chen Y, Chao Y, Li Y, Lin Q, Bai J, Tang L, Wang S, Ying R, Qiu R (2016) Survival strategies of the plant-associated bacterium Enterobacter sp. strain EG16 under cadmium stress. Appl Environ Microbiol 82(6):1734–1744. https://doi.org/10.1128/aem.03689-15
Mergeay M, Nies D, Schlegel HG, Gerits J, Charles P, Van Gijsegem F (1985) Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J Bacteriol 162(1):328–334
Chellaiah ER (2018) Cadmium (heavy metals) bioremediation by Pseudomonas aeruginosa: a minireview. Appl Water Sci 8(6):154. https://doi.org/10.1007/s13201-018-0796-5
Ullah A, Heng S, Munis MFH, Fahad S, Yang X (2015) Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: a review. Environ Exp Bot 117:28–40. https://doi.org/10.1016/j.envexpbot.2015.05.001
Baca BE, Elmerich C (2007) Microbial production of plant hormones. In: Elmerich C, Newton WE (eds) Associative and endophytic nitrogen-fixing bacteria and cyanobacterial associations. Springer, Netherlands, pp 113–143
Khan A, Hossain MT, Park HC, Yun D-J, Shim SH, Chung YR (2016) Development of root system architecture of Arabidopsis thaliana in response to colonization by Martelella endophytica YC6887 depends on auxin signaling. Plant Soil 405(1):81–96. https://doi.org/10.1007/s11104-015-2775-z
Hammad Y, Nalin R, Marechal J, Fiasson K, Pepin R, Berry AM, Normand P, Domenach A-M (2003) A possible role for phenyl acetic acid (PAA) on Alnus glutinosa nodulation by Frankia. Plant Soil 254(193):193–205. https://doi.org/10.1023/A:1024971417777
Kang JG, Kim ST, Kang KY (1999) Production of the antifungal compound phenylacetic acid by antagonistic bacterium Pseudomonas sp. J Appl Biol Chem 42(2):197–201
Hwang BK, Lim SW, Kim BS, Lee JY, Moon SS (2001) Isolation and in vivo and in vitro antifungal activity of phenylacetic acid and sodium phenylacetate from Streptomyces humidus. Appl Environ Microbiol 67(8):3739–3745. https://doi.org/10.1128/AEM.67.8.3739-3745.2001
Sajid I, Shaaban KA, Hasnain S (2011) Identification, isolation and optimization of antifungal metabolites from the Streptomyces malachitofuscus ctf9. Braz J Microbiol 42(2):592–604. https://doi.org/10.1590/S1517-838220110002000024
Johnston-Monje D, Raizada MN (2011) Plant and endophyte relationships: nutrient management. In: Moo-Young M (ed) Comprehensive biotechnology, 2nd edn. Elsevier, Oxford, pp 713–727
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169(1):30–39. https://doi.org/10.1016/j.micres.2013.09.009
Osmolovskaya N, Dung VV, Kuchaeva L (2018) The role of organic acids in heavy metal tolerance in plants. Bio Comm 63(1):9–16. https://doi.org/10.21638/spbu03.2018.103
Zhou C, Zhu L, Ma Z, Wang J (2017) Bacillus amyloliquefaciens SAY09 increases cadmium resistance in plants by activation of auxin-mediated signaling pathways. Genes 8(7):173. https://doi.org/10.3390/genes8070173
Funding
The work reported was funded by grants from CONACYT (Fondo Sectorial de Investigación para la Educación, CB2017-2018 A1-S-40454), Fondo de Apoyo a la Investigación (UASLP 2019 C19-FAI-05-40.40), and Fondos Concurrentes de la UASLP (FCR UASLP 210920280) to Alejandro Hernández-Morales. Gisela Adelina Rolón-Cárdenas (CVU 712240) thanks CONACYT-Mexico for the financial support given to carry out her Ph.D. studies.
Author information
Authors and Affiliations
Contributions
GARC (Ph. D. student) performed the research, analyzed data, and wrote the paper. JLAG, JRPA, and JVM participated in experimental design, contributed to bacterial characterization, and analyzed data. AHM conceived the study, analyzed data, and participated in the paper writing. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible Editor: Ieda Carvalho Mendes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rolón-Cárdenas, G.A., Arvizu-Gómez, J.L., Pacheco-Aguilar, J.R. et al. Cadmium-tolerant endophytic Pseudomonas rhodesiae strains isolated from Typha latifolia modify the root architecture of Arabidopsis thaliana Col-0 in presence and absence of Cd. Braz J Microbiol 52, 349–361 (2021). https://doi.org/10.1007/s42770-020-00408-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-020-00408-9