Abstract
Versatile strategies have been developed to construct electrospun fiber-based drug delivery systems for tissue regeneration and cancer therapy. We first introduce the construction of electrospun fiber scaffolds and their various structures, as well as various commonly used types of drugs. Then, we discuss some representative strategies for controlling drug delivery by electrospun fibers, with specific emphasis on the design of endogenous and external stimuli-responsive drug delivery systems. Afterwards, we summarize the recent progress on controlling drug delivery with electrospun fiber scaffolds for tissue engineering, including soft tissue engineering (such as skin, nerve, and cardiac repair) and hard tissue engineering (such as bone, cartilage, and musculoskeletal systems), as well as for cancer therapy. Furthermore, we provide future development directions and challenges facing the use of electrospun fibers for controlled drug delivery, aiming to provide insights and perspectives for the development of smart drug delivery platforms and improve clinical therapeutic effects in tissue regeneration and cancer therapy.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Living systems are based around complex and precise regulatory rules that modulate the on-demand release or alteration of important biologically active substances in a spatiotemporally controlled manner to maintain normal metabolic balance [1]. However, the limited capability of many human tissues to perform self-regulation and/or self-repair can cause the occurrence of many diseases [2]. In these cases, the assistance of biologically active substances or drugs is necessary to inhibit or eliminate the internal and external factors that are unfavorable to health, effectively treating diseases and promoting regeneration of damaged tissues [3]. As a key component of drug delivery systems (DDSs), drugs play pivotal roles and are responsible for achieving satisfactory therapeutic effects [4, 5]. However, most drugs are administered systemically, require frequent administration and are characterized by short-term effectiveness, potentially leading to adverse cytotoxic side effects and the development of drug resistance. In addition, drug concentration and therapeutic effects at targeted tissues cannot be guaranteed [6]. Meanwhile, the tissue regeneration and cancer treatment involve complex physiological processes [7], so it is difficult for a single type of drug to achieve an ideal therapeutic effect; rather, effective treatment usually requires multiple types of drugs to be released in a coordinated and periodically controlled manner.
With the rapid development of nanotechnology, various DDSs have been developed to address problems such as burst and discontinuous drug release, unsatisfactory drug loading efficiency, and low drug stability and utilization efficiency in vivo [8,9,10]. Compared with DDSs based on liposomes, micelles, nanoparticles and hydrogels, electrospun fibers have attracted increasing attention as promising drug carriers [11]. DDSs based on electrospun fibers have been studied and explored due to the maneuverability of the electrospinning process and the subsequent customizable design of the fiber-based scaffolds [12, 13]. Electrospun fiber scaffolds have characteristics that include simple preparation, high material universality, and favorable surface chemical properties for drug adsorption [14, 15]. In addition, the high porosity and large specific surface area of electrospun fiber scaffolds make them beneficial to increasing drug-loading efficiency and the response speed of stimuli-delivered drugs [16]. The extracellular matrix (ECM)-like morphology of electrospun fibers inherently guides cellular drug uptake [1]. These advantages allow multifunctional electrospun fiber scaffolds to support customizable drug delivery platforms that can achieve the sustained and programmed release of multiple drugs for tissue regeneration and cancer therapy.
In principle, almost all polymers and many additional functional components can be integrated into the electrospun fiber platform [13, 17]. As for drug delivery, a variety of technologies derived from electrospinning, including coaxial electrospinning [18], multiaxial electrospinning [19], electrospraying [20], etc., have been developed to prepare drug-loaded electrospun fiber platforms. In addition, electrospinning can be used to fabricate porous micro- and nanofibers, as well as various types of hierarchically controlled fibrous structures, ranging from 1 to 3D fibrous scaffolds. A growing number of customized electrospun fiber scaffolds have been used for drug delivery to facilitate tissue regeneration and cancer therapy. By modifying loading strategies, drugs can be released in a fast, sustained, heterogeneous or controlled manner by being combined with polymers, adsorbing on the fiber surface, or indirectly encapsulating onto electrospun fibers [21, 22]. Similarly, multifunctional electrospun fiber scaffolds that allow the sequential release of multiple drugs or in a spatiotemporally controllable manner, can be created to meet a variety of in vivo needs [23, 24].
Critically, a combination of strategies is needed for tissue engineering and cancer therapy, including the development of multifunctional scaffolds to provide biomimetic topographical cues and mechanical support, as well as the simultaneous delivery of small molecule drugs, growth factors, and other biochemical signals [25,26,27]. In addition to serving as a drug carrier, electrospun fibers can also be engineered to manipulate cell morphology and migration, neurite elongation, and stem cell differentiation by controlling their structure and array. Given that the realization of multiple functions in the body requires high levels of temporal and spatial precision, electrospun fiber scaffolds that enable the precise delivery of drug doses with spatiotemporal control have received great attention [28, 29]. It is well known that responsive DDSs can exploit intrinsic endogenous stimuli in living systems to develop new strategies for drug delivery with electrospun fiber scaffolds based on factors such as drug sensitivity to pH [30], reactive oxygen species (ROS) [31], enzymes [32], and glucose [33]. Likewise, external stimuli such as temperature [34], light [35], electricity [36], magnetic fields [37], and ultrasound [38] have also been used to modulate cellular behaviors, inducing tissue regeneration, and to remotely control drug delivery [39]. Based on this, stimuli-responsive electrospun platforms can serve as precise on-demand drug release repositories to mimic the function of living systems as much as possible and develop new tissue regeneration methods via the design of fiber structural features, thereby expanding the application of electrospun fibers for drug delivery in the fields of tissue regeneration and disease treatment.
Herein, we summarize the recent progress on controlling drug delivery from electrospun fiber scaffolds for tissue engineering, including soft tissue engineering (such as skin, nerve, cardiac, blood vessels) and hard tissue engineering (such as bone, cartilage, musculoskeletal, dental), as well as for cancer therapy. Meanwhile, emerging strategies for combining drugs with electrospun fibers and the resultant mechanism of drug delivery are discussed, and the effects of endogenous and external stimuli on drug release are emphasized (Fig. 1). Typically, “drugs” refer not only to traditional small-molecule drugs but also to bioactive components with specific therapeutic and regenerative functions, which can be divided into small molecular drugs and bioactive substances (e.g., growth factors, protein polypeptides, gene nucleic acids, and liposomes), as well as nanoparticles with therapeutic effects. Finally, the future directions of electrospun fibers for controlled drug delivery in tissue regeneration and cancer therapy are prospected, providing insights and perspectives for the development of smart drug release and highlighting the challenges to accelerate clinical translation.
Construction of Electrospun Fibers for Drug Delivery
The setup of electrospinning consists of a high-voltage power supply, a syringe pump, a spinneret, and a conductive collector. Firstly, the solution is extruded from the spinneret and forms a hanging droplet due to surface tension. When the high voltage power supply is applied, electrostatic repulsion among the same charges formed on the droplet surface turns it into a Taylor cone, from which a charged jet is ejected. Because of the behavior of bending instability, the jet undergoes a whipping motion after initially extending in a straight line. The jet will get slimmer and solidify quickly when the diameter of jet is optimal under the action of the electric field, then finally deposit on the surface of the conductive collector [17]. To meet various applications, different electrospun fiber sizes and morphologies can be obtained by changing the spinning process and parameters such as the voltage, solution composition, and collector design [40]. Meanwhile, satisfactory fiber structures can also be obtained by post-treatment procedures, such as weaving [41], twisting [42], foaming [43] and others. In this section, electrospun fibers are divided into three categories as follows: 1D pristine fiber (an individual fiber with different morphologies and structures), 2D hybrid fiber (in which material is loaded on the lumen/surface of an individual fiber), and 3D fiber architecture (arrangement and combination of fibers in 3D space).
The morphology and structure of 1D pristine fibers are the most basic units, and the most common and easily obtained morphology is the fiber with a smooth surface (Fig. 2a), however, at the same time, its simpleness makes it unsuitable for many applications. Many emerging structures, such as beaded, grooved, multi-channel, core–shell, and porous structures, have been designed and constructed, with various advantages described. Contrary to previous perception, beaded fibers tend to be produced due to a decrease in surface tension or an uneven distribution of solution concentration (Fig. 2b) [44], which is considered to be a structural defect that needs to be avoided and removed. However, it has been found that the presence of beads changes the surface roughness of the fiber, and this high surface roughness can have some beneficial effects on cell differentiation [45]. The fabrication of fiber by multi-fluid control technology is a new method in recent years [46]. A porous or grooved structure can be formed by electrospinning and stretching two incompatible polymers, then removing one of the components with a specific solvent [47] (Fig. 2c, d). Compared with the original fibers, both of these structures possess increased surface roughness and area, which are beneficial cell adhesion [48]. In addition, the grooved surface can provide topographic cues to promote the directional migration of cells. Meanwhile, some additional properties can be integrated into the hollow lumen of core–shell structures [46] and multi-channel structures [49] to meet the unique conditions for the regeneration of different tissues (Fig. 2e, f).
The versatile structure of electrospun fibers. a Solid fiber. Reproduced with permission from Ref. [200]; Copyright 2020, Wiley–VCH Limited. b Beaded fiber. Reproduced with permission from Ref. [44]; Copyright 2008, Wiley–VCH Limited. c Porous fiber. Reproduced with permission from Ref. [47]; Copyright 2008, Wiley–VCH Limited. d Grooved fiber. Reproduced with permission from Ref. [48]; Copyright 2020, Wiley–VCH Limited. e Core-sheath fiber. Reproduced with permission from Ref. [46]; Copyright 2010, American Chemical Society Limited. f Multi-channel fiber. Reproduced with permission from Ref. [49]; Copyright 2007, American Chemical Society Limited. g Fiber loaded with bioactive molecules. Reproduced with permission from Ref. [50]; Copyright 2014, Wiley–VCH Limited. h Cell-encapsulated fiber. Reproduced with permission from Ref. [51]; Copyright 2020, Elsevier Limited. i Nanoparticles-embedded fiber. Reproduced with permission from Ref. [53]; Copyright 2015, Elsevier Limited. j Nanoparticles anchored fiber. Reproduced with permission from Ref. [54]; Copyright 2020, Elsevier Limited. (k) Nanosheet-grown fiber. Reproduced with permission from Ref. [55]; Copyright 2021, Elsevier Limited. l Nanorod-grown fiber. Reproduced with permission from Ref. [56]; Copyright 2018, Elsevier Limited. m Radially aligned fiber array. Reproduced with permission from Ref. [57]; Copyright 2010, American Chemical Society Limited. n Bionic patterned fiber array. Reproduced with permission from Ref. [41]. Copyright 2020, Wiley–VCH Limited. o Complex pattern fiber array. Reproduced with permission from Ref. [58]; Copyright 2011, Wiley–VCH Limited. p Fiber mat with grooved surface. Reproduced with permission from Ref. [59]; Copyright 2021, American Association for the Advancement of Science Limited. q Tubular conduit. Reproduced with permission from Ref. [60]; Copyright 2017, Wiley–VCH Limited. r Multi-tubular conduit. Reproduced with permission from Ref. [61]; Copyright 2018, Wiley–VCH Limited. s 3D porous fiber scaffold. Reproduced with permission from Ref. [62]; Copyright 2019, American Chemical Society Limited. t Multifilament electrospun fiber yarns. Reproduced with permission from Ref. [42]; Copyright 2018, Elsevier Limited
Distinct from pristine fibers, 2D hybrid fibers can combine nanoparticles, cells, and bioactive factors to provide some specific effects. For example, embedding bioactive factors in fibers can promote cell migration, proliferation, and differentiation [50] (Fig. 2g). Packing cells in fibers can not only maintain high cellular activity and the ability to secrete immune molecules but can also provide a suitable environment for tissue regeneration [51] (Fig. 2h). In addition, embedding nanoparticles, such as iron oxide nanoparticles [52], graphene and organic materials [53] in fibers, can increase the intensity of external signals, thereby enhancing stimulation to promote tissue repair (Fig. 2i). Attaching specific substances to the fiber surface by post-treatment or in-situ growth is also a common method for broadening the applications of electrospun fibers [54,55,56] (Fig. 2j–l). For example, the introduction of polydopamine (PDA) coatings to electrospun nanofiber membrane could provide nucleation sites and active centers for zinc oxide (ZnO) nano-seeds to form nanorod structures, endowing nanofiber membrane with excellent photocatalysis and antibacterial properties [56].
Compared with the 2D hybrid fibers, the growth, morphology, differentiation, and function of cells in 3D scaffolds are closer to those found in in vivo microenvironments. Using the previously described pristine fibers or hybrid fibers, 3D scaffolds with different structures can be fabricated, typically by changing the collector, or by post-processing procedures such as crimping, weaving, molding, and others. By using different collectors, such as rollers and conductive rings [57], fibers arranged in an orderly 3D direction can be prepared (Fig. 2m), and the collector substrate pattern can be changed to fabricate various patterned scaffolds (Fig. 2n, o), making these scaffolds conducive to the development of cells in vivo [41, 58]. In addition, post-treatment methods can be used to change the surface morphology of scaffolds. For example, molding and photoetching can form grooves on the surface, providing topographical cues for cell migration (Fig. 2p) [59]. Another simple method, rolling-up, is often used to fabricate conduits from fiber membrane to connect defective nerves and build a bridge conducive to nerve regeneration (Fig. 2q) [60]. To improve the ability of bionics to simulate physiological cues, several small tubes were sequentially embedded in a larger tube to simulate the multi-fascicle structure of normal nerves, effectively avoiding misaligned axons growth (Fig. 2r) [61]. Foaming technology is another important method to fabricate 3D scaffolds composed of fibers. Scaffolds with radial arrangement structure can be fabricated through customizable control technology, which can effectively promote the migration of cells from the periphery to the center (Fig. 2s) [62]. Moreover, this 3D structure provides larger porosity and pore size, broadening the applications of electrospun fiber scaffolds in tissue engineering [62, 63]. Yarn can be made of electrospun fibers by weaving and twisting, leading to better permeability and drafting behavior (Fig. 2t), as well as increased suitability for tissue suturing [42].
Electrospun fiber scaffolds have wide application prospects in tissue regeneration and cancer therapy due to their porosity, high loading capability, adjustable mechanical properties, and excellent biocompatibility [64]. The integration of emerging 3D printing technology can support the design of more scaffolds with different structures and patterns, which can play unique roles in specific tissue regeneration processes [65]. Furthermore, advanced 4D electrospun fiber scaffolds can be developed by incorporating shape memory or stimuli-responsive properties for biomedical applications [66]. Accordingly, the optimization of electrospun fiber scaffold-based DDSs has attracted increasing attention. Therefore, it is believed that a wide variety of electrospun fibers will be developed to support better and multifunctional drug delivery platforms.
Drug Delivery for Tissue Engineering and Cancer Therapy
In recent years, the loading of functional therapeutic agents into electrospun fibers has attracted research attention. Functional therapeutic agents can be classified into (i) small molecular drugs, such as ciprofloxacin [67, 68], doxorubicin (DOX) [69], and ibuprofen [70], (ii) bioactive substances, such as peptides [71], proteins [72], nucleic acids [73], and liposomes [74], and (iii) nanoparticles with therapeutic efficacy, such as Ag nanoparticles [75], mesoporous silica nanoparticles (MSNs) [76], nano-enzymes [77, 78], and bioglass [79, 80].
Small molecular drugs are typically signal transduction inhibitors that can treat diseases by blocking corresponding signaling pathways [81,82,83]. Small molecular drugs are mainly chemically synthesized or derived from natural extracts, and their molecular weights are usually less than 1,000 [84]. They have a wide range of applications due to their low cost, ease of storage and transport, high tissue permeability and minimal immunogenicity [85]. Although small molecular drugs have excellent therapeutic effects, most have poor pharmacokinetics and are easily metabolized into other substances in the body [86]. Therefore, it is essential to effectively deliver small molecular drugs using a suitable platform. Loading small molecular drugs into fibers can significantly overcome the above challenges by improving water solubility and stability, increasing drug concentrations at disease sites, and reducing side effects.
Compared with small molecular drugs, bioactive substances have relatively larger molecular weights, more complex structures, and better water solubility. The immobilization or encapsulation of these bioactive substances onto the surface and into the interior of fibers can overcome limitations associated with systemic administration or local injection. Proteins and growth factors are involved in many physiological processes in the human body. For example, bone morphogenetic protein-2 (BMP-2) and insulin-like growth factor-1 [72, 87] have the ability to promote bone tissue repair, while vascular endothelial growth factor (VEGF) [88], epidermal growth factor (EGF) [89], and nerve growth factor (NGF) [90] can promote skin and nerve tissue repair. Unlike macromolecule proteins, peptides are not specifically absorbed by the reticuloendothelial system or liver, leading to fewer toxic side effects and more mature peptide synthesis technology. Therefore, many types of peptides are widely used in tissue engineering repair, including ε-polylysine peptides with antibacterial properties [91] and peptides for angiogenesis [71], as well as many other types of anti-bacterial peptides. For the delivery of nucleic acids, the key outstanding issue is protecting nucleic acid activity from the surrounding environment [92,93,94,95]; currently, commonly used nucleic acids primarily include plasmid DNA, microRNA, and small interfering RNA, among others [96]. Most bioactive molecules are easily damaged by the external environment due to their chemical instability, relatively short half-lives, and vulnerability, so it is often difficult to effectively encapsulate bioactive molecules in nanofibers with conventional electrospinning technology. Therefore, it is particularly important to develop new technologies to enable the effective encapsulation and delivery of bioactive molecules by nanofibers.
In addition to the above-mentioned drugs, a number of functional nanoparticles have the ability to promote tissue repair and cancer treatments. For example, Ag nanoparticles have excellent antibacterial effects [75], and manganese dioxide nanoparticles can effectively scavenge excess hydrogen peroxide in the body [91]. Similarly, metal–organic framework materials, such as magnesium organic frameworks, can effectively scavenge ROS, slow down the inflammatory response, and promote angiogenesis [97]. Compared with small molecular drugs and bioactive substances, these therapeutic nanoparticles have more stable chemical properties and are easier to load into electrospun fibers. More importantly, they can also act as drug carriers to facilitate the controlled release of drugs from electrospun fibers [76, 98].
Manipulation of Electrospun Fibers to Control Drug Delivery
Strategies for Encapsulating Drugs in Electrospun Fibers
Due to their ECM-like structure, high specific surface area, high porosity, high drug loading capability, and controlled drug delivery function, electrospun fiber-based scaffolds have outstanding advantages for the delivery of functional therapeutic agents [99,100,101,102]. Functional therapeutic agents can be loaded into electrospun fibers by blending electrospinning, second carrier electrospinning, emulsion electrospinning, coaxial electrospinning, electrospraying, physical adsorption, and covalent immobilization. In selecting the fiber matrix for drug loading to achieve a typical drug release profile, the interaction between the drug and the fiber scaffolds should be considered. The composition, molecular weight, hydrophilicity, and degradation rate of the fiber polymer matrix all affect the drug release behavior. In addition, the relative molecular mass, crystallinity and solubility of the drug, and other properties of the drug also affect the release behavior. In addition to hydrogen bonds and electrostatic interactions, covalent bonds can also be used to link the drug with the fibers. Evaluations of the bioactivities of both the drug-loaded scaffolds and the released drug are necessary. Typically, the activities of both the drug-loaded scaffolds and the drug can be evaluated by co-culturing with cells or bacteria to observe the influence of the scaffolds on the adhesion, growth, migration and differentiation of cells or the growth of bacteria, depending on the application direction.
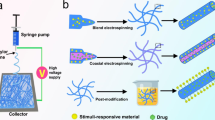
Blend electrospinning is the most straightforward technique for loading drugs into nanofibers (Fig. 3a). Specifically, a number of drugs or functional nanoparticles with chemical stability and organic solvent resistance can be mixed with polymers to form a homogeneous electrospinning solution. Then, micro- or nanofibers loaded with one or multiple drugs can be fabricated by electrospinning [70, 103]. Due to the random distribution of drugs on the fiber surface and inside the fibers, the release process is generally characterized by an initial burst release and subsequent slow release [104]. Since most fibers have high specific surface area and large porosity, the drug is often completely released from the fibers within a few hours or days, incompatible with long-term administration at the tissue. In these cases, polymers that can form electrostatic adsorption interactions with the drugs can be used to delay drug release. It is difficult to induce interactions between some chemical drugs or biologically active molecules and polymers, so new technologies are urgently needed to prolong release time. One solution is to load chemical drugs and bioactive molecules into a secondary carrier, after which the drug-loaded electrospun fibers can be prepared by blending electrospinning. These secondary carriers can be nanoparticles [105, 106], micelles [107], vesicles [108], microspheres and other forms. For example, drug-loaded halloysite clay nanotubes were doped into polycaprolactone (PCL)/gelatin nanofibers, achieving sustained drug release over 20 days, which was greatly extended compared to directly loading the drugs in pristine electrospun fibers [109].
Schematic illustration showing the different fabrication methods of drug-loaded electrospun fibers, including a blend electrospinning, second carrier electrospinning, emulsion electrospinning, b coaxial electrospinning, c electrospinning combined with electrospraying, and d post-processing by physical adsorption and covalent immobilization
Chemically unstable and easily inactivated bioactive factors, such as growth factors, proteins and nucleic acids, function only when they are able to enter cells. Therefore, it is important to avoid contact between bioactive factors and organic solvents, as well as to deliver bioactive molecules successfully to the cellular interior without inactivation [110, 111]. The above-mentioned problems can be solved using emulsion electrospinning technology [112]. In emulsion electrospinning, there is no direct contact between the molecules and the dissolved organic matter, as the bioactive substances are partitioned into the aqueous phase, thus greatly enhancing molecular activity. The loading of drugs into the fiber interior by emulsion electrospinning effectively mitigates the explosive release of drugs at early stages. In addition, emulsion electrospinning enables the simultaneous loading of multiple drugs. Furthermore, emulsion electrospinning can realize the simultaneous loading of multiple drugs and alleviate the problem of explosive drug release in early stages [113]. For instance, emulsion electrospinning was applied to fabricate nanofibers loaded with hydrophobic 10-hydroxycamptothecin (HCPT) in the sheath layer and with hydrophilic tea polyphenols in the core layer [114]. In the initial 4 days, the release of HCPT reached about 61.5%, while the release of tea polyphenols was only about 20.4%. Although emulsion electrospinning has significant advantages, it still has some problems, including poor solution stability and low drug-loading efficiency. In this case, microsol-electrospinning technology can be used to achieve efficient loading and slow release of hydrophilic drugs or easily deactivated biomolecules [115, 116]. For example, when microsol-electrospinning was used to load VEGF in electrospun nanofibers, only 36.8% of VEGF was released in the initial two days, followed by sustained release over 4 weeks [117].
Similar to emulsion electrospinning, coaxial electrospinning can successfully be used to encapsulate bioactive molecules with unstable chemical properties into electrospun fibers (Fig. 3b) [118]. For coaxial electrospinning, a core layer spinning solution composed of biomolecules and a sheath layer spinning solution composed of polymers form two separate jets from the coaxial needle to fabricate core-sheath nanofibers. Compared with commonly used electrospinning methods, coaxial nanofibers are prepared in a way that minimizes interactions between the organic polymer solution and the water-based biomolecules, maintaining the biological activity of unstable biomolecules. Meanwhile, compared with blend electrospinning, coaxial electrospinning can reduce the explosive early-stage release of drugs, realize the simultaneous loading of hydrophilic and hydrophobic drugs, and avoid the biological toxicity caused by late crosslinking of hydrophilic polymers [18, 119]. Since coaxial electrospinning can be used to prepare core-sheath electrospun fibers, it is feasible to load different drugs or bioactive molecules into the core and sheath layers, respectively. The drug in the core layer needs to pass through the sheath layer to be released, slowing its release rate compared to that of the sheath layer [102]. As an example, an in vitro release study showed that the amount of doxorubicin hydrochloride released from the sheath of nanofibers reached 62.2% in the first 200 hours, while the amount of matrix metalloproteinase-2 released from the core layer was only about 50% through 960 hours [120]. To further delay the rate of drug release, the drugs can also be encapsulated into a secondary carrier before preparation of the nanofiber membrane by coaxial electrospinning [121].
Electrospray technology can integrate nanoparticles loaded with bioactive molecules or drugs into and/or onto fibers (Fig. 3c) [122]. For electrospraying, it allows the deposition of particles loaded with bioactive molecules or drugs on the fibers. It can not only encapsulate drugs with bioactive molecules, microspheres, and micelles to protect their activity and prolong drug release but can also allow the design of on-demand DDSs responsive to external stimuli. Compared with the passive release of drugs from other electrospun fibers, fibers that enable spatiotemporally controlled drug release can be prepared by integrating electrospinning and electrospraying technologies. For example, collagen particles loaded with neurotrophin-3 (NT-3) can be sandwiched between two nanofiber layers using electrospray technology, realizing the sustained and controllable release of NT-3 [123]. In addition, the combination of masked electrospray technology and electrospinning can achieve a gradient distribution of biomacromolecular particles on fibers [124].
In addition to the above methods, a number of drugs can also be loaded onto fibers by physical adsorption (Fig. 3d) [125, 126]. Especially for certain biomolecules, physical adsorption is not only the simplest way to load biomolecules into fibers but can also effectively maintain the activity of the biomaterials. Although this method of drug loading is relatively simple, the drugs cannot be released in a sustained manner [127]. In particular, drugs and bioactive molecules that do not make electrostatic interactions with nanofibers are difficult to load by this method [15]. For example, recombinant human BMP-2 was adsorbed on the surface of poly(D,L-lactide-co-glycolide)/hydroxylapatite composite nanofibers by the physical adsorption method [128]. The in vitro BMP-2 release profile showed that 75% of BMP-2 was released within the first 5 days. In addition, layer-by-layer self-assembly (LBL) is another common physical adsorption method for drug loading onto fibers based on the alternating adsorption of polyelectrolytes on the matrix through electrostatic interactions, hydrogen bonds or other interactions. For example, positively charged chitosan and negatively charged type I collagen can be assembled onto electrospun silk fibroin fiber membrane by LBL technology for scar-free wound repair [129].
In addition to physical absorption, drugs or biomacromolecules that can react with functional groups on the fiber surface can be bound to nanofibers by covalent immobilization [125, 129]. Such drugs and biomacromolecules can also be released in vivo by endogenous stimuli. For example, a polypeptide containing a carboxyl group reacted with the amino group on the surface of chitosan hydrogel nanofibers to form an amide bond; thus, the polypeptide was successfully loaded onto the nanofibers [71].
At present, the development of a multi-functional electrospinning platform is conducive to the delivery of multiple drugs. Sequential electrospinning is a technique used to construct multilayer nanofibers, and a variety of drugs can be loaded into the different nanofiber layers, so as to control the release rates of different drugs [130]. For example, sequential electrospinning was used to prepare a three-layer nanofiber scaffold in which the inner and middle layers were loaded with microRNA-126 and microRNA-145, respectively, leading to the sequential release of microRNA-126 followed by microRNA-145 [131].
Stimuli-Responsive Drug Delivery Systems
Electrospun fiber platforms incorporating stimuli-response are emerging as a major driving force in the development of smart drug delivery [132]. Just like many important functions in the human body are achieved in a site-specific and time-controlled manner, the responses to intrinsic endogenous and external stimuli provide more possibilities for the development of new drug delivery strategies [29, 39]. To this end, it can be realized to significantly improve the selectivity and targeting of drugs, deliver appropriate drug concentrations to the target site at a specific time, effectively reduce side effects, and meet the requirements of tissue regeneration and cancer treatment. Herein, typical endogenous and external stimuli are summarized, and their potential to deliver precise amounts of drugs in a spatiotemporally controllable manner is also described.
Endogenous Stimuli-Responsive Drug Delivery Systems
Differences in pH, enzyme expression, ROS levels, and glucose content in pathological environments compared to normal physiology can provide some ideas for the development of smart drug delivery platforms. Indeed, various types of nanofiber delivery systems responsive to endogenous stimuli have been developed based on microenvironmental changes in cells or tissues [68, 133].
By selecting the appropriate type of polymer and post-processing method, electrospun fibers can be endowed with pH-responsive drug release characteristics [134, 135]. pH-sensitive polymeric nanofibers change their own volume in response to external pH changes, enabling intelligent and responsive drug delivery [136]. Some nanofiber membranes contain chemical groups that are sensitive to hydrogen and hydroxide ions, enabling the controlled release of drugs by changing intermolecular forces of the polymers when external pH changes. For instance, under acidic conditions, the amino and acetyl amino groups of chitosan undergo a protonation reaction to form an amine cation [137]. Swelling of the nanofiber membrane is increased due to mutual repulsion between ammonia cations and hydrogen ions. Thus, the interaction forces between allicin and chitosan or polyvinyl alcohol (PVA) are weakened, accelerating the release of allicin from the fibrous membrane into the surrounding environment (Fig. 4a). However, in alkaline environments, interactions between hydroxide ions, chitosan and PVA are not obvious, reducing the degree of fiber membrane swelling. In addition to fiber swelling, pH-responsive drug release can be triggered by external pH change through chemical bond breakage. For example, IL-4-loaded liposomes containing aldehyde groups were grafted onto the surface of fibers containing amino groups via Schiff base reactions (Fig. 4b) [138]. In acidic environments, these chemical bonds were broken due to hydrolysis reactions, and the liposomes were released from the nanofibers. The in vitro release profile showed that the release rate of liposomes was significantly faster at pH = 5.8 than at either pH = 7.4 or pH = 6.6.
Endogenous stimuli-responsive drug delivery fiber systems. a Schematic illustration and SEM image showing the microstructure of CS/PVA/GO/Alli fiber mat, and the release of Alli from the fiber mat at different pH values. Reproduced with permission from Ref. [137]; Copyright 2020, Elsevier Limited. b Schematic illustration showing the pH-responsive release of liposomes from the surface of a nanofiber, and the release curves of liposomes from the electrospun fiber mat at different pH values. Reproduced with permission from Ref. [138]; Copyright 2020, Springer Nature Limited. c Schematic illustration showing the degradation-triggered release of esterase-sensitive prodrug from electrospun fiber mat followed by the enzyme-triggered release of IBU from the prodrug, and the release curve of IBU from the different types of scaffolds in the presence or absence of enzyme. Reproduced with permission from Ref. [143]; Copyright 2015, Elsevier Limited. d Schematic illustration showing that the thioketal linkers in polyurethane containing thioketal (PUTK) can be cleaved in response to ROS, and the cumulatively released percentage of MP in vitro from the electrospun fiber patch in PBS solution (pH = 7.4) and 1 mM H2O2 solution at 37 °C, respectively. Reproduced with permission from Ref. [31]; Copyright 2020, Elsevier Limited
In addition to the above methods for preparing pH-responsive nanofibers, a number of pH-responsive nanomaterials, such as liposomes, micelles and others, can also be encapsulated into nanofibers to realize controllable and responsive drug release. For example, electrospun PVA nanofibers were loaded with a reduction-responsive Pt (IV) prodrug micelle and dichloroacetate [139]. Simulation of the cancer cell state with s acetate buffer solution and sodium ascorbate better triggered the release of Pt (II), and levels of cleaved Pt rapidly accumulated to 50% within 24 h.
Enzymes play important roles in different biological processes and usually have high specificity, with various species of enzymes distributed across different tissues at specific concentrations. Therefore, in response to abnormal concentrations of enzymes, enzyme-responsive DDSs provide a way to increase selectivity and sensitivity [140]. In inflammatory locations and tumor tissues, some specific enzymes are significantly different from those in normal tissues, so it is feasible to exploit this feature to enable enzyme-responsive controlled drug release [141, 142]. For example, an esterase-sensitive prodrug was loaded in electrospun nanofibers to realize enzyme-triggered release of ibuprofen, an anti-inflammatory drug [143]. As shown in Fig. 4c, in the presence of lipase, nanofibers loaded with prodrug-of-ibuprofen exhibited enzyme-triggered drug release, and the cumulative release of ibuprofen reached 100% within 14 weeks; in contrast, only a small amount of drug was released in the absence of the lipase enzyme.
ROS can regulate intracellular biological behaviors as signaling molecules [144, 145]. However, excessive ROS production usually causes severe oxidative damage to cells and tissues. At present, due to the high concentrations of ROS in pathological environments, a variety of ROS-responsive DDSs have been developed [146, 147]. As shown in Fig. 4d, ROS-responsive nanofibers can be prepared by electrospinning a biodegradable elastomer containing thioketone [31]. Nanofibers loaded with glucocorticoid methylprednisolone (MP) were incubated in 1 mM H2O2 solution for 2 weeks, and the release of MP was significantly higher than that of any other groups.
Since hyperglycemia patients have excess blood glucose in their plasma, a glucose-responsive DDS can be established based on a gradient in blood glucose levels [148]. At present, many researchers have realized the release of insulin triggered by hyperglycemia and have applied glucose oxidase to reduce the pH or oxygen content in hyperglycemic regions through an enzymatic reaction, thereby promoting insulin release [149, 150]. These glucose-responsive nanofibers are mostly used for monitoring blood glucose. By encapsulating glucose oxidase or glucose dehydrogenase into nanofiber scaffolds, blood glucose levels can be quickly and sensitively monitored [28]. Obviously, a system responsive only to an individual type of endogenous stimulus is unable to meet current needs. Therefore, to deliver therapeutic agents to the right place at the right time in physiologically relevant doses, it is particularly critical to develop endogenous stimuli-responsive nanofiber scaffolds that can respond synergistically to multiple signals.
External Stimuli-Responsive Drug Delivery Systems
External stimuli, such as heat, light, electricity, magnetic fields, and ultrasound, have attracted much attention due to their non-invasive nature, high tissue penetration depth, and spatiotemporal controllability [38]. All these strategies can be combined with electrospun drug-loaded scaffolds to enable stimuli response and synchronize drug release profiles under real physiological conditions by manipulating the external environment, providing new avenues for tissue regeneration and cancer therapy.
Temperature controls almost all physical, chemical, and biological reactions, in addition to being critical regulatory parameter for the human body. Temperature-responsive materials can enable the controlled release of drugs by modulating the critical solution temperature (LCST) of thermosensitive polymers through volumetric phase transitions. As shown in Fig. 5a, a mixture of PCL and temperature stimuli-responsive nanogel was used to form the outer shell [151]. The nanogel was composed of temperature-responsive poly(N-isopropylacrylamide) copolymerized with acrylic acid, which could shrink or expand with ambient temperature changes. Therefore, the existence or disappearance of nanochannels between the nanogel and PCL could be controlled by varying the temperature. In this case, the drug-encapsulating shell acted as a valve to control ordered drug release. Three-cycle low-to-high temperature transition images of drug release demonstrated better temperature-responsive drug release properties when nanogels were encapsulated in the shell compared to when nanogels were omitted.
External stimuli-responsive drug delivery fiber systems. a Schematic illustration showing the mechanism of thermal switch-controlled drug release system, and the release rate of drug upon multiple cycles of low-to-high temperature transitions. Reproduced with permission from Ref. [151]; Copyright 2015, Wiley–VCH Limited. b Schematic illustration showing the thermal-responsive release of fluorescein from nanofibers containing gold nanorods upon NIR irradiation, and the release curves with NIR irradiation at different power densities. Reproduced with permission from Ref. [153]; Copyright 2021, Multidisciplinary Digital Publishing Institute Limited. c Schematic illustration showing the temperature-responsive release of DOX, and the cumulatively released percentages of DOX with alternating cycles of “ON–OFF” switching of AMF. Reproduced with permission from Ref. [156]; Copyright 2013, Wiley–VCH Limited. d Schematic illustration showing the electrical-responsive release of DEX from PLGA nanofibers, and the cumulative mass release of DEX under the control of electrical stimulation. Reproduced with permission from Ref. [162]; Copyright 2006, Wiley–VCH Limited. e Schematic illustration showing the multimodal-responsive release of DEX from piezoelectric nanofibers by breaking the silica (SiO2) capsules under the action of ultrasound, the images showing the situation after 60 s of sonication. Reproduced with permission from Ref. [32]; Copyright 2018, American Chemical Society Limited. f Schematic illustration of synergistic sono-photodynamic therapy for breast cancer via 808 nm laser and 1 MHz ultrasound, as well as the live/dead staining images. Reproduced with permission from Ref. [166]; Copyright 2020, American Chemical Society Limited
Considering the advantages of long-range and stronger penetration, near-infrared (NIR) light has been increasingly adopted as a light source in drug release-assisted tissue regeneration [152]. By introducing photothermal agents, electrospun fibers can be endowed with excellent photothermal properties, enabling the effecting delivery of nutrients and drugs [52]. Gold-based nanorods (GNRs) can also generate heat through the plasmonic resonance effect under NIR irradiation. As shown in Fig. 5b, GNRs-loaded poly(N-isopropylacrylamide) (PNIPAM) composite nanofibers were used to allow the controlled release of drugs by NIR irradiation [153]. The heat generated by the GNRs ensured the shrinkage of thermally responsive PNIPAM nanofibers to allow for the drug release, and this on-demand DDS could be regulated by the NIR power density. This convenient, remote-controllable, non-invasive approach provides new ideas for the on-demand delivery of required doses of drugs.
Magnetic fields, electric fields, and ultrasound are also research foci due to their relevance to corresponding stimuli and ease of operation. For magnetic fields, superparamagnetic iron oxide nanoparticles (IONPs) have been applied for osteogenic differentiation and axon extension [154, 155]. The hyperthermia caused by IONPs under magnetic field is also beneficial for reducing drug transmission loss and enhancing targeted delivery [37]. In one study, a nanofiber scaffold composed of temperature-responsive polymers, magnetic nanoparticles (MNPs), and an anticancer drug (DOX) was designed (Fig. 5c) [156]. The MNPs generated heat under an alternating magnetic field (AMF), which dissociated the polymer network in the nanofibers and allowed the release of DOX. By switching the “on–off” properties of the magnetic field, the drug could be delivered on demand. The generated heat and chemotherapeutic effects of the released DOX rapidly induced cancer cell apoptosis.
External electrical stimulation has also been used for bone repair [157], nerve regeneration [158], and drug delivery [159]. For example, electrical stimulation can modulate curcumin (CUR) delivery through volume changes induced by the voltammetric response of PEDOT nanoparticles [160]. A PPy-PVDF electrospun system was used as a carrier to load growth factor complexed with streptavidin, and the release curve of the growth factor showed an obvious electro-sensitive release behavior [161]. As shown in Fig. 5d, dexamethasone (DEX) was released from the PEDOT nanotubes by controlling the contraction or expansion of PEDOT by electrical stimulation [162]. The blue curve represents the cumulative mass release of DEX from PEDOT-encapsulated poly (lactic-co-glycolic acid) (PLGA) nanofibers when 1 V electrical stimulation was applied at five specific times.
As for ultrasound, it usually provides a sustained thermal effect from continuous oscillation of microbubbles and a mechanical effect upon rupture [163]. Ultrasound has been shown to trigger the release of drugs, as well as promote deep drug penetration with minimal thermal damage to surrounding tissues [164]. By encapsulating drugs into ultrasound-sensitive microcapsules, scaffolds can be effectively combined for multimodal triggered release [32]. Figure 5e shows that silica microcapsules in the fibers were destroyed, and TRITC-BSA was effectively released under the stimulation of ultrasonic waves. This approach can be extended to both exogenous (NIR irradiation, electrical stimulation) and endogenous (enzymatic treatment) stimuli to improve the precise delivery of multiple drugs.
Recently, attention has been drawn to the idea of applying multiple stimuli synergistically to deliver drugs. For example, a smart hyperthermic nanofiber has been developed with the ability to simultaneously switch two-stage drug release in response to AMF and heat [34]. In addition to their own effects on cell behavior and tissue regeneration, some related therapies, such as photothermal therapy, magnetothermal therapy, electromagnetic thermotherapy, and sonodynamic therapy, have also been derived from these strategies and show to have synergistic effects with drugs [165]. As shown in Fig. 5f, the synergistic sono-photodynamic therapy significantly promoted the generation of ROS and achieved a 95.8% inactivation rate of breast cancer cells under 808 nm NIR irradiation and 1 MHz ultrasound treatment [166]. These potential integrative mechanisms should be incorporated into drug-loaded electrospun fiber scaffolds to facilitate the development of future nanomedicines and promote tissue regeneration and cancer therapy.
Applications for Tissue Regeneration and Cancer Therapy
Electrospun fibers for DDSs have been developed and explored based on the diversity and simplicity of the preparation methods for drug-loaded electrospun fiber scaffolds, as well as the design of fiber structures, the selection of electrospinning parameters, post-treatment methods, and the combination of various stimuli. These products have been widely applied to tissue regeneration, including soft tissues (such as skin, nerve, cardiac, and blood vessels) and hard tissues (such as bone, cartilage, and musculoskeletal and dental systems), as well as cancer therapy.
Skin Tissue Engineering
Skin regeneration and wound healing are dynamic and complex processes that usually include four overlapping and different periods: hemostasis, inflammation, proliferation, and remodeling [167]. To promote wound repair, functional drug-loaded fibrous scaffolds can be prepared through electrospinning technology, which can effectively avoid wound infection, shorten the inflammatory stage, promote tissue proliferation and remodeling, and prevent granulation tissue proliferation and scar formation.
Bacterial infection is an inevitable and urgent problem during wound healing [168, 169]. Therefore, many researchers have loaded antimicrobial agents or nanoparticles into nanofibers by blending electrospinning technology to improve antibacterial function [170, 171]. For example, tetracycline hydrochloride has been loaded into poly (ω-pentadecalactone-co-ε-caprolactone)/gelatin/chitosan nanofibers to achieve excellent antibacterial effects against gram-positive and gram-negative bacteria [172]. However, given the increasing bacterial resistance and the burst release of drugs, it remains a great challenge to achieve sustained and efficient antibacterial activity at the wound site using the aforementioned methods [173]. In addition to exploring and synthesizing new types of alternative antimicrobial agents, antimicrobial peptides have also been incorporated in fibers to achieve deep bactericidal effects [174]. For instance, a Janus-type antibacterial dressing loaded with antimicrobial peptides was prepared by combining electrospinning nanofiber membranes with dissolvable microneedle arrays [175]. This antibacterial dressing could penetrate bacterial biofilms to effectively kill bacteria. To further enhance the antibacterial effect of these materials, as well as to achieve the controlled release of drugs, some drug-loaded fiber platforms have been explored with respect to external stimuli [176]. The obtained nanocomposite fiber scaffolds exhibited excellent NIR light-triggered controlled drug release behavior. As shown in Fig. 6a, the dressing caused irreversible damage to bacterial biofilms under NIR irradiation, thus effectively inhibiting infection by drug-resistant bacteria.
Drug delivery systems based on electrospun fiber scaffolds for skin tissue engineering. a Illustration of dual stimuli-responsive fibrous membranes for drug-resistant bacterial infection, and SEM images of E. coli and MRSA incubated with or without NIR irradiation. Scale bar = 5 μm. Reproduced with permission from Ref. [176]; Copyright 2022, Elsevier Limited. b Illustration and macroscopic images of the different samples after in vivo hemostasis in an ear artery injury model and a liver trauma model of rabbits. Reproduced with permission from Ref. [177]; Copyright 2021, Wiley–VCH Limited. c Schematic illustration of PCL nanofibers loaded with dimethyloxalylglycine can significantly promote angiogenesis and re-epithelialization, and expression levels of IL-6 and IL-4 were detected in macrophages cultured for 2 days. Reproduced with permission from Ref. [184]; Copyright 2017, American Chemical Society Limited. d Schematic illustration of H&E, Masson’s and CD31 immunohistochemical staining images of different groups of wound areas at 14 days. The black arrow indicates the blood vessel, the semi-black arrow indicates the keratinous basal cells, and the dotted circle shows the epithelial spike. Reproduced with permission from Ref. [185]; Copyright 2019, American Chemical Society Limited. e Schematic diagram of the synthesis of careob-like 5-Fu@dMBG/PEO@PEEUU nanofibers (((F@B)/P)@PU) and the quantitative analysis of relative occupied area of collagen I at post-surgery. Reproduced with permission from Ref. [189]; Copyright 2022, Elsevier Limited. f Schematic of the fabrication of on-skin electronic devices and temperature-sensitive on-demand drug release, the release profiles of MOX, and photographs of agar plates onto which S. aureus suspensions. Reproduced with permission from Ref. [196]; Copyright 2019, Wiley–VCH Limited
Hemostasis is a critical period in the wound healing process. Although the body has an inherent hemostatic system, it cannot stop bleeding quickly [43]. Therefore, many hemostatic agents have been incorporated into hemostatic dressings by electrospinning technology, and this approach has attracted wide attention. For example, an ultralight 3D gelatin sponge prepared by conjugate electrospinning technology was able to aggregate a large number of activated platelets and accelerate the formation of platelet clots [177]. An in vivo study showed that this gelatin nanofiber sponge could rapidly induce stable blood clots in a rabbit ear model of artery injury and was associated with reduced bleeding compared to gelatin nanofiber membrane (Fig. 6b).
Sustained inflammation also seriously postpones wound healing [178,179,180]. Especially in chronic wounds, high ROS levels often result in a failure of wound healing. Therefore, removing excessive ROS can reduce oxidative stress to effectively promote collagen deposition and ECM remodeling [181]. For example, poly (l-lactide-co-caprolactone)/gelatin core-sheath nanofibers loaded with epigallocatechin-3-O-gallate (EGCG) exhibited excellent ROS-scavenging ability, promoting skin regeneration and inhibiting subsequent wound infection [180].
In addition to excessive ROS, high expression of various pro-inflammatory chemokines, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), which are secreted by neutrophils and macrophages, can also severely delay wound healing [182]. It has been shown that anti-inflammatory drugs released at a wound can effectively down-regulate expression of IL-6 and TNF-α [183]. At the same time, this approach can reduce inflammatory response at the wound, promote fibroblast proliferation, and accelerate the reconstruction of granulation tissue. For example, PCL nanofibers loaded with dimethyloxalylglycine can significantly promote angiogenesis and improve the re-epithelialization ratio [184]. Meanwhile, at the molecular level, this approach promoted wound healing by enhancing the expression of anti-inflammatory factors (IL-4) and reducing the expression of pro-inflammatory factors (IL-6) (Fig. 6c).
Tissue regeneration and remodeling involve angiogenesis, granulation tissue formation and re-epithelialization [167]. During the process of wound healing, angiogenesis is beneficial for the continuous delivery of oxygen and nutrients to the wound. As shown in Fig. 6d, an oriented, aligned PCL nanofiber membrane loaded with tazarotene promoted angiogenesis and significantly accelerated wound healing and re-epithelialization ratio [185]. In addition, various types of growth factors, peptides, and RNA can be delivered from electrospun nanofibers to promote angiogenesis [71, 74]. During the tissue regeneration stage, loading growth factors into nanofibers is an effective way to improve the wound healing rate. For example, PCL/PEG core–shell nanofibers loaded with EGF and basic fibroblast growth factor can significantly promote fibroblast proliferation and enhance collagen deposition and keratin synthesis [186].
If a wound is not treated properly, scar formation is very likely. Scar formation is primarily due to excessive inflammation, myofibroblast proliferation, and over-deposition of collagen [187]. Loading of nanofibers with scar inhibitors can effectively inhibit the formation of scars. At present, common scar inhibitors include TGF-β inhibitor [188], 5-fluorouracil [189], α-lactalbumin [190], 20(R)-ginsenoside Rg3 [191], palmatine, and triamcinolone acetonide [192, 193]. Typically, 5-fluorouracil (5-Fu)-loaded dendritic mesoporous bioglass nanoparticles (dMBG) are loaded in electrospun nanofibers by coaxial electrospinning, and the obtained scaffolds can significantly promote wound healing and inhibit scar formation (Fig. 6e) [189].
Currently, another challenge for wound dressings is that it is difficult to monitor the real-time state of wound repair while simultaneously meeting the needs of wound healing treatment [194]. With the emerging development of bioelectronics, many integrated electronic dressings have been developed to integrate diagnosis, monitoring, and treatment [195]. These techniques also allow the monitoring of wound status and on-demand controlled drug delivery based on changes in the wound microenvironment. As shown in Fig. 6f, a flexible and breathable thermal-responsive nanofiber membrane can monitor the temperature of wound tissue in real time and trigger the on-demand release of antibiotics from the fibers according to temperature changes [196].
Nerve Tissue Engineering
Injuries to the nervous system, including both the peripheral nervous system and the central nervous system, often lead to nerve cell death and tissue destruction, resulting in permanent loss of nerve function [197]. Although recent developments are promising, it nevertheless remains a great challenge to treat nerve injuries using tissue engineering scaffolds.
For peripheral nerve repair, nerve guidance conduits (NGCs) constructed from electrospun fibers are considered to be optimal nerve graft substitutes because of their excellent biocompatibility, tunable mechanical properties, porosity, and capacity to provide guidance cues [64]. Unmodified fiber-based NGCs often fail to overcome the barriers of limited regenerative capacity and disordered axonal growth, especially when used to repair thick nerves with large gaps [198]. To this end, integrating NGCs with topographic cues [48, 197] and biological signals [123, 124, 199, 200] is often done to overcome these barriers. One current potential strategy is to create nerve conduits based on topographical cues in combination with drugs, with different drug loading modes controlling drug release [3, 16]. For example, drugs physically attached to a scaffold usually have faster release rates, while drugs embedded in microspheres or fibers are hindered by complex cross-linking networks [201, 202].
Typically, gradient structures can provide chemotactic or haptotactic cues for accelerating cell migration and neurite extension. As shown in Fig. 7a, a concentration gradient of active functional groups was first generated on nanofiber surface, after which an NGF density gradient was successfully constructed based on the amphiphilic nature of heparin, ultimately promoting the directional outgrowth of neurites from DRG along the direction of increasing NGF concentration [200]. In addition to the adsorption or immobilization of growth factor on the fiber surface, bioactive particles have also been deposited on fibers. Figure 7b shows the application of a masked electrospray method to construct a density gradient of biomacromolecular nanoparticles on the surface of uniaxially aligned fibers by manipulating the deposition period with a movable physical mask [124]. The aligned fibers could guide neurite extension along the fiber alignment, while the density gradient of biological macromolecules further promoted directional extension of neurites along the direction of increasing particle density.
Drug delivery systems based on electrospun fiber scaffolds for nerve tissue regeneration. a Schematic illustration showing the construction of concentration gradient of NGF on aligned fibers by amino and heparin functionalization, and fluorescence micrographs showing the extension of neurites from DRGs on the uniform and gradient scaffolds. Scale bar = 500 μm. Reproduced with permission from Ref. [200]; Copyright 2020, Wiley–VCH Limited. b Schematic illustration showing the generation of density gradient of biomolecular nanoparticles on the surface of uniaxially aligned electrospun fibers using masked electrospray method, and fluorescence micrographs showing the extension of neurites from DRGs on the uniform and gradient scaffolds. The neurites were stained with Tuj1 (green). Scale bar = 500 μm. Reproduced with permission from Ref. [124]; Copyright 2020, Wiley–VCH Limited. c Schematic of external stimulation device, SEM images showing the morphology of pristine fibers and SPION-grafted fibers, and fluorescence micrographs showing the extension of neurites from DRGs on the blank and SPION-grafted scaffolds. The neurites were stained with neurofilament (green). Scale bar = 500 μm. Reproduced with permission from Ref. [206]; Copyright 2021, Elsevier Limited. d Fluorescence micrographs showing the extension of neurites from PC12 cells on the scaffolds without/with electrical stimulation, and the chart showing the promoting effect of electrical stimulation on neurite growth. Scale bar = 50 μm. Reproduced with permission from Ref. [158]; Copyright 2014, The Royal Society of Chemistry Limited. e Schematic illustration of simultaneous loading of NGF and VEGF in the scaffold, the chart showing the different diffusion rate of different growth factors, and both of SFI value and Tissue section staining images showing the scaffold with NGF and VEGF has a good ability to promote repair. Scale bar = 25 μm. Reproduced with permission from Ref. [207]; Copyright 2018, Elsevier Limited. f Schematic illustration of scaffold loaded with MP and polysialic acid (PSA) implanted in the model of spinal cord injury in mice, and both of BBB score and Tissue section staining images showing the scaffold has the abilities to inhibit inflammation and promote spinal regeneration. Scale bar = 100 μm. Reproduced with permission from Ref. [217]; Copyright 2018, Elsevier Limited. g Schematic illustration of scaffold containing LysoGM1 implanted in traumatic brain injury model, and the scaffold have abilities to promote cell migration and differentiation. Tissue section staining image also present the enhancement of nerve regeneration. Scale bar = 500 μm. Reproduced with permission from Ref. [220]; Copyright 2020, American Chemical Society Limited
Another therapeutic approach is to combine external stimuli, such as light [203], electricity [204, 205], or magnetic field [206], to regulate cell behavior and induce tissue regeneration. Under the action of AMF, superparamagnetic iron oxide nanoparticles could be uniformly distributed in fibers, and the fabricated hybrid fibers could respond to a magnetic field and promote neurite extension (Fig. 7c) [206]. In addition, as electrically active tissues, neurite extension can be promoted by applying electrical stimulation at an appropriate intensity. For example, electrically conductive electrospun fibers can be loaded with NGF and combined with electrical stimulation to further accelerate the extension of neurites from PC12 cells along the direction of the electrical field (Fig. 7d) [158].
For peripheral nerve repair, some researchers rely on different drug release rates to design NGCs. As shown in Fig. 7e, core-sheath fibers loaded with two growth factors were prepared by coaxial electrospinning [207]. The fast release of VEGF from the sheath layer promotes the migration, proliferation, and differentiation of endothelial cells, while the slow release of NGF promotes long-term axonal elongation. With the release of VEGF, intraneural vascularization, an important prerequisite for nerve regeneration [208], could be achieved. More importantly, the generated blood vessels could provide guidance for cell migration and transport oxygen and nutrients to axons and Schwann cells [60, 209], which is particularly significant for the repair of thick nerve defects. Polymer microspheres [210] and inorganic nanoparticles [211] can also serve as carriers of neurotrophic factors and be incorporated with electrospun fibers to regulate conduit delivery behavior.
Furthermore, hydrogels can encapsulate a variety of bioactive substances, and their degradability can be varied by regulating the degree of cross-linking [2, 212]. In addition, hydrogels can simulate the ECM of natural tissues and generate a 3D microenvironment conducive to nerve tissue regeneration [213]. Therefore, the development of electrospun fiber-hydrogel drug loading systems has significant potential. Multiple small conduits can be sequentially embedded within a larger conduit to simulate the multi-bundle structure of human nerves, effectively reducing side effects associated with disordered axon growth [61]; furthermore, this type of NGC can be combined with drug loaded-hydrogels to additionally promote nerve repair. Distinct properties can be introduced into each lumen to optimize conduit performance, so this highly bionic structure will be ideal for nerve tissue regeneration.
Distinct from peripheral nerves, neurons of the central nervous system (CNS) can hardly regenerate axons due to the harsh microenvironment after CNS injury [138, 214]; therefore, remodeling the microenvironment can support CNS regeneration [215]. Furthermore, more endogenous cells should be promoted to migrate, infiltrate into the damaged area and differentiate into neurons to rebuild the damaged neural circuit network [216]. The main approach is to release anti-inflammatory drugs to reduce inflammation and regulate the acidic microenvironment. MP, a strong anti-inflammatory drug, can be loaded on the electrospun fiber scaffold with polysialic acid to promote axonal regeneration [217]. As shown in Fig. 7f, MP can effectively inhibit inflammatory reactions and glial cell proliferation while ensuring axon growth. In contrast to direct release, anti-inflammatory drugs can be loaded in liposomes and grafted onto scaffolds by chemical bonds that can be broken in response to the acidic pH of the inflammatory environment [138], thereby reducing the risk to benign areas. In addition, proteoglycans in the ECM of neurons condense around neurons to prevent the influence of harmful substances. However, proteoglycan condensation becomes a physical barrier to nerve recovery after spinal cord injury, so the addition of proteases could decompose these proteoglycans and break down this barrier [214]. Thus, protease- and neurotrophic factor-based fiber scaffolds can build renewable bridges in spinal cord defects. In summary, electrospun fiber scaffolds for the repair of spinal cord injury require a combination of multiple optimization factors to regulate the microenvironment and promote nerve regeneration [218].
Brain tissue is a complex nerve tissue, and common brain diseases include traumatic brain injury [219, 220], stroke [221] and others. In the repair of brain injury, it is also important to promote endogenous cells to migrate toward the damaged area and differentiate into neurons to rebuild the damaged nervous system [216]. Neurotrophic factors play an important role in the protection and migration of nerve cells. However, they cannot be delivered to injured sites because of their lack of permeability through the blood–brain barrier (BBB) [222, 223]. Engineering bioactive electrospun fiber scaffolds can enable the treatment and repair of brain diseases. Monosialotetrahexosylganglioside (LysoGM1) is one kind of drug that can protect neurons and promote nerve regeneration [224]. By chemically grafting LysoGM1 onto fiber scaffolds, its biological activity can be maintained, and the diffusion effect could be weakened to some extent [220]. As shown in Fig. 7g, the scaffold could continuously deliver drugs to the injured area in a traumatic brain injury model, contributing to a reduction in the number of astrocytes and good regeneration of nerve tissue. Meanwhile, neurodegenerative diseases, including Alzheimer's disease [225] and Parkinson's disease [226], are common mental disorders caused mainly by the decreased ability of neurons and glial cells to secrete nutritional factors. Therefore, the ability of electrospun fibers to deliver nutritional factors to brain tissue is highlighted again, and the release profile of factors is more suitable than that associated with current clinical drug administration methods; thus, the frequency of drug administration could be reduced [227]. Of note, electrospun fibers also play an important role in detecting diseases, including potential diagnosis of neurodegenerative diseases through a variety of physiological indicators [228, 229]. For instance, coupling dopamine receptors to electrospun fibers can enable the detection of neurodegenerative disorders with high sensitivity and rapid responsiveness [229].
Great progress has been achieved in applying drug-loaded electrospun fiber scaffolds to promote nerve regeneration, but some side effects remain due to fast drug diffusion, resulting in high local drug concentrations. In addition, short drug half-lives also present a major challenge for nerve repair [209]. Therefore, the long-term maintenance of drug activity in vivo and precise response to microenvironmental changes at various stages remain the key foci of follow-up research. Moreover, axonal myelin formation is another key factor in functional recovery, and ways to regulate the phenotype of Schwann cells should be included in future scaffold design [230]. In addition, scaffold neuroimaging will have great potential in future applications, as it is indisputable that non-invasive imaging helps monitor nerve regeneration and enables real-time adjustments based on individual regeneration differences.
Cardiac Tissue Engineering
Due to the limited regenerative capacity of cardiac tissue, it is difficult for ischemic myocardial tissue to repair itself after myocardial infarction (MI) [231]. Although heart transplantation is the most effective way to restore cardiac function, the shortage of donor organs and side effects after transplantation seriously limit its application [232]. Electrospun nanofibers can mimic the natural ECM structure, provide temporary physical support for damaged heart tissue, and limit ventricular dilatation and remodeling [233]. Therefore, it is possible to use nanofibers for cardiac tissue engineering because of their controllable fiber structure and capacity to improve the retention rate of bioactive substances.
During the necrotic phase after MI, a large number of cardiomyocytes necrotize while releasing excessive ROS and other cellular contents into the surrounding microenvironment [234, 235]. As a result, many immune cells are recruited to the damaged cardiac tissue. After MI, this inflammatory environment disrupts cell homeostasis, leading to more severe oxidative damage. Therefore, to avoid the occurrence of inflammation, scavenging of excessive ROS can effectively inhibit pathological remodeling of the left ventricle. For example, MP-loaded polyurethane fiber patches can release anti-inflammatory drugs to remove excess ROS [147]. As shown in Fig. 8a, fiber patches containing MP could significantly promote cardiac functional repair and angiogenesis while reducing fibrosis and cardiac remodeling. In addition to the local delivery of anti-inflammatory drugs by nanofibers to reduce inflammation, many anti-inflammatory nanoparticles can be loaded into nanofibers to effectively remove excessive ROS and relieve inflammation. For example, a cerium oxide nanoparticles-loaded PCL/gelatin nanofiber scaffold can significantly reduce ROS levels in the MI area and inhibit cardiomyocyte hypertrophy [236].
Drug delivery systems based on electrospun fiber scaffolds for cardiac tissue engineering. a Schematic illustration showing the application of a methylprednisolone (MP)-loaded PUTK fiber patch to suppress inflammation, and Masson and Sirius red staining shows the pathological examination of the hearts. Reproduced with permission from Ref. [31]; Copyright 2020, Elsevier Limited. b Schematic illustration showing the application of chitosan/silk fibroin-modified nanofiber patch seeded with mesenchymal stem cells for preventing heart remodeling post-MI in rats. Reproduced with permission from Ref. [244]; Copyright 2018, Elsevier Limited. c Schematic and cross-sectional SEM images illustrating the structure of the rGO/silk fibroin scaffolds, from the bottom to the top, consisting of a randomly arranged layer, randomly oriented layer and oriented layer, the thickness between different layers, and the conductive anisotropy that can be transmitted through the patch through implantation were used to reconstruct the anisotropic electrical microenvironment of the infarcted myocardium. Reproduced with permission from Ref. [248]; Copyright 2022, Elsevier Limited. d Schematic illustration of using computational methods to design patient-specific electrospun fiber-based cardiac patches for pediatric heart failure, representative images of patches attached to the RV in tissue sections collected after 4 weeks following implantation, and quantification of vessel density and myocyte hypertrophy. Scar bar = 200 μm. Reproduced with permission from Ref. [256]; Copyright 2022, Elsevier Limited
After MI, the hypoxic state is highly susceptible to oxidative stress and irreversible cardiomyocyte death, so the restoration of oxygen supply is extremely important [237,238,239]. Therefore, a bilayer cardiac patch loaded with calcium peroxide and adipose stem cell exosomes was fabricated to enable continuous oxygen supply, alleviate oxidative stress, and promote angiogenesis [240]. In addition to reducing inflammation, an ideal cardiac patch also needs to provide adequate blood supply to the left ventricle. To this end, a cardiac patch was prepared by covalently combining nitrate pharmacological functional groups with biodegradable PCL [241]. To further reduce cardiac fibrosis, an alternative strategy induces fibrotic cardiomyocytes into new cardiomyocytes. In particular, functionalized nanofibers can effectively support the proliferation and adhesion of cardiomyocytes to promote the self-repair of cardiac tissue. For example, PLGA nanofibers covalently coupled with two adhesion peptides, YIGSR and RGD, could promote the adhesion and proliferation of cardiomyocytes [242].
Functionalized nanofibers also support the adhesion and proliferation of other cells that can differentiate into cardiomyocytes. For example, a VEGF-coated nanofiber scaffold can significantly promote the differentiation of human mesenchymal stem cells into cardiomyocytes [243]. In addition, as shown in Fig. 8b, a chitosan/serin protein-modified cellulose nanofiber patch not only improved the survival rate of adipose tissue-derived mesenchymal stem cells but also reduced myocardial fibrosis and inhibited ventricular remodeling after MI [244]. Beyond exogenous cell therapy, two muscle-specific microRNAs can be delivered using nanofibers with different topologies, after which they reprogram cardiac fibroblasts into cardiomyocyte-like cells and reduce myocardial fibrosis [245].
After MI, the electrical microenvironment usually undergoes pathological changes, such as abnormal contraction, disruption of the conductive network, and irregular propagation of electrical signals, which severely limit repair of the damaged myocardium [246]. Usually, various conductive agents can be added to improve the electrical microenvironment in the MI area [247]. However, it is difficult to achieve real electrical anisotropy matching the natural myocardium by simply loading conductive materials or adjusting the orientation of the fiber structure. In one study, a reduced graphene oxide functional silk fibroin nanofiber patch was developed with a similar anisotropic conductivity to natural myocardium to improve the electrical microenvironment of infarcted myocardium (Fig. 8c) [248]. In addition to improving the electrical microenvironment of the myocardium, it is particularly important for the myocardium to beat synchronously and rhythmically [249]. Although it has been verified that spontaneous cardiomyocyte contraction can be observed when cardiomyocytes are cultured on fibers, it is also essential that they beat synchronously with the natural myocardium. The mechanical properties, arrangement structure, chemical composition and electrical conductivity of fibers significantly affect the beating of cardiomyocytes on fibers [250]. When cultured on parallelly aligned conductive polyaniline/PLGA nanofibers, all cardiomyocytes within a single cluster were found to beat synchronously [251].
Congenital heart disease is a congenital disease distinct from MI [252], and autologous cardiomyocyte therapy is the main treatment method. Due to the low retention rate of injected cells, one promising solution for the treatment of congenital heart disease is the delivery of c-Kit cardiac progenitor cells via nanofiber scaffolds [253, 254]. For example, nanofibers coated with gelatin and/or fibronectin effectively enhance the metabolism of c-Kit + cardiac progenitor cells [255]. However, the quality of c-Kit+ progenitor cells differs significantly across patients. To solve these problems, a computational modeling approach has been used to determine the repair mechanisms of cardiac-derived c-Kit cells and understand how these mechanisms can be used to design biomaterials to improve cardiac patch performance [256]. As shown in Fig. 8d, a nanofiber patch for pediatric heart failure patients was designed and prepared by computational methods and was confirmed to effectively achieve antifibrosis and angiogenesis.
DDSs based on electrospun nanofiber scaffolds can be engineered with anti-inflammatory capabilities to promote myocardial cell adhesion and proliferation and achieve cardiac phenotype and function for cardiac tissue engineering. To enable versatility and achieve these functions simultaneously and comprehensively, multiple regulatory signals need to be integrated on a single platform. Moreover, achieving synchronized contraction and electrical anisotropy that matches the natural myocardium remain major challenges. Electrically active biomaterials can combine electrical stimulation with scaffolds to promote cardiac tissue regeneration and maintain synchronized beating contractions of heart tissue. Continuous delivery of different bioactive factors at typical time points is also important to improve repair efficacy. In situ measurement of delivered drug concentrations during the delivery period remains difficult. In addition, real-time monitoring of the regeneration process is important. The integration of imaging techniques can further address both issues and has important implications for the exploration of physiological processes in cardiac tissue regeneration, as well as the study of the regulatory behaviors of materials in vivo.
Bone Tissue Engineering
As a typical hard tissue, bone tissue exhibits a complex and highly stratified structure with high density and involves various growth factors and endogenous signals [257]. Bone tissue engineering also involves many aspects, including osteogenic differentiation, angiogenesis, bone healing, and the treatment of bone-related diseases [258]. Electrospun fiber scaffolds provide a structure that simulates the bone environment and have drug-delivery advantages (for small molecule drugs and growth factors, etc.), which are expected to solve problems related to limited donor sites for autologous transplantation and to provide a promising avenue for bone tissue engineering.
To promote bone tissue regeneration, the primary task is to enhance osteogenic activity. To this end, researchers have investigated a series of electrospun fibers combined with the delivery of bioactive substances that promote osteogenesis [259,260,261]. For example, a layered micro/nanofiber biomimetic periosteum with sustainable release of VEGF has been developed (Fig. 9a) [117]. VEGF was encapsulated in hyaluronan (HA)-poly(l-lactide) acid (PLLA) core-sheath fibers and released in a sustained manner to promote angiogenesis, and collagen self-assembly on the fibers greatly mimicked the microenvironment necessary for intramembranous osteogenesis. The 3D reconstruction images of the defect at 4 and 8 weeks post-surgery showed that the repair effect of the mat was the most satisfactory, suggesting a synergistic effect of hierarchical structure and VEGF in promoting osteogenesis. In view of the complexity of the bone repair process, co-delivery or sequential delivery of multiple drugs is generally required, so it is particularly important to design electrospun fiber scaffolds that can carry multiple drugs and allow their release in a controlled manner [262]. Coaxial electrospinning and LBL provide good methods for this. For example, a nanofiber mat made of core-sheath fibers with PVA as the core and SF/PCL as the shell was prepared, BMP-2 introduced into the core, and connective tissue growth factor (CTGF) was bound to the surface of the nanofibers through LBL technology (Fig. 9b) [263]. Fluorescent labeling in vivo showed the sustained release of BMP-2 over 30 days, while CTGF rapidly dropped to minimum levels within 6 days, indicative of an early, transient release. In vivo studies showed that areas of alkaline phosphatase (ALP) positive tissues and angiogenesis were both significantly increased compared with a single BMP-2 release system. Similarly, the combination of DEX and BMP-2 also had a synergistic effect on ALP expression and osteogenesis [264].
Drug delivery systems based on electrospun fiber scaffolds for bone tissue engineering. a Schematic illustration showing the construction of a nanofiber-based biomimetic periosteum for periosteum and bone regeneration and 3D reconstructed images of the regenerated bone after implantation for 4 and 8 weeks, respectively, in a rat calvarial critical size defect model. Reproduced with permission from Ref. [117]; Copyright 2020, Elsevier Limited. b Spatiotemporally controlled release of BMP-2 and CTGF for bone repair by combining coaxial electrospinning and LBL technology, and in vivo tracing of fluorescent dye-labeled BMP-2 and CTGF, as well as ALP-positive tissue areas in a model of ectopic osteogenesis. Reproduced with permission from Ref. [263]; Copyright 2019, American Chemical Society Limited. c Schematic illustration showing design of nanofiber for simultaneously dual delivery of ALN and silicate, as well as tuning the balance between bone resorption and bone formation. Reproduced with permission from Ref. [106]; Copyright 2019, The Royal Society of Chemistry Limited. d Schematic illustration showing PCL/MoS2 nanofibrous mat with photothermal property, the relative expressions of OCN, OPN, and HSPs genes with or without NIR irradiation, as well as photos of the rat tibias with implants and H&E staining of the tissues after implanting with PCL/1%MoS2 electrospun mat for 4 and 8 weeks with or without NIR irradiation, respectively. Reproduced with permission from Ref. [270]; Copyright 2021, Wiley–VCH Limited. e Schematic illustrations showing a dual function nanofibrous scaffold for tumor suppression and bone repair by loading DOX and modifying PDA, as well as the release route of DOX from the scaffold. Reproduced with permission from Ref. [271]; Copyright 2021, American Chemical Society Limited
The regulation of the activity balance between osteoblasts and osteoclasts is another important factor to be considered in bone repair [265]. As shown in Fig. 9c, the scaffold could achieve the simultaneous dual delivery of alendronate (ALN) and silicate to further adjust the balance between bone resorption and bone formation, thus accelerating bone repair. ALN encapsulated in MSN was released from nanofibers and inhibited the bone resorption process by preventing the expression of GTP-related proteins, while silicate released upon MSN hydrolysis accelerated the bone formation process by promoting angiogenesis and bone calcification [106]. Coaxial electrospinning can also provide a similar dual-delivery system to modulate the osteogenesis–osteoclastogenesis balance. In particular, the rapid release of substance P enhanced the migration and osteogenic differentiation of BMSCs, while the sustained release of ALN reduced bone resorption [266]. Of note, delivery systems programmed to match the spatiotemporal specificity of bone healing are increasingly being developed.
The ability of exogenous stimuli to regulate cells is gradually being appreciated. Most commonly, bioelectrical signals in native bone serve as key factors in regulating bone growth, structural reconstruction, and healing [36]. Some studies have confirmed that electrical stimulation treatment promotes the adhesion, growth, and proliferation of osteoblasts, as well as significantly enhancing calcium and phosphorus deposition [267, 268]. Similarly, the heat generated by NIR not only penetrates the tissue but also regulates the expression of heat shock proteins (HSPs) and enhances the expression of osteogenesis-related proteins [269]. By incorporating MoS2, an osteogenesis promoter and photothermal agent, into electrospun fibers, the obtained scaffold exhibits stronger cell growth and osteogenic ability in combination with photothermal therapy [270]. Under NIR-triggered mild photothermal treatment for 30 or 60 s, the expression levels of OPN and OCN, osteogenesis-related genes, were up-regulated in BMSCs after 7 and 14 days of culture, and the ability to accelerate osteogenesis and bone healing was also demonstrated in vivo in a rat tibial defect model (Fig. 9d). In addition to the introduction of stimulatory components, the design of 3D-structured fiber scaffolds can also better simulate the bone environment. For example, a radial 3D scaffold obtained by NaBH4 foaming not only provides topographical clues and a good bone repair environment but also allows the loading of various growth factors to promote the bone healing process [63]. In the future, it remains a key challenge to incorporate stimulation into 3D electrospun fiber scaffolds to develop 4D bone tissue scaffolds.
One of the main causes of bone defects is bone tumors, so the design of bone tissue scaffolds requires the consideration of bone repair and prevention of bone tumor recurrence. For example, DOX was intercalated into lamellar hydroxyapatite and dissolved in PLGA for electrospinning, after which the surface of the electrospun fibers was further coated with PDA to obtain a PDA@DH/PLGA scaffold. The PDA coating prolonged the drug release (Fig. 9e) [271]. More importantly, the PDA@DH/PLGA scaffold significantly inhibited tumor cells growth initially, then subsequently improved osteoblast proliferation and promoted the repair of bone defects caused by tumor resection in vivo. The development of electrospun drug-loaded fiber scaffolds for bone tumor treatment is still worthy of further investigation, while the extensive ability to incorporate drugs into electrospun fibers provides a good alternative for bone tumor treatment and postoperative repair.
Cartilage Tissue Engineering
Articular cartilage, which is primarily composed of chondrocytes and ECM, is responsible for reducing interface friction and assisting in bearing loads. Due to its low level of regeneration and limited self-healing capacity [272], the clinical pressures of cartilage-related diseases have been increased with population aging. Thus, there is an urgent need to develop new biomaterial scaffolds to treat cartilage damage, and various drugs and growth factors have been explored for application to cartilage regeneration. To avoid rapid drug clearance and ensure controlled release [273], electrospun fibers are widely applied due to their customizable structures and selectable properties with regards to drug binding, enabling these scaffolds to mimic the different morphologies of cartilage ECM and control drug delivery [274].
In general, PLLA [275,276,277] and PLGA [278, 279] are rarely used due to their potential to cause inflammation during degradation, while PCL [280,281,282] and polyhydroxybutyrate [283] can be applied after modification or blending. Methylsulfonylmethane, a typical drug to inhibit inflammation and promote chondrocytes differentiation [284, 285], was loaded on PLGA fiber mats to accelerate cartilage regeneration [279]. However, the release curve reached stability within 24 h after the initial burst release, indicating a deficiency in sustained release. Candidate drugs [278] and effective natural plant components [286] have faced similar problems. To this end, the design of fiber structures and in-depth exploration of drug combinations remain under way. For example, Kartogenin [287, 288], has been shown to promote cartilage defect repair, was loaded into coaxial fibers [289]. As shown in Fig. 10a, the existence of the PCL sheath successfully alleviated burst release and lengthened the drug release process to more than 20 days. At 14 days, levels of glycosaminoglycan deposited on the coaxial fibers were lower than those found on monoaxial ones, but they were modestly higher at 21 days, implying the advantages of long-lasting sustained release. For the same purpose, nanoparticles loaded with small molecular drugs [290] and growth factors [277] have been combined with electrospun fiber scaffolds to maintain bioactivity and enable long-term controllable delivery. Multidrug delivery systems have also been developed to recruit endogenous cells and promote cartilage regeneration [291]. Cartilage-derived extracellular matrix (cECM), which contains various growth factors and natural components, possesses an imaginably unique potential. In one study, cECM was mixed with PCL for electrospinning, and the as-obtained scaffold was seeded with chondrocytes and implanted into nude mice [292]. Figure 10b shows the repair effects, as well as H&E staining at different times, indicating that cECM has a positive effect on regenerated cartilage after 24 weeks, and more interestingly, the Young’s modulus of the regenerated cartilage reached approximately native auricular levels.
Drug delivery systems based on electrospun fiber scaffolds for cartilage tissue engineering. a Schematic of kartogenin-loaded monoaxial and coaxial nanofibers, and comparison of their release profile. Reproduced with permission from Ref. [289]; Copyright 2020, Elsevier Limited. b The gross view and H&E staining images of regenerated cartilage after implanting the cECM-loaded PCL membrane into mice. Reproduced with permission from Ref. [292]; Copyright 2020, Elsevier Limited. c Gas-foamed chondroitin sulfate crosslinked PLCL/SF-based three-dimensional scaffold enhances cartilage regeneration, with gross view at 12 weeks and stain results of COL-II revealing its effects. Reproduced with permission from Ref. [295]; Copyright 2021, Elsevier Limited. d Schematic illustration of the structure of the biomimicking multilayer scaffold loaded with FGF-2, BMP-2, as well as some other components promotes deep cartilage defect from regenerating, and results of micro-CT examination at different time points. Reproduced with permission from Ref. [296]; Copyright 2018, Elsevier Limited. e PCL nanomembranes realized sustained release of lignin, thus suppress inflammation factors, scavenge ROS, restrain osteoarthritis from deteriorating by suppressing expression of IL-1β, MMP13 and Keap1, at the same time upregulate the expression of ATG4 to adjust autophagy. Reproduced with permission from Ref. [301]; Copyright 2020, Elsevier Limited
It is well known that larger pores are favorable to cell infiltration and proliferation [293]. Nevertheless, 2D membranes are relatively dense and unsuitable for deep defects. As a result, studies aiming at developing 2D membranes to 3D scaffolds have arisen. For instance, 3D structures fabricated using NaBH4 exhibit large pores that facilitate cell attachment and proliferation [294]. Chondroitin sulfate (CS), a common component extracted from cartilage, can be grafted to the matrix by chemical modification to further enhance the effects of 3D scaffolds [295]. The lowest levels of inflammatory cytokines and the highest glycosaminoglycan content were detected in 3D scaffolds crosslinked with CS in vitro, and the optimal repair effects were confirmed by the results of morphological analysis and immunohistochemical staining, as shown in Fig. 10c. With respect to deep osteochondral defects, multilayer scaffolds can be developed to meet variable needs. A four-layer hydrogel-fiber composite was fabricated in which a fibrous membrane was used as a barrier to limit cell migration [296], and BMP-2 loaded coaxial fibers were incorporated to promote subchondral bone formation. Micro-CT images in Fig. 10d reveals that the boundaries between the defect and surrounding tissues almost disappeared at 12 weeks, with the exception of the blank group. In addition, electrospun fibers can be combined with freeze-drying [284, 297, 298] and 3D printing [299, 300] approaches to develop multi-dimensional scaffolds that promote cartilage regeneration.
Injured cartilage gradually leads to osteoarthritis, with inflammation serving as the main culprit. As a durable antioxidant, lignin can efficiently alleviate excessive oxidative stress. In one study, modified lignin was mixed with PCL for electrospinning to prevent the development of osteoarthritis. As shown in Fig. 10e, non-significant differences were found among the groups without H2O2 stimulation, while PCL-lignin50 increased the expression of inflammatory factors (MMP13 and IL-1β) after H2O2 treatment, thereby effectively preventing hydrogen peroxide-induced chondrocyte inflammation. Moreover, lignin can upregulate the relative expression of allied enzyme under the influence of H2O2, and higher expression of ATG4 indicated that lignin can also prevent chondrocytes from experiencing excessive oxidative stress by activating autophagy. Their work also demonstrated that low intensity pulsed ultrasound (LIPUS) may further enhance regeneration capacity [301]. With the deepening of research on injectable hydrogels, it is becoming possible to disperse electrospun fibers in hydrogels to form injectable DDSs, which is an excellent potential approach.
Other Tissue Engineering
In addition to the above-mentioned applications, drug-loaded electrospun fiber scaffolds have also been developed for the engineering of vascular, dental and musculoskeletal tissues, among others. The high specific surface area and porosity of electrospun fiber scaffold ensure excellent gas exchange and nutrient transport properties, making it to become a good choice for artificial vascular grafts [302]. Considering the structure of natural blood vessels, tubular morphologies with multilayered vessel walls have attracted much attention due to their mimicry. As shown in Fig. 11a, a conduit composed of a tri-layer electrospun fiber (R-126/R-145/PCL) was prepared by encapsulating microRNA-126 and microRNA-145 in the inner and middle layers of poly(ethylene glycol)-b-poly(l-lactide-co-ε-caprolactone) fibers, respectively, in combination with an outer layer of PCL fibers [131]. The fiber mat enables the fast release of microRNA-126 and slow release of microRNA-145. Tri-layered electrospun grafts can promote the growth and intracellular nitric oxide production of vascular endothelial cells, modulate the phenotype of vascular smooth muscle cells, and suppress calcification. More importantly, color doppler ultrasound imaging demonstrated prominent vascular patency in the fiber graft. Although the functions of electrospun fiber-based vascular scaffold are continuously optimized, thrombosis and intimal hyperplasia still inevitably occurred [303]. The addition of a variety of bioactive substances can be beneficial to accelerating the replacement of autologous blood vessels and improving the treatment of cardiovascular diseases [304].
Drug delivery systems based on electrospun fiber scaffolds for other tissue engineering. a Illustration of three-layered vascular grafts prepared by successive three-step electrospinning to encapsulate miR-126 and miR-145 in the inner and middle layers of fibers, respectively, as well as its multiple efficacies, including of patency testing after in vivo implantation for 4 weeks. Arrows indicate blood flow (yellow) and suture sites (blue). Reproduced with permission from Ref. [131]; Copyright 2020, American Chemical Society Limited. b Depiction of periodontal defect in rats with fibrous membrane implantation, P. gingivalis colony forming units (CFUs) on membrane surface and micro-CT analysis of rat maxilla after implantation for 6 weeks. Reproduced with permission from Ref. [314]; Copyright 2018, Wiley–VCH Limited. c Schematic illustration of pea-like bi-stranded nanofibers prepared by electrospinning in the repair of chronic rotator cuff tears, the effect of lithium ion on the expression of osteogenesis and adipogenic-related proteins in vitro was indicated by western blotting, and the repair effect of each group was shown by micro-CT image after implantation for 2 weeks and 8 weeks. Reproduced with permission from Ref. [321]; Copyright 2020, Elsevier Limited. d Schematic diagram of the preparation of HCPT and diclofenac sodium (DS) composite membrane and the synergistic anti-adhesion combined with physical isolation and drug treatment, as well as H&E and Masson’s trichome staining of repair sites after 14 days. Reproduced with permission from Ref. [324]; Copyright 2018, American Chemical Society Limited
As described above for bone tissue, a combination of electrospun fibers and osteogenic factors can also be used to guide dental bone regeneration [305,306,307]. In this case, it is necessary to consider the impact of the oral environment [308]. Guided bone regeneration membranes with dual functions of anti-infection and osteogenesis have been extensively studied [309,310,311,312]. In particular, in addition to the good antibacterial properties of metronidazole, some inorganic particles can exhibit good anti-infective effects [313]. For example, ZnO can endow PCL fiber membrane with good osteo-conductivity and antibacterial properties (Fig. 11b) [314]. The number of Colony forming units of Pseudomonas gingivalis on the membrane surface was reduced, and micro-CT analysis of the rat maxilla confirmed the effectiveness of ZnO-loaded PCL fibers in periodontitis-related bone regeneration. This drug-loaded electrospun fiber membrane can also be applied as an oral drug delivery patch for the treatment of oral diseases [315], this approach not only maintains good oral adhesion but also provides continuous and controllable drug delivery capabilities to kill bacteria and eliminate inflammation [316,317,318].
Tendon is an important part of musculoskeletal tissues, and tendon grafts and tendon sutures used in surgical treatment cannot satisfy requirements relating to flexibility, anti-adhesion, and permanent remodeling [319]. To address these problems, electrospun drug-loaded scaffolds can serve as a potential alternative for the treatment and regeneration of injured tendon tissue. In one study, thymosin β4 (Tβ4)-loaded oriented fibers not only mimicked the ultrastructure of natural tendon tissue but also showed 28-day sustained release that promoted the migration and proliferation of human adipose-derived mesenchymal stem cells and supported tendon differentiation [320]. In addition to direct drug delivery, nanoparticles can also be doped in electrospun fibers to deliver therapeutic ions for tendon repair. For example, inspired by the structure of cowpea, lithium was loaded in MSNs and doped in electrospun poly(ester urethane) urea (PEUU) nanofibers (Li+@MSNS/PEUU), allowing the slow release of Li+ to inhibit rotator cuff fat penetration and promote tendon and bone healing (Fig. 11c) [321]. Western blotting results showed that Gsk3β was inhibited, while Wnt5a and β-catenin were up-regulated under the influence of Li+ ions. At the proximal tendon-bone junction, the osteogenic effects of the Li+-containing fiber patch were significantly higher than those of the fiber patch without Li+ ions. Micro-CT analysis showed that the bone mineral density (BMD) and bone volume fraction (bone volume/total volume, BV/TV) of the group using Li+@MSNS/PEUU nanofiber patch were significantly higher than those in other groups after implanted for 2 and 8 weeks, respectively.
Tissue anti-adhesion is another research hotspot, especially with respect to tendon tissues. By loading engineered growth factors and related small molecular drugs into electrospun fibers, not only can the purpose of controlled release be achieved, but also the formation of adhesion can also be inhibited [322, 323]. As shown in Fig. 11d, the synergistic prevention of peritoneal adhesions can be enabled by loading HCPT and diclofenac sodium (DS) into the sheath and core of nanofibers, respectively, to exert anti-fibrin proliferative and anti-inflammatory effects [324]. Histological staining confirmed that collagenous tissue was compartmentalized in the group loaded with HCPT and DS, with little adhesion formation. Although electrospun drug-loaded scaffolds have widespread applications in tissue engineering, it is worthwhile to continue to develop new DDSs based on electrospun fiber scaffolds to broaden tissue regeneration strategies.
Cancer Therapy
Surgical resection of the tumor in combination with systemic chemotherapy is one of the most common strategies for cancer treatment [325,326,327]. However, as a result of its systemic administration and poorly targeted delivery, conventional chemotherapy often causes serious side effects to other normal tissues [328, 329]. Therefore, it is urgent to develop a new anticancer drug delivery platform to solve the above problems. Electrospun fibers can allow the local delivery of anticancer drugs, so they have been widely applied in tumor therapy [12]. In a recent study, CUR was incorporated into MSNs and embedded into PLGA nanofibers by blending electrospinning [76]. The nanofibers had an excellent ability to scavenge tumor cells. In addition to the passive release of anti-cancer drugs from drug-loaded nanofibers, many researchers have designed pH-responsive fibers to deliver anti-cancer drugs based on the acidic tumor microenvironment [12]. For instance, DOX-loaded MSNs were doped into nanofibers, and CaCO3 was used as an “inorganic cap” to control the opening of the MSN hole inlet [330]. In the acidic tumor microenvironment, CaCO3 reacted with hydrogen ions to generate carbon dioxide, promoting the release of DOX from MSNs. This type of intelligent-response drug delivery can be further endowed with targeting capacities to enhance the utilization efficacy of chemotherapeutic drugs. Hydrophobic DOX was first encapsulated in folic acid-coupled PCL self-assembled micelles, after which core–shell nanofibers loaded with the micelles were prepared by coaxial electrospinning (Fig. 12a) [331]. Compared with repeated intravenous injection, the delivery of targeted micelles can greatly reduce the dose, administration frequency, and side effects of chemotherapy drugs.
Drug delivery systems based on electrospun fiber scaffolds for cancer therapy. a Mechanism diagram of tumor clearance by the implantable DOX loaded active-targeting micelle-nanofiber platform and the micelles transfer from nanofiber matrix to tumor tissue, and finally to tumor cells. Reproduced with permission from Ref. [331]; Copyright 2015, American Chemical Society Limited. b The release of dual drugs from fibers and their synergistic treatment of tumor, and CLSM image of cavity and fluorescence colocalization analysis. Reproduced with permission from Ref. [337]; Copyright 2020, Wiley–VCH Limited. c Schematic illustration of chemical relief of the hypoxia environment with in situ release of ACM nanoparticles for oesophageal cancer photodynamic therapy as well as Ki-67 and TUNEL-stained slices of oesophageal cancer of each group. Black arrows indicate tracheas. Reproduced with permission from Ref. [341]; Copyright 2019, Wiley–VCH Limited. d Schematic illustration of the application of Tra-CSO-PP scaffolds and representative photographs of tumors and skin wounds on days 0, 4, 8, and 14. Reproduced with permission from Ref. [343]; Copyright 2018, American Chemical Society Limited. e Schematic illustration of DOX@PLGA fibrous rings for simultaneous tumor therapy and metastasis inhibition, and T1-weighted MR images at different time points. Reproduced with permission from Ref. [346]; Copyright 2022, Elsevier Limited
Compared to chemotherapy with a single type of drug, multi-drug combination chemotherapy has obvious advantages [332]. Combination chemotherapy allows the use of multiple chemotherapeutic drugs to improve the therapeutic effects of chemotherapy. Meanwhile, combination chemotherapy can induce apoptosis of tumor cells through different signaling pathways, exerting a synergistic effect in killing tumor cells [333]. For example, pluronic F127-modified nanofibers loaded with camptothecin and CUR could achieve the simultaneous and sustained release of the two drugs [334]. Camptothecin can convert topoisomerase I into a cytotoxic agent by inhibiting the movement of replication forks, leading to tumor death, while CUR inhibits tumor cell growth by inhibiting the kB and Wnt signaling pathways [335, 336]. The use of combination chemotherapy can effectively inhibit the growth of colon cancer cells by inhibiting different signal pathways in tumor cells. Drug delivery platforms loaded with multiple drugs can facilitate different therapeutic effects and avoid drug toxicity and side effects associated with prolonged overuse of a single drug. For instance, hierarchical nanofibers loaded with DOX and matrix metalloproteinases-2 were fabricated through coaxial electrospinning [120]. With this approach, the rapid release of DOX from the fibrous nuclear layer could kill remaining tumor cells, while the loading of matrix metalloproteinase-2 inhibitor disulfiram in the fibrous shell could effectively inhibit tumor erosion and prevent metastasis. In addition, the time-programmed release of multiple drugs is the most critical factor in combination chemotherapy. For example, as shown in Fig. 12b, DOX formed periodic chambers inside the fibers, while the double walls of the fibers were made of polylactic acid and PCL containing the angiogenesis inhibitor apatinib. In vivo experiments showed that a good synergistic effect was obtained by transplanting the fiber into subcutaneous tissue near the tumor site in mice [337].
Although chemotherapy has great advantages in cancer treatment, the tolerance of tumors to chemotherapy drugs highlights the urgency of integrating these approaches with other types of technologies. The delivery of photothermal agents or photosensitizers by nanofibers can effectively kill tumor cells [338,339,340]. As illustrated in Fig. 12c, the efficacy of nanofiber scaffolds loaded with albumin-chloro-6-manganese dioxide nanoparticles (ACM) for tumor treatment was evaluated using an in situ rabbit model of esophageal cancer [341]. In the presence of endogenous hydrogen peroxide, a nanofiber scaffold implanted into the area of tissue damage could produce oxygen and alleviate tumor hypoxia. At the same time, ACM nanoparticles gradually diffused out from the scaffold to the tumor, resulting in effective photodynamic therapy for cancer treatment.
For tumors in the skin, bone and breast, surgical resection causes serious tissue defects. Therefore, the removal of remaining tumor cells needs to be accompanied by the promotion of tissue regeneration [342]. Therefore, it is particularly important to functionalize nanofiber scaffolds to enable them to scavenge tumor cells and promote tissue regeneration. In the study shown in Fig. 12d, drug-loaded copper silicate hollow microspheres were loaded into nanofiber scaffolds, which exhibited excellent photothermal effects and the capability to trigger drug release under NIR irradiation [343]. Upon NIR irradiation, the scaffold could both eliminate tumors and promote skin tissue healing.
Circulating tumor cells are cancer cells that are shed from the tumor and enter the circulatory system; therefore, capturing circulating tumor cells is critical to delaying cancer metastasis [344]. However, it is quite difficult to capture tumor cells from circulating blood in vivo. Due to the advantages of high specific surface area and flexible surface modification of nanofibers, the targeted aptamer-modified nanofiber surface can be used to capture circulating tumor cells. For example, coating anti-CD146 antibodies-to-melanoma on the surface of PLGA nanofibers can enable the capture of circulating melanoma cells [345]. As shown in Fig. 12e, to achieve cancer treatment, tumor metastasis inhibition, and magnetic resonance imaging, DOX-loaded PLGA fiber mats were immersed in a salt solution to form fibrous rings [346]. The fibrous rings were functionalized with the chelating agent gadolinium and DNA aptamers via the ethylenediamine-mediated coupling reaction. Analysis showed that the multifunctional fibrous rings could simultaneously deliver tumor chemotherapy, enable magnetic resonance imaging, and anchor circulating tumor cells, ultimately inhibiting the migration and erosion of tumor cells.
Conclusions and Perspectives
Over the past decades, electrospun fibers have been increasingly applied for controlled drug delivery in the fields of tissue regeneration and cancer therapy. To meet the needs of target tissues, electrospinning provides customizable process parameters, collection devices, and post-processing procedures. Correspondingly, electrospun fiber scaffolds with multiple structures, architectures, and dimensions, including aligned, core-sheath, porous, grooved, and gradient features, have been fabricated to regulate cellular states and match the anatomical structures of regenerating tissues. By combining various therapeutic drugs, electrospun fiber-controlled drug delivery platforms with customizable characteristics have gradually broadened the potential applications of soft tissue engineering, hard tissue engineering and cancer treatment. Thanks to the unremitting efforts of scientific researchers and developments in nanoscience, advances have been made to the design of electrospun fiber structures, the in-depth exploration of combinations of electrospun fibers and drugs, and the combination of stimuli with controlled properties. Herein, some representative examples of electrospun fibers loaded with functional therapeutic agents and their applications are listed in Table 1. It is believed that the electrospun fiber drug-loading platform can provide continued possibilities for the development of new drug delivery strategies, characterized by the valuable capacity to deliver precise amounts of drugs at specific locations and times in tissue regeneration and cancer therapy.
For smart drug-loading platforms, electrospun fiber scaffolds are offering predictable potential. The emergence of technologies such as special collectors and gas foaming has enabled 3D electrospun fiber scaffolds to maintain their original nano-morphology with larger porosity and pore size, broadening the applications of electrospun drug-loaded scaffolds in tissue engineering [347, 348]. In addition, by introducing stimuli-responsive components, drugs can be precisely programmed for release in vivo. The combination of drugs with photothermal therapy, photodynamic therapy, magnetothermal therapy, sonodynamic therapy and multiple therapies trigger by exogenous stimuli can achieve better therapeutic effects. Similarly, the introduction of unique imaging properties enables drug delivery to simultaneously support long-term stable tracking and real-time monitoring, thereby greatly advancing the process of drug visualization therapy [346, 349,350,351]. Furthermore, artificial intelligence (AI) has also expanded the future of intelligent DDSs. Some strategies integrating electronic components into scaffolds have been recently proposed, and these techniques allow remote monitoring of tissue function and intervention through stimulation and controlled drug release [352, 353]. We foresee that the integration of microelectronic devices into electrospun drug-loaded scaffolds will provide a better way to monitor patient health [354,355,356]. These additional advantages are likely to further optimize electrospun fibers-controlled DDSs as ideal disease treatment platforms and individually customizable therapeutic regimens.
Although the design of DDSs based on electrospun fiber scaffolds has reached a new stage, there is still a long way to go to translate into clinical and commercial applications. The first consideration is the biosafety of drug-loaded electrospun fiber scaffolds. All components should be stable and nontoxic, and the long-term immune and host responses after in vivo implantation should be well understood. It is also a great challenge to match the degradation properties of electrospun fiber scaffolds with tissue repair rates in practical applications, which is expected to be solved by optimizing combinations of substrate materials, adding other components or modifying the scaffolds. During these processes, the implanted scaffold material can be adjusted by monitoring tissue repair through real-time imaging. In addition, the fate and pharmaceutic kinetics of drugs after entering the body remain unclear. More detailed studies of fiber- and drug-induced inflammatory responses and healing mechanisms are also required. Furthermore, the design of drug-loaded systems is currently based mostly on experimental trials and experience, so great advances could be made by applying in-depth machine learning and AI to predict interactions between drugs and fibers and optimize drug selection, release behavior, and simulation of tissue degradation, among other factors, to direct system construction. Finally, there is still a gap in the industrialization of drug-loaded electrospun fiber scaffolds. It is important to realize the high-throughput preparation of electrospun fibers and maintain quality control during the scale-up process, as this also determines the effectiveness and repeatability of drug release behavior. In the chain of manufacturing-experimental validation-clinical trial-commercialization, it encourages more dialogue and in-depth cooperation among scientists working in the areas of materials science, nanotechnology, pharmacy, biomedicine, and clinical medicine to solve these challenges.
References:
Chen ML, Li YF, Besenbacher F. Electrospun nanofibers-mediated on-demand drug release. Adv Healthc Mater 2014;3:1721.
Li W, Tang J, Lee D, Tice TR, Schwendeman SP, Prausnitz MR. Clinical translation of long-acting drug delivery formulations. Nat Rev Mater 2022;7:406.
Lan XZ, Wang H, Bai JF, Miao XM, Lin Q, Zheng JP, Ding SK, Li XR, Tang YD. Multidrug-loaded electrospun micro/nanofibrous membranes: fabrication strategies, release behaviors and applications in regenerative medicine. J Controlled Release 2021;330:1264.
Huang W, Xiao YC, Shi XY. Construction of electrospun organic/inorganic hybrid nanofibers for drug delivery and tissue engineering applications. Adv Fiber Mater 2019;1:32.
Ding YP, Li W, Zhang F, Liu ZH, Ezazi NZ, Liu DF, Santos HA. Electrospun fibrous architectures for drug delivery, tissue engineering and cancer therapy. Adv Funct Mater 2019;29:1802852.
Manzari MT, Shamay Y, Kiguchi H, Rosen N, Scaltriti M, Heller DA. Targeted drug delivery strategies for precision medicines. Nat Rev Mater 2021;6:351.
Muzzio N, Moya S, Romero G. Multifunctional scaffolds and synergistic strategies in tissue engineering and regenerative medicine. Pharmaceutics 2021;13:792.
Hong S, Choi DW, Kim HN, Park CG, Lee W, Park HH. Protein-based nanoparticles as drug delivery systems. Pharmaceutics 2020;12:604.
Edis Z, Wang JL, Waqas MK, Ijaz M, Ijaz M. Nanocarriers-mediated drug delivery systems for anticancer agents: an overview and perspectives. Int J Nanomed 2021;16:1313.
Topuz F, Uyar T. Electrospinning of cyclodextrin functional nanofibers for drug delivery applications. Pharmaceutics 2019;11:6.
Luraghi A, Peri F, Moroni L. Electrospinning for drug delivery applications: a review. J Control Release 2021;334:463.
Zhao JW, Cui WG. Functional electrospun fibers for local therapy of cancer. Adv Fiber Mater 2020;2:229.
Xue JJ, Xie JW, Liu WY, Xia YN. Electrospun nanofibers: New concepts, Materials, and applications. Acc Chem Res 2017;50:1976.
Bhattarai RS, Bachu RD, Boddu SHS, Bhaduri S. Biomedical applications of electrospun nanofibers: Drug and nanoparticle delivery. Pharmaceutics 2019;11:5.
Feng XR, Li JN, Zhang X, Liu TJ, Ding JX, Chen XS. Electrospun polymer micro/nanofibers as pharmaceutical repositories for healthcare. J Control Release 2019;302:19.
Yang G, Li XL, He Y, Ma JK, Ni GL, Zhou SB. From nano to micro to macro: electrospun hierarchically structured polymeric fibers for biomedical applications. Prog Polym Sci 2018;81:80.
Xue JJ, Wu T, Dai YQ, Xia YN. Electrospinning and electrospun nanofibers: methods, materials, and applications. Chem Rev 2019;119:5298.
Pant B, Park M, Park SJ. Drug delivery applications of core-sheath nanofibers prepared by coaxial electrospinning: A review. Pharmaceutics 2019;11:305.
Zhang XD, Chi C, Chen JJ, Zhang XD, Gong M, Wang X, Yan JH, Shi R, Zhang LQ, Xue JJ. Electrospun quad-axial nanofibers for controlled and sustained drug delivery. Mater Des 2021;206:9.
Xue JJ, Zhu CL, Li JH, Li HX, Xia YN. Integration of phase-change Materials with electrospun fibers for promoting neurite outgrowth under controlled release. Adv Funct Mater 2018;28:1705563.
Buck E, Maisuria V, Tufenkji N, Cerruti M. Antibacterial properties of PLGA electrospun scaffolds containing ciprofloxacin incorporated by blending or physisorption. ACS Appl Bio Mater 2018;1:627.
Mashayekhi S, Rasoulpoor S, Shabani S, Esmaeilizadeh N, Serati-Nouri H, Sheervalilou R, Pilehvar-Soltanahmadi Y. Curcumin-loaded mesoporous silica nanoparticles/nanofiber composites for supporting long-term proliferation and stemness preservation of adipose-derived stem cells. Int J Pharm 2020;587:25.
Yang YY, Chang SY, Bai YF, Du YT, Yu DG. Electrospun triaxial nanofibers with middle blank cellulose acetate layers for accurate dual-stage drug release. Carbohydr Polym 2020;243:11647.
Singh B, Kim K, Park MH. On-demand drug delivery systems using nanofibers. Nanomaterials 2021;11:3411.
Wang Z, Cui WG. Two sides of electrospun fiber in promoting and inhibiting biomedical processes. Adv Ther 2021;4:1.
Doostmohammadi M, Forootanfar H, Ramakrishna S. Regenerative medicine and drug delivery: Progress via electrospun biomaterials. Mat Sci Eng C-Mater 2020;109:110521.
Pina S, Ribeiro VP, Marques CF, Maia FR, Silva TH, Reis RL, Oliveira JM. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials 2019;12:1824.
Huang C, Soenen SJ, Rejman J, Lucas B, Braeckmans K, Demeester J, De Smedt SC. Stimuli-responsive electrospun fibers and their applications. Chem Soc Rev 2011;40:2417.
Municoy S, Echazu MIA, Antezana PE, Galdoporpora JM, Olivetti C, Mebert AM, Foglia ML, Tuttolomondo MV, Alvarez GS, Hardy JG, Desimone MF. Stimuli-responsive materials for tissue engineering and drug delivery. Int J Mol Sci 2020;21:4724.
Zhang ZW, Wells CJR, King AM, Bear JC, Davies GL, Williams GR. pH-Responsive nanocomposite fibres allowing MRI monitoring of drug release. J Mater Chem B 2020;8:7264.
Yao YJ, Ding J, Wang ZY, Zhang HL, Xie JQ, Wang YC, Hong LJ, Mao ZW, Gao JQ, Gao CY. ROS-responsive polyurethane fibrous patches loaded with methylprednisolone (MP) for restoring structures and functions of infarcted myocardium in vivo. Biomaterials 2020;232:119726.
Timin AS, Muslimoy AR, Zyuzin MV, Peltek OO, Karpoy TE, Sergeev IS, Dotsenko AI, Goncharenko AA, Yolshin ND, Sinelnik A, Krause B, Baumbach T, Surmeneya MA, Chernozem RV, Sukhorukoy GB, Surmeney RA. Multifunctional scaffolds with improved antimicrobial properties and osteogenicity based on piezoelectric electrospun fibers decorated with bioactive composite microcapsules. ACS Appl Mater Interfaces 2018;10:34849.
Hosseinian H, Hosseini S, Martinez-Chapa SO, Sher M. A meta-analysis of wearable contact lenses for medical applications: Role of electrospun fiber for drug delivery. Polymers 2022;14:185.
Samadzadeh S, Babazadeh M, Zarghami N, Pilehvar-Soltanahmadi Y, Mousazadeh H. An implantable smart hyperthermia nanofiber with switchable, controlled and sustained drug release: possible application in prevention of cancer local recurrence. Mat Sci Eng C-Mater 2021;118:111384.
Qu YC, Lu KY, Zheng YJ, Huang CB, Wang GN, Zhang YX, Yu Q. Photothermal scaffolds/surfaces for regulation of cell behaviors. Bioact Mater 2022;8:449.
Zhang X, Li L, Ouyang J, Zhang L, Xue J, Zhang H, Tao W. Electroactive electrospun nanofibers for tissue engineering. Nano Today 2021;39:101196.
Nikolaou M, Avraam K, Kolokithas-Ntoukas A, Bakandritsos A, Lizal F, Misik O, Maly M, Jedelsky J, Savva I, Balanean F, Krasia-Christoforou T. Superparamagnetic electrospun microrods for magnetically-guided pulmonary drug delivery with magnetic heating. Mat Sci Eng C-Mater 2021;126:112117.
Tu L, Liao Z, Luo Z, Wu Y-L, Herrmann A, Huo S. Ultrasound-controlled drug release and drug activation for cancer therapy. Exploration 2021;1:20210023.
Mertz D, Harlepp S, Goetz J, Begin D, Schlatter G, Begin-Colin S, Hebraud A. Nanocomposite polymer scaffolds responding under external stimuli for drug delivery and tissue engineering applications. Adv Ther 2020;3:2.
Sun Y, Cheng S, Lu W, Wang Y, Zhang P, Yao Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv 2019;9:25712.
Zheng Y, Panatdasirisuk W, Liu J, Tong A, Xiang Y, Yang S. Patterned, Wearable UV indicators from electrospun photochromic fibers and yarns. Adv Mater Technol 2020;5:11.
Abhari RE, Mouthuy PA, Vernet A, Schneider JE, Brown CP, Carr AJ. Using an industrial braiding machine to upscale the production and modulate the design of electrospun medical yarns. Polym Test 2018;69:188.
Zhang K, Bai X, Yuan Z, Cao X, Jiao X, Li Y, Qin Y, Wen Y, Zhang X. Layered nanofiber sponge with an improved capacity for promoting blood coagulation and wound healing. Biomaterials 2019;204:70.
Liu Y, He J-H, Yu J-y, Zeng H-m. Controlling numbers and sizes of beads in electrospun nanofibers. Polym Int 2008;57:632.
Ding S, Li J, Luo C, Li L, Yang G, Zhou S. Synergistic effect of released dexamethasone and surface nanoroughness on mesenchymal stem cell differentiation. Biomater Sci 2013;1:1091.
Chen H, Wang N, Di J, Zhao Y, Song Y, Jiang L. Nanowire-in-microtube structured core/shell fibers via multifluidic coaxial electrospinning. Langmuir 2010;26:11291.
Xie JW, Li XR, Xia YN. Putting electrospun nanofibers to work for biomedical research. Macromol Rapid Commun 2008;29:1775.
Wu T, Xue JJ, Xia YN. Engraving the surface of electrospun microfibers with nanoscale grooves promotes the outgrowth of neurites and the migration of Schwann cells. Angew Chem Int 2020;59:15626.
Zhao Y, Cao XY, Jiang L. Bio-mimic multichannel microtubes by a facile method. J Am Chem Soc 2007;129:764.
Yang G, Wang J, Li L, Ding S, Zhou SB. Electrospun micelles/drug-loaded nanofibers for time-programmed multi-agent release. Macromol Biosci 2014;14:965.
Feng K, Huang RM, Wu RQ, Wei YS, Zong MH, Linhardt RJ, Wu H. A novel route for double-layered encapsulation of probiotics with improved viability under adverse conditions. Food Chem 2020;310:125977.
Xiong RH, Hua DW, Van Hoeck J, Berdecka D, Leger L, De Munter S, Fraire JC, Raes L, Harizaj A, Sauvage F, Goetgeluk G, Pille M, Aalders J, Belza J, Van Acker T, Bolea-Fernandez E, Si T, Vanhaecke F, De Vos WH, Vandekerckhove B, van Hengel J, Raemdonck K, Huang CB, De Smedt SC, Braeckmans K. Photothermal nanofibres enable safe engineering of therapeutic cells. Nat Nanotechnol 2021;16:1281.
Li L, Zhou GL, Wang Y, Yang G, Ding S, Zhou SB. Controlled dual delivery of BMP-2 and dexamethasone by nanoparticle-embedded electrospun nanofibers for the efficient repair of critical-sized rat calvarial defect. Biomaterials 2015;37:218.
Baek SH, Roh J, Park CY, Kim MW, Shi R, Kailasa SK, Park TJ. Cu-nanoflower decorated gold nanoparticles-graphene oxide nanofiber as electrochemical biosensor for glucose detection. Mat Sci Eng C-Mater 2020;107:110273.
Li J, Hu B, Nie P, Shang X, Jiang W, Xu K, Yang J, Liu J. Fe-regulated δ-MnO2 nanosheet assembly on carbon nanofiber under acidic condition for high performance supercapacitor and capacitive deionization. Appl Surf Sci 2021;542:148715.
Kim JH, Joshi MK, Lee J, Park CH, Kim CS. Polydopamine-assisted immobilization of hierarchical zinc oxide nanostructures on electrospun nanofibrous membrane for photocatalysis and antimicrobial activity. J Colloid Interface Sci 2018;513:566.
Xie J, Macewan MR, Ray WZ, Liu W, Siewe DY, Xia Y. Radially aligned, electrospun nanofibers as dural substitutes for wound closure and tissue regeneration applications. ACS Nano 2010;4:5027.
Xie J, Liu W, MacEwan MR, Yeh YC, Thomopoulos S, Xia Y. Nanofiber membranes with controllable microwells and structural cues and their use in forming cell microarrays and neuronal networks. Small 2011;7:293.
Li G, Zheng T, Wu L, Han Q, Lei Y, Xue L, Zhang L, Gu X, Yang Y. Bionic microenvironment-inspired synergistic effect of anisotropic micro-nanocomposite topology and biology cues on peripheral nerve regeneration. Sci Adv 2021;7:eabi5812.
Zhu L, Wang K, Ma T, Huang L, Xia B, Zhu S, Yang Y, Liu Z, Quan X, Luo K, Kong D, Huang J, Luo Z. Noncovalent bonding of RGD and YIGSR to an electrospun poly(epsilon-caprolactone) conduit through peptide self-assembly to synergistically promote sciatic nerve regeneration in rats. Adv Healthc Mater 2017;6:1600860.
Xue J, Li H, Xia Y. Nanofiber-based multi-tubular conduits with a honeycomb structure for potential application in peripheral nerve repair. Macromol Biosci 2018;18:1800090.
Chen SX, Wang HJ, McCarthy A, Yang Z, Kim HJ, Carlson MA, Xia YN, Xie JW. Three-dimensional objects consisting of hierarchically assembled nanofibers with controlled alignments for regenerative medicine. Nano Lett 2019;19:2059.
Chen SX, Wang HJ, Mainardi VL, Talo G, McCarthy A, John JV, Teusink MJ, Hong L, Xie JW. Biomaterials with structural hierarchy and controlled 3D nanotopography guide endogenous bone regeneration. Sci Adv 2021;7:eabg3089.
Balusamy B, Celebioglu A, Senthamizhan A, Uyar T. Progress in the design and development of “fast-dissolving” electrospun nanofibers based drug delivery systems—a systematic review. J Control Release 2020;326:482.
Vijayavenkataraman S, Kannan S, Cao T, Fuh JYH, Sriram G, Lu WF. 3D-printed PCL/PPy conductive scaffolds as three-dimensional porous nerve guide conduits (NGCs) for peripheral nerve injury repair. Front Bioeng Biotechnol 2019;7:266.
Wang J, Xiong H, Zhu T, Liu Y, Pan H, Fan C, Zhao X, Lu WW. Bioinspired multichannel nerve guidance conduit based on shape memory nanofibers for potential application in peripheral nerve repair. ACS Nano 2020;14:12579.
Kataria K, Gupta A, Rath G, Mathur RB, Dhakate SR. In vivo wound healing performance of drug loaded electrospun composite nanofibers transdermal patch. Int J Pharm 2014;469:102.
Morey M, Pandit A. Responsive triggering systems for delivery in chronic wound healing. Adv Drug Deliv Rev 2018;129:169.
Yan E, Fan Y, Sun Z, Gao J, Hao X, Pei S, Wang C, Sun L, Zhang D. Biocompatible core–shell electrospun nanofibers as potential application for chemotherapy against ovary cancer. Mat Sci Eng C-Mater 2014;41:217.
Jamshidi-Adegani F, Seyedjafari E, Gheibi N, Soleimani M, Sahmani M. Prevention of adhesion bands by ibuprofen-loaded PLGA nanofibers. Biotechnol Prog 2016;32:990.
Rao F, Wang Y, Zhang D, Lu C, Cao Z, Sui J, Wu M, Zhang Y, Pi W, Wang B, Kou Y, Wang X, Zhang P, Jiang B. Aligned chitosan nanofiber hydrogel grafted with peptides mimicking bioactive brain-derived neurotrophic factor and vascular endothelial growth factor repair long-distance sciatic nerve defects in rats. Theranostics 2020;10:1590.
Niu B, Li B, Gu Y, Shen X, Liu Y, Chen L. In vitro evaluation of electrospun silk fibroin/nano-hydroxyapatite/BMP-2 scaffolds for bone regeneration. J Biomater Sci Polym Ed 2017;28:257.
Lei C, Cui Y, Zheng L, Chow PK, Wang CH. Development of a gene/drug dual delivery system for brain tumor therapy: potent inhibition via RNA interference and synergistic effects. Biomaterials 2013;34:7483.
Hu K, Xiang L, Chen J, Qu H, Wan Y, Xiang D. PLGA-liposome electrospun fiber delivery of miR-145 and PDGF-BB synergistically promoted wound healing. Chem Eng J 2021;422:129951.
Chen X, Zhang H, Yang X, Zhang W, Jiang M, Wen T, Wang J, Guo R, Liu H. Preparation and application of quaternized chitosan- and AgNPs-base synergistic antibacterial hydrogel for burn wound healing. Molecules 2021;26:4037.
Mohebian Z, Babazadeh M, Zarghami N, Mousazadeh H. Anticancer efficiency of curcumin-loaded mesoporous silica nanoparticles/nanofiber composites for potential postsurgical breast cancer treatment. J Drug Deliv Sci Technol 2021;61:102170.
Peng Y, He D, Ge X, Lu Y, Chai Y, Zhang Y, Mao Z, Luo G, Deng J, Zhang Y. Construction of heparin-based hydrogel incorporated with Cu5.4O ultrasmall nanozymes for wound healing and inflammation inhibition. Bioact Mater 2021;6:3109.
Zhang D-Y, Liu H, Li C, Younis MR, Lei S, Yang C, Lin J, Li Z, Huang P. Ceria nanozymes with preferential renal uptake for acute kidney injury alleviation. ACS Appl Mater Interfaces 2020;12:56830.
Han Y, Li Y, Zeng Q, Li H, Peng J, Xu Y, Chang J. Injectable bioactive akermanite/alginate composite hydrogels for in situ skin tissue engineering. J Mater Chem B 2017;5:3315.
Zhou Y, Gao L, Peng J, Xing M, Han Y, Wang X, Xu Y, Chang J. Bioglass activated albumin hydrogels for wound healing. Adv Healthc Mater 2018;7:1800144.
Hoi S, Tsuchiya H, Itaba N, Suzuki K, Oka H, Morimoto M, Takata T, Isomoto H, Shiota G. WNT/β-catenin signal inhibitor IC-2–derived small-molecule compounds suppress TGF-β1–induced fibrogenic response of renal epithelial cells by inhibiting SMAD2/3 signalling. Clin Exp Pharmacol P 2020;47:940.
Peukert S, Miller-Moslin K. Small-molecule inhibitors of the hedgehog signaling pathway as cancer therapeutics. ChemMedChem 2010;5:500.
Rozpedek W, Nowak A, Pytel D, Diehl AJ, Majsterek I. Molecular basis of human diseases and targeted therapy based on small-molecule inhibitors of ER stress-induced signaling pathways. Curr Mol Med 2017;17:118.
Roskoski R. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol Res 2019;144:19.
He F, Wen N, Xiao D, Yan J, Xiong H, Cai S, Liu Z, Liu Y. Aptamer-based targeted drug delivery systems: current potential and challenges. Curr Med Chem 2020;27:2189.
Yi X, Zeng W, Wang C, Chen Y, Zheng L, Zhu X, Ke Y, He X, Kuang Y, Huang Q. A step-by-step multiple stimuli-responsive metal-phenolic network prodrug nanoparticles for chemotherapy. Nano Res 2022;15:1205.
Locatelli V, Bianchi VE. Effect of GH/IGF-1 on bone metabolism and osteoporsosis. Int J Endocrinol 2014;2014:235060.
Song J, Chen Z, Liu Z, Yi Y, Tsigkou O, Li J, Li Y. Controllable release of vascular endothelial growth factor (VEGF) by wheel spinning alginate/silk fibroin fibers for wound healing. Mater Des 2021;212:110231.
Golchin A, Nourani MR. Effects of bilayer nanofibrillar scaffolds containing epidermal growth factor on full-thickness wound healing. Polym Adv Technol 2020;31:2443.
Fang Z, Ge X, Chen X, Xu Y, Yuan W-E, Ouyang Y. Enhancement of sciatic nerve regeneration with dual delivery of vascular endothelial growth factor and nerve growth factor genes. J Nanobiotechnology 2020;18:46.
Wang S, Zheng H, Zhou L, Cheng F, Liu Z, Zhang H, Wang L, Zhang Q. Nanoenzyme-reinforced injectable hydrogel for healing diabetic wounds infected with multidrug resistant bacteria. Nano Lett 2020;20:5149.
Chernikov IV, Vlassov VV, Chernolovskaya EL. Current development of siRNA bioconjugates: From research to the clinic. Front Pharmacol 2019;10:444.
Guimaraes PPG, Zhang R, Spektor R, Tan M, Chung A, Billingsley MM, El-Mayta R, Riley RS, Wang L, Wilson JM, Mitchell MJ. Ionizable lipid nanoparticles encapsulating barcoded mRNA for accelerated in vivo delivery screening. J Control Release 2019;316:404.
McCall J, Smith JJ, Marquardt KN, Knight KR, Bane H, Barber A, DeLong RK. ZnO nanoparticles protect RNA from degradation better than DNA. Nanomaterials 2017;7:378.
Lorden ER, Levinson HM, Leong KW. Integration of drug, protein, and gene delivery systems with regenerative medicine. Drug Deliv Transl Res 2015;5:168.
Puhl DL, Mohanraj D, Nelson DW, Gilbert RJ. Designing electrospun fiber platforms for efficient delivery of genetic material and genome editing tools. Adv Drug Deliv Rev 2022;183:114161.
Yin M, Wu J, Deng M, Wang P, Ji G, Wang M, Zhou C, Blum NT, Zhang W, Shi H, Jia N, Wang X, Huang P. Multifunctional magnesium organic framework-based microneedle patch for accelerating diabetic wound healing. ACS Nano 2021;15:17842.
Adhikari C, Mishra A, Nayak D, Chakraborty A. Drug delivery system composed of mesoporous silica and hollow mesoporous silica nanospheres for chemotherapeutic drug delivery. J Drug Deliv Sci Technol 2018;45:303.
Zhang X, Meng Y, Gong B, Wang T, Lu Y, Zhang L, Xue JJ. Electrospun nanofibers for manipulating the soft tissue regeneration. J Mater Chem B 2022. https://doi.org/10.1039/d2tb00609j .
Ding J, Zhang J, Li J, Li D, Xiao C, Xiao H, Yang H, Zhuang X, Chen X. Electrospun polymer biomaterials. Prog Polym Sci 2019;90:1.
Prabaharan M, Jayakumar R, Nair SV. Electrospun nanofibrous scaffolds-current status and prospects in drug delivery. Adv Polym Sci 2012;246:241.
Lan X, Wang H, Bai J, Miao X, Lin Q, Zheng J, Ding S, Li X, Tang Y. Multidrug-loaded electrospun micro/nanofibrous membranes: Fabrication strategies, release behaviors and applications in regenerative medicine. J Control Release 2021;330:1264.
Schaub NJ, Corey JM. A method to rapidly analyze the simultaneous release of multiple pharmaceuticals from electrospun fibers. Int J Pharm 2020;574:118871.
Ajmal G, Bonde GV, Mittal P, Khan G, Pandey VK, Bakade BV, Mishra B. Biomimetic PCL-gelatin based nanofibers loaded with ciprofloxacin hydrochloride and quercetin: a potential antibacterial and anti-oxidant dressing material for accelerated healing of a full thickness wound. Int J Pharm 2019;567:118480.
Xie Z, Paras CB, Weng H, Punnakitikashem P, Su L-C, Vu K, Tang L, Yang J, Nguyen KT. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater 2013;9:9351.
Wang Y, Cui W, Zhao X, Wen S, Sun Y, Han J, Zhang H. Bone remodeling-inspired dual delivery electrospun nanofibers for promoting bone regeneration. Nanoscale 2019;11:60.
Hu J, Zeng F, Wei J, Chen Y, Chen Y. Novel controlled drug delivery system for multiple drugs based on electrospun nanofibers containing nanomicelles. J Biomater Sci Polym Ed 2014;25:257.
Li W, Luo T, Yang Y, Tan X, Liu L. Formation of controllable hydrophilic/hydrophobic drug delivery systems by electrospinning of vesicles. Langmuir 2015;31:5141.
Xue J, Niu Y, Gong M, Shi R, Chen D, Zhang L, Lvov Y. Electrospun microfiber membranes embedded with drug-loaded clay nanotubes for sustained antimicrobial protection. ACS Nano 2015;9:1600.
Pachuau L. Recent developments in novel drug delivery systems for wound healing. Expert Opin Drug Deliv 2015;12:1895.
Garcia-Orue I, Gainza G, Gutierrez FB, Aguirre JJ, Evora C, Pedraz JL, Hernandez RM, Delgado A, Igartua M. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int J Pharm 2017;523:556.
Chen S, Li R, Li X, Xie J. Electrospinning: An enabling nanotechnology platform for drug delivery and regenerative medicine. Adv Drug Deliv Rev 2018;132:188.
Chen X, Wang J, An Q, Li D, Liu P, Zhu W, Mo X. Electrospun poly(l-lactic acid-co-ɛ-caprolactone) fibers loaded with heparin and vascular endothelial growth factor to improve blood compatibility and endothelial progenitor cell proliferation. Colloids Surf B 2015;128:106.
Li J, Xu W, Li D, Liu T, Zhang YS, Ding J, Chen X. Locally deployable nanofiber patch for sequential drug delivery in treatment of primary and advanced orthotopic hepatomas. ACS Nano 2018;12:6685.
Xia G, Zhang H, Cheng R, Wang H, Song Z, Deng L, Huang X, Santos HA, Cui W. Localized controlled delivery of gemcitabine via microsol electrospun fibers to prevent pancreatic cancer recurrence. Adv Healthc Mater 2018;7:1800593.
Zhou L, Zhu C, Cui W, Yang H, Li B. Long-term release of water-soluble drugs using microsol-electrospun nanofiber sheets. J Control Release 2015;213:e10.
Wu L, Gu Y, Liu L, Tang J, Mao J, Xi K, Jiang Z, Zhou Y, Xu Y, Deng L, Chen L, Cui W. Hierarchical micro/nanofibrous membranes of sustained releasing VEGF for periosteal regeneration. Biomaterials 2020;227:119555.
Liao IC, Chew SY, Leong KW. Aligned core–shell nanofibers delivering bioactive proteins. Nanomedicine 2006;1:465.
Jin G, Prabhakaran MP, Kai D, Ramakrishna S. Controlled release of multiple epidermal induction factors through core–shell nanofibers for skin regeneration. Eur J Pharm Biopharm 2013;85:689.
Li X, Xu F, He Y, Li Y, Hou J, Yang G, Zhou S. A hierarchical structured ultrafine fiber device for preventing postoperative recurrence and metastasis of breast cancer. Adv Funct Mater 2020;30:2004851.
Beck-Broichsitter M, Thieme M, Nguyen J, Schmehl T, Gessler T, Seeger W, Agarwal S, Greiner A, Kissel T. Novel “nano in nano” composites for sustained drug delivery: biodegradable nanoparticles encapsulated into nanofiber non-wovens. Macromol Biosci 2010;10:1527.
Xue J, Wu T, Li J, Zhu C, Xia Y. Promoting the outgrowth of neurites on electrospun microfibers by functionalization with electrosprayed microparticles of fatty acids. Angew Chem Int Ed 2019;58:3948.
Donsante A, Xue J, Poth KM, Hardcastle NS, Diniz B, O’Connor DM, Xia Y, Boulis NM. Controlling the release of neurotrophin-3 and chondroitinase ABC enhances the efficacy of nerve guidance conduits. Adv Healthc Mater 2020;9:2000200.
Xue J, Wu T, Qiu J, Rutledge S, Tanes ML, Xia Y. Promoting cell migration and neurite extension along uniaxially aligned nanofibers with biomacromolecular particles in a density gradient. Adv Funct Mater 2020;30:40.
He C, Nie W, Feng W. Engineering of biomimetic nanofibrous matrices for drug delivery and tissue engineering. J Mater Chem B 2014;2:7828.
Beachley V, Wen X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog Polym Sci 2010;35:868.
Koh HS, Yong T, Chan CK, Ramakrishna S. Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials 2008;29:3574.
Nie H, Soh BW, Fu YC, Wang CH. Three-dimensional fibrous PLGA/HAp composite scaffold for BMP-2 delivery. Biotechnol Bioeng 2008;99:223.
Wu G, Ma X, Fan L, Gao Y, Deng H, Wang Y. Accelerating dermal wound healing and mitigating excessive scar formation using LBL modified nanofibrous mats. Mater Des 2020;185:108265.
Yin X, Tan P, Luo H, Lan J, Shi Y, Zhang Y, Fan H, Tan L. Study on the release behaviors of berberine hydrochloride based on sandwich nanostructure and shape memory effect. Mat Sci Eng C-Mater 2020;109:110541.
Wen ML, Zhi DK, Wang LN, Cui C, Huang ZQ, Zhao YH, Wang K, Kong DL, Yuan XY. Local delivery of dual microRNAs in trilayered electrospun grafts for vascular regeneration. ACS Appl Mater Interfaces 2020;12:6863.
Zhou X, Saiding Q, Wang X, Wang J, Cui W, Chen X. Regulated exogenous/endogenous inflammation via “inner-outer” medicated electrospun fibers for promoting tissue reconstruction. Adv Healthc Mater 2022;11:2102534.
Williams L, Hatton FL, Willcock H, Mele E. Electrospinning of stimuli-responsive polymers for controlled drug delivery: pH- and temperature-driven release. Biotechnol Bioeng 2022;119:1177.
Demirci S, Celebioglu A, Aytac Z, Uyar T. pH-responsive nanofibers with controlled drug release properties. Polym Chem 2014;5:2050.
Zhang RY, Zaslavski E, Vasilyev G, Boas M, Zussman E. Tunable pH-responsive chitosan-poly(acrylic acid) electrospun fibers. Biomacromol 2018;19:588.
Schoeller J, Itel F, Wuertz-Kozak K, Fortunato G, Rossi RM. pH-Responsive electrospun nanofibers and their applications. Polym Rev 2021;62:351.
Liu Y, Song R, Zhang X, Zhang D. Enhanced antimicrobial activity and pH-responsive sustained release of chitosan/poly (vinyl alcohol)/graphene oxide nanofibrous membrane loading with allicin. Int J Biol Macromol 2020;161:1405.
Xi K, Gu Y, Tang J, Chen H, Xu Y, Wu L, Cai F, Deng L, Yang H, Shi Q, Cui W, Chen L. Microenvironment-responsive immunoregulatory electrospun fibers for promoting nerve function recovery. Nat Commun 2020;11:4504.
Zhang Z, Wu Y, Kuang G, Liu S, Zhou D, Chen X, Jing X, Huang Y. Pt(iv) prodrug-backboned micelle and DCA loaded nanofibers for enhanced local cancer treatment. J Mater Chem B 2017;5:2115.
Guo D-S, Wang K, Wang Y-X, Liu Y. Cholinesterase-responsive supramolecular vesicle. J Am Chem Soc 2012;134:10244.
Mu J, Lin J, Huang P, Chen X. Development of endogenous enzyme-responsive Nanomaterials for theranostics. Chem Soc Rev 2018;47:5554.
Ye J, Zhang X, Xie W, Gong M, Liao M, Meng Q, Xue J, Shi R, Zhang L. An enzyme-responsive prodrug with inflammation-triggered therapeutic drug release characteristics. Macromol Biosci 2020;20:2000116.
Pan G, Liu S, Zhao X, Zhao J, Fan C, Cui W. Full-course inhibition of biodegradation-induced inflammation in fibrous scaffold by loading enzyme-sensitive prodrug. Biomaterials 2015;53:202.
Krylatov AV, Maslov LN, Voronkov NS, Boshchenko AA, Popov SV, Gomez L, Wang H, Jaggi AS, Downey JM. Reactive oxygen species as intracellular signaling molecules in the cardiovascular system. Curr Cardiol Rev 2018;14:290.
Diebold L, Chandel NS. Mitochondrial ROS regulation of proliferating cells. Free Radic Biol Med 2016;100:86.
Liang J, Liu B. ROS-responsive drug delivery systems. Bioeng Transl Med 2016;1:239.
Zhang Y, Xiao C, Li M, Ding J, He C, Zhuang X, Chen X. Core-cross-linked micellar nanoparticles from a linear-dendritic prodrug for dual-responsive drug delivery. Polym Chem 2014;5:2801.
Marathe PH, Gao HX, Close KL. American diabetes association standards of medical care in diabetes 2017. J Diabetes 2017;9:320.
Gu Z, Dang TT, Ma M, Tang BC, Cheng H, Jiang S, Dong Y, Zhang Y, Anderson DG. Glucose-Responsive microgels integrated with enzyme nanocapsules for closed-loop insulin delivery. ACS Nano 2013;7:6758.
Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS, Buse JB, Gu Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci 2015;112:8260.
Li L, Yang G, Zhou GL, Wang Y, Zheng XT, Zhou SB. Thermally switched release from a nanogel-in-microfiber device. Adv Healthc Mater 2015;4:1658.
Tee SY, Ye EY, Teng CP, Tanaka Y, Tang KY, Win KY, Han MY. Advances in photothermal Nanomaterials for biomedical, environmental and energy applications. Nanoscale 2021;13:14268.
Singh B, Shukla N, Kim J, Kim K, Park MH. Stimuli-responsive nanofibers containing gold nanorods for on-demand drug delivery platforms. Pharmaceutics 2021;13:1319.
Chen HM, Sun JF, Wang ZB, Zhou Y, Lou ZC, Chen B, Wang P, Guo ZR, Tang H, Ma JQ, Xia Y, Gu N, Zhang FM. Magnetic cell-scaffold interface constructed by superparamagnetic IONP enhanced osteogenesis of adipose-derived stem cells. ACS Appl Mater Interfaces 2018;10:44279.
Johnson CDL, Ganguly D, Zuidema JM, Cardina TJ, Ziemba AM, Kearns KR, McCarthy SM, Thompson DM, Ramanath G, Borca-Tasciuc DA, Dutz S, Gilbert RJ. Injectable, magnetically orienting electrospun fiber conduits for neuron guidance. ACS Appl Mater Interfaces 2019;11:356.
Kim YJ, Ebara M, Aoyagi T. A smart hyperthermia nanofiber with switchable drug release for inducing cancer apoptosis. Adv Funct Mater 2013;23:5753.
Ribeiro C, Correia DM, Rodrigues I, Guardao L, Guimaraes S, Soares R, Lanceros-Mendez S. In vivo demonstration of the suitability of piezoelectric stimuli for bone reparation. Mater Lett 2017;209:118.
Zhang JG, Qiu KX, Sun BB, Fang J, Zhang KH, Ei-Hamshary H, Al-Deyab SS, Mo XM. The aligned core-sheath nanofibers with electrical conductivity for neural tissue engineering. J Mater Chem B 2014;2:7945.
Croitoru AM, Karacelebi Y, Saatcioglu E, Altan E, Ulag S, Aydogan HK, Sahin A, Motelica L, Oprea O, Tihauan BM, Popescu RC, Savu D, Trusca R, Ficai D, Gunduz O, Ficai A. Electrically triggered drug delivery from novel electrospun poly(lactic acid)/graphene oxide/quercetin fibrous scaffolds for wound dressing applications. Pharmaceutics 2021;13:957.
Puiggali-Jou A, Cejudo A, Del Valle LJ, Aleman C. Smart drug delivery from electrospun fibers through electroresponsive polymeric nanoparticles. ACS Appl Bio Mater 2018;1:1594.
Miar S, Ong JL, Bizios R, Guda T. Electrically stimulated tunable drug delivery from polypyrrole-coated polyvinylidene fluoride. Front Chem 2021;9:599631.
Abidian MR, Kim DH, Martin DC. Conducting-polymer nanotubes for controlled drug release. Adv Mater 2006;18:405.
Yudina A, de Smet M, Lepetit-Coiffe M, Langereis S, Van Ruijssevelt L, Smirnov P, Bouchaud V, Voisin P, Grull H, Moonen CTW. Ultrasound-mediated intracellular drug delivery using microbubbles and temperature-sensitive liposomes. J Control Release 2011;155:442.
Song B, Wu C, Chang J. Ultrasound-triggered dual-drug release from poly(lactic-co-glycolic acid)/mesoporous silica nanoparticles electrospun composite fibers. Regen Biomater 2015;2:229.
Li L, Zhang X, Zhou J, Zhang L, Xue J, Tao W. Non-Invasive thermal therapy for tissue engineering and regenerative medicine. Small 2022;18:e2107705. https://doi.org/10.1002/smll.202107705.
Sun D, Zhang ZY, Chen MY, Zhang YP, Amagat J, Kang SF, Zheng YY, Hu B, Chen ML. Co-Immobilization of Ce6 sono/photosensitizer and protonated graphitic carbon nitride on PCL/gelation fibrous scaffolds for combined sono-photodynamic cancer therapy. ACS Appl Mater Interfaces 2020;12:40728.
Hao R, Cui Z, Zhang X, Tian M, Zhang L, Rao F, Xue J. Rational design and preparation of functional hydrogels for skin wound healing. Front Chem 2021;9:839055.
Xia G, Zhai D, Sun Y, Hou L, Guo X, Wang L, Li Z, Wang F. Preparation of a novel asymmetric wettable chitosan-based sponge and its role in promoting chronic wound healing. Carbohydr Polym 2020;227:115296.
Wang C, Wang M, Xu T, Zhang X, Lin C, Gao W, Xu H, Lei B, Mao C. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics 2019;9:65.
Wang Y, Wu Y, Long L, Yang L, Fu D, Hu C, Kong Q, Wang Y. Inflammation-Responsive drug-loaded hydrogels with sequential hemostasis, antibacterial, and anti-inflammatory behavior for chronically infected diabetic wound treatment. ACS Appl Mater Interfaces 2021;13:33584.
Luo ZQ, Che JY, Sun LY, Yang L, Zu Y, Wang H, Zhao YJ. Microfluidic electrospray photo-crosslinkable κ-Carrageenan microparticles for wound healing. Eng Regener 2021;2:257.
Ulker Turan C, Guvenilir Y. Electrospun poly(ω-pentadecalactone-co-ε-caprolactone)/gelatin/chitosan ternary nanofibers with antibacterial activity for treatment of skin infections. Eur J Pharm Sci 2022;170:106113.
Song J, Yuan C, Jiao T, Xing R, Yang M, Adams DJ, Yan X. Multifunctional antimicrobial biometallohydrogels based on amino acid coordinated self-assembly. Small 2020;16:1907309.
Xu M, Khan A, Wang T, Song Q, Han C, Wang Q, Gao L, Huang X, Li P, Huang W. Mussel-Inspired hydrogel with potent in vivo contact-active antimicrobial and wound healing promoting activities. ACS Appl Bio Mater 2019;2:3329.
Su Y, Mainardi VL, Wang H, McCarthy A, Zhang YS, Chen S, John JV, Wong SL, Hollins RR, Wang G, Xie J. Dissolvable microneedles coupled with nanofiber dressings eradicate biofilms via effectively delivering a database-designed antimicrobial peptide. ACS Nano 2020;14:11775.
Zhang S, Ye J, Liu X, Wang G, Qi Y, Wang T, Song Y, Li Y, Ning G. Dual stimuli-responsive smart fibrous membranes for efficient photothermal/photodynamic/chemo-Therapy of drug-resistant bacterial infection. Chem Eng J 2022;432:134351.
Xie X, Li D, Chen Y, Shen Y, Yu F, Wang W, Yuan Z, Morsi Y, Wu J, Mo X. Conjugate electrospun 3D gelatin nanofiber sponge for rapid hemostasis. Adv Healthc Mater 2021;10:2100918.
Zhao H, Huang J, Li Y, Lv X, Zhou H, Wang H, Xu Y, Wang C, Wang J, Liu Z. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials 2020;258:120286.
Tu Z, Chen M, Wang M, Shao Z, Jiang X, Wang K, Yao Z, Yang S, Zhang X, Gao W, Lin C, Lei B, Mao C. Engineering bioactive M2 macrophage-polarized anti-inflammatory, antioxidant, and antibacterial scaffolds for rapid angiogenesis and diabetic wound repair. Adv Funct Mater 2021;31:2100924.
Li A, Li L, Zhao B, Li X, Liang W, Lang M, Cheng B, Li J. Antibacterial, antioxidant and anti-inflammatory PLCL/gelatin nanofiber membranes to promote wound healing. Int J Biol Macromol 2022;194:914.
Jia Z, Gong J, Zeng Y, Ran J, Liu J, Wang K, Xie C, Lu X, Wang J. Bioinspired conductive silk microfiber integrated bioelectronic for diagnosis and wound healing in diabetes. Adv Funct Mater 2021;31:2010461.
Gao Y, Qiu Z, Liu L, Li M, Xu B, Yu D, Qi D, Wu J. Multifunctional fibrous wound dressings for refractory wound healing. J Polym Sci 2022. https://doi.org/10.1002/pol.20220008 .
Chen X, Wang X, Wang S, Zhang X, Yu J, Wang C. Mussel-inspired polydopamine-assisted bromelain immobilization onto electrospun fibrous membrane for potential application as wound dressing. Mat Sci Eng C-Mater 2020;110:110624.
Zhang Q, Oh JH, Park CH, Baek JH, Ryoo HM, Woo KM. Effects of dimethyloxalylglycine-embedded poly(ε-caprolactone) fiber meshes on wound healing in diabetic rats. ACS Appl Mater Interfaces 2017;9:7950.
Zhu Z, Liu Y, Xue Y, Cheng X, Zhao W, Wang J, He R, Wan Q, Pei X. Tazarotene released from aligned electrospun membrane facilitates cutaneous wound healing by promoting angiogenesis. ACS Appl Mater Interfaces 2019;11:36141.
Choi JS, Choi SH, Yoo HS. Coaxial electrospun nanofibers for treatment of diabetic ulcers with binary release of multiple growth factors. J Mater Chem 2011;21:5258.
Mulholland EJ. Electrospun biomaterials in the treatment and prevention of scars in skin wound healing. Front Bioeng Biotechnol 2020;8:481.
Zhang J, Zheng Y, Lee J, Hua J, Li S, Panchamukhi A, Yue J, Gou X, Xia Z, Zhu L, Wu X. A pulsatile release platform based on photo-induced imine-crosslinking hydrogel promotes scarless wound healing. Nat Commun 2021;12:1670.
Zhang H, Guo M, Zhu T, Xiong H, Zhu L-M. A careob-like nanofibers with a sustained drug release profile for promoting skin wound repair and inhibiting hypertrophic scar. Compos B Eng 2022;236:109790.
Guo X, Liu Y, Bera H, Zhang H, Chen Y, Cun D, Foderà V, Yang M. α-Lactalbumin-based nanofiber dressings improve burn wound healing and reduce scarring. ACS Appl Mater Interfaces 2020;12:45702.
Xu T, Yang R, Ma X, Chen W, Liu S, Liu X, Cai X, Xu H, Chi B. Bionic poly(γ-glutamic acid) electrospun fibrous scaffolds for preventing hypertrophic scars. Adv Healthc Mater 2019;8:1900123.
Jiang Z, Zhao L, He F, Tan H, Li Y, Tang Y, Duan X, Li Y. Palmatine-loaded electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds accelerate wound healing and inhibit hypertrophic scar formation in a rabbit ear model. J Biomater Appl 2021;35:869.
Yang B, Dong Y, Shen Y, Hou A, Quan G, Pan X, Wu C. Bilayer dissolving microneedle array containing 5-fluorouracil and triamcinolone with biphasic release profile for hypertrophic scar therapy. Bioact Mater 2021;6:2400.
Zhang X, Lv R, Chen L, Sun R, Zhang Y, Sheng R, Du T, Li Y, Qi Y. A multifunctional janus electrospun nanofiber dressing with biofluid draining, monitoring, and antibacterial properties for wound healing. ACS Appl Mater Interfaces 2022;14:12984.
Zeng Q, Qi X, Shi G, Zhang M, Haick H. Wound dressing: From Nanomaterials to diagnostic dressings and healing evaluations. ACS Nano 2022;16:1708.
Gong M, Wan P, Ma D, Zhong M, Liao M, Ye J, Shi R, Zhang L. Flexible breathable nanomesh electronic devices for on-demand therapy. Adv Funct Mater 2019;29:1902127.
Deng R, Luo Z, Rao Z, Lin Z, Chen S, Zhou J, Zhu Q, Liu X, Bai Y, Quan D. Decellularized extracellular matrix containing electrospun fibers for nerve regeneration: a comparison between core-shell structured and preblended composites. Adv Fiber Mater 2022;4:503.
Pi W, Zhang Y, Li L, Li C, Zhang M, Zhang W, Cai Q, Zhang P. Polydopamine-coated polycaprolactone/carbon nanotube fibrous scaffolds loaded with brain-derived neurotrophic factor for peripheral nerve regeneration. Biofabrication 2022;14:035006.
Tanes ML, Xue J, Xia Y. A General strategy for generating gradients of bioactive proteins on electrospun nanofiber mats by masking with bovine serum albumin. J Mater Chem B 2017;5:5580.
Zhu L, Jia S, Liu T, Yan L, Huang D, Wang Z, Chen S, Zhang Z, Zeng W, Zhang Y, Yang H, Hao D. Aligned PCL fiber conduits immobilized with nerve growth factor gradients enhance and direct sciatic nerve regeneration. Adv Funct Mater 2020;30:2002610.
Ye K, Kuang H, You Z, Morsi Y, Mo X. Electrospun nanofibers for tissue engineering with drug loading and release. Pharmaceutics 2019;11:182.
Saudi A, Zebarjad SM, Alipour H, Katoueizadeh E, Alizadeh A, Rafienia M. A study on the role of multi-walled carbon nanotubes on the properties of electrospun poly(caprolactone)/poly(glycerol sebacate) scaffold for nerve tissue applications. Mater Chem Phys 2022;282:125868.
Zhang Z, Jorgensen ML, Wang Z, Amagat J, Wang Y, Li Q, Dong M, Chen M. 3D anisotropic photocatalytic architectures as bioactive nerve guidance conduits for peripheral neural regeneration. Biomaterials 2020;253:120108.
Jin F, Li T, Yuan T, Du L, Lai C, Wu Q, Zhao Y, Sun F, Gu L, Wang T, Feng ZQ. Physiologically self-regulated, fully implantable, battery-free system for peripheral nerve restoration. Adv Mater 2021;33:2104175.
Wang J, Cheng Y, Chen L, Zhu T, Ye K, Jia C, Wang H, Zhu M, Fan C, Mo X. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration. Acta Biomater 2019;84:98.
Funnell JL, Ziemba AM, Nowak JF, Awada H, Prokopiou N, Samuel J, Guari Y, Nottelet B, Gilbert RJ. Assessing the combination of magnetic field stimulation, iron oxide nanoparticles, and aligned electrospun fibers for promoting neurite outgrowth from dorsal root ganglia in vitro. Acta Biomater 2021;131:302.
Xia B, Lv Y. Dual-delivery of VEGF and NGF by emulsion electrospun nanofibrous scaffold for peripheral nerve regeneration. Mat Sci Eng C-Mater 2018;82:253.
Qian Y, Song J, Zhao X, Chen W, Ouyang Y, Yuan W, Fan C. 3D fabrication with integration molding of a graphene oxide/polycaprolactone nanoscaffold for neurite regeneration and angiogenesis. Adv Sci 2018;5:1700499.
Qian Y, Lin H, Yan Z, Shi J, Fan C. Functional NanoMaterials in peripheral nerve regeneration: Scaffold design, chemical principles and microenvironmental remodeling. Mater Today 2021;51:165.
Jahromi HK, Farzin A, Hasanzadeh E, Barough SE, Mahmoodi N, Najafabadi MRH, Farahani MS, Mansoori K, Shirian S, Ai J. Enhanced sciatic nerve regeneration by poly-L-lactic acid/multi-wall carbon nanotube neural guidance conduit containing Schwann cells and curcumin encapsulated chitosan nanoparticles in rat. Mat Sci Eng C-Mater 2020;109:110564.
Zuidema JM, Dumont CM, Wang J, Batchelor WM, Lu YS, Kang J, Bertucci A, Ziebarth NM, Shea LD, Sailor MJ. Porous silicon nanoparticles embedded in poly(lactic-co-glycolic acid) nanofiber scaffolds deliver neurotrophic payloads to enhance neuronal growth. Adv Funct Mater 2020;30:2002560.
Ayoubi-Joshaghani MH, Seidi K, Azizi M, Jaymand M, Javaheri T, Jahanban-Esfahlan R, Hamblin MR. Potential applications of advanced nano/hydrogels in biomedicine: Static, dynamic, multi-stage, and bioinspired. Adv Funct Mater 2020;30:2004098.
Wang L, Wu Y, Hu T, Ma PX, Guo B. Aligned conductive core-shell biomimetic scaffolds based on nanofiber yarns/hydrogel for enhanced 3D neurite outgrowth alignment and elongation. Acta Biomater 2019;96:175.
Colello RJ, Chow WN, Bigbee JW, Lin C, Dalton D, Brown D, Jha BS, Mathern BE, Lee KD, Simpson DG. The incorporation of growth factor and chondroitinase ABC into an electrospun scaffold to promote axon regrowth following spinal cord injury. J Tissue Eng Regen Med 2016;10:656.
Tian L, Prabhakaran MP, Ramakrishna S. Strategies for regeneration of components of nervous system: scaffolds, cells and biomolecules. Regen Biomater 2015;2:31.
Fernandez D, Guerra M, Lisoni JG, Hoffmann T, Araya-Hermosilla R, Shibue T, Nishide H, Moreno-Villoslada I, Flores ME. Fibrous materials made of poly(epsilon-caprolactone)/poly(ethylene oxide)-b-poly(epsilon-caprolactone) blends support neural stem cells differentiation. Polymers 2019;11:1621.
Zhang S, Wang XJ, Li WS, Xu XL, Hu JB, Kang XQ, Qi J, Ying XY, You J, Du YZ. Polycaprolactone/polysialic acid hybrid, multifunctional nanofiber scaffolds for treatment of spinal cord injury. Acta Biomater 2018;77:15.
Wang S, Hashemi S, Stratton S, Arinzeh TL. The effect of physical cues of biomaterial scaffolds on stem cell behavior. Adv Healthc Mater 2021;10:2001244.
Mahumane GD, Kumar P, Pillay V, Choonara YE. Repositioning N-acetylcysteine (NAC): NAC-loaded electrospun drug delivery scaffolding for potential neural tissue engineering application. Pharmaceutics 2020;12:934.
Tang W, Fang F, Liu K, Huang Z, Li H, Yin Y, Wang J, Wang G, Wei L, Ou Y, Wang Y. Aligned biofunctional electrospun PLGA-LysoGM1 scaffold for traumatic brain injury repair. ACS Biomater Sci Eng 2020;6:2209.
Zhang Y, Wang J, Xiao J, Fang T, Hu N, Li M, Deng L, Cheng Y, Zhu Y, Cui W. An electrospun fiber-covered stent with programmable dual drug release for endothelialization acceleration and lumen stenosis prevention. Acta Biomater 2019;94:295.
He L, Huang G, Liu H, Sang C, Liu X, Chen T. Highly bioactive zeolitic imidazolate framework-8-capped nanotherapeutics for efficient reversal of reperfusion-induced injury in ischemic stroke. Sci Adv 2020;6:9751.
Houlton J, Abumaria N, Hinkley SFR, Clarkson AN. Therapeutic potential of neurotrophins for repair after brain injury: A helping hand from biomaterials. Front Neurosci 2019;13:790.
Huang M, Hu M, Song Q, Song H, Huang J, Gu X, Wang X, Chen J, Kang T, Feng X, Jiang D, Zheng G, Chen H, Gao X. GM1-modified lipoprotein-like nanoparticle: multifunctional nanoplatform for the combination therapy of Alzheimer’s disease. ACS Nano 2015;9:10801.
AnjiReddy K, Karpagam S. Chitosan nanofilm and electrospun nanofiber for quick drug release in the treatment of Alzheimer’s disease: In vitro and in vivo evaluation. Int J Biol Macromol 2017;105:131.
Bloem BR, Okun MS, Klein C. Parkinson’s disease. The Lancet 2021;397:2284.
Bukhary H, Williams GR, Orlu M. Fabrication of electrospun levodopa-carbidopa fixed-dose combinations. Adv Fiber Mater 2020;2:194.
Wu Y, Zhang Q, Wang H, Wang M. Multiscale engineering of functional organic polymer interfaces for neuronal stimulation and recording. Mater Chem Front 2020;4:3444.
Siafaka PI, Ozcan Bulbul E, Dilsiz P, Karantas ID, Okur ME, Ustundag ON. Detecting and targeting neurodegenerative disorders using electrospun nanofibrous matrices: current status and applications. J Drug Target 2021;29:476.
Jessen KR, Mirsky R. The success and failure of the Schwann cell response to nerve injury. Front Cell Neurosci 2019;13:33.
Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science 2009;324:98.
Roshanbinfar K, Vogt L, Ruther F, Roether JA, Boccaccini AR, Engel FB. Nanofibrous composite with tailorable electrical and mechanical properties for cardiac tissue engineering. Adv Funct Mater 2020;30:1908612.
Jin G, He R, Sha B, Li W, Qing H, Teng R, Xu F. Electrospun three-dimensional aligned nanofibrous scaffolds for tissue engineering. Mat Sci Eng C-Mater 2018;92:995.
Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 2014;11:255.
Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction. Circ Res 2016;119:91.
Jain A, Behera M, Mahapatra C, Sundaresan NR, Chatterjee K. Nanostructured polymer scaffold decorated with cerium oxide nanoparticles toward engineering an antioxidant and anti-hypertrophic cardiac patch. Mat Sci Eng C-Mater 2021;118:111416.
Wang F, Guan J. Cellular cardiomyoplasty and cardiac tissue engineering for myocardial therapy. Adv Drug Deliv Rev 2010;62:784.
Li Z, Guan J. Hydrogels for cardiac tissue engineering. Polymers 2011;3:740.
Spinale FG, Janicki JS, Zile MR. Membrane-associated matrix proteolysis and heart failure. Circ Res 2013;112:195.
Ahmad Shiekh P, Anwar Mohammed S, Gupta S, Das A, Meghwani H, Kumar Maulik S, Kumar Banerjee S, Kumar A. Oxygen releasing and antioxidant breathing cardiac patch delivering exosomes promotes heart repair after myocardial infarction. Chem Eng J 2022;428:132490.
Zhu D, Hou J, Qian M, Jin D, Hao T, Pan Y, Wang H, Wu S, Liu S, Wang F, Wu L, Zhong Y, Yang Z, Che Y, Shen J, Kong D, Yin M, Zhao Q. Nitrate-functionalized patch confers cardioprotection and improves heart repair after myocardial infarction via local nitric oxide delivery. Nat Commun 2021;12:4501.
Yu J, Lee A-R, Lin W-H, Lin C-W, Wu Y-K, Tsai W-B. Electrospun PLGA fibers incorporated with functionalized biomolecules for cardiac tissue engineering. Tissue Eng Part A 2014;20:1896.
Kai D, Prabhakaran MP, Jin G, Tian L, Ramakrishna S. Potential of VEGF-encapsulated electrospun nanofibers for in vitro cardiomyogenic differentiation of human mesenchymal stem cells. J Tissue Eng Regen Med 2017;11:1002.
Chen J, Zhan Y, Wang Y, Han D, Tao B, Luo Z, Ma S, Wang Q, Li X, Fan L, Li C, Deng H, Cao F. Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats. Acta Biomater 2018;80:154.
Muniyandi P, Palaninathan V, Mizuki T, Mohamed MS, Hanajiri T, Maekawa T. Scaffold mediated delivery of dual miRNAs to transdifferentiate cardiac fibroblasts. Mat Sci Eng C-Mater 2021;128:112323.
Fleischer S, Shevach M, Feiner R, Dvir T. Coiled fiber scaffolds embedded with gold nanoparticles improve the performance of engineered cardiac tissues. Nanoscale 2014;6:9410.
Karimi SNH, Aghdam RM, Ebrahimi SAS, Chehrehsaz Y. Tri-layered alginate/poly(epsilon-caprolactone) electrospun scaffold for cardiac tissue engineering. Polym Int 2022. https://doi.org/10.1002/pi.6371 .
Zhao G, Feng Y, Xue L, Cui M, Zhang Q, Xu F, Peng N, Jiang Z, Gao D, Zhang X. Anisotropic conductive reduced graphene oxide/silk matrices promote post-infarction myocardial function by restoring electrical integrity. Acta Biomater 2022;139:190.
Kapnisi M, Mansfield C, Marijon C, Guex AG, Perbellini F, Bardi I, Humphrey EJ, Puetzer JL, Mawad D, Koutsogeorgis DC, Stuckey DJ, Terracciano CM, Harding SE, Stevens MM. Auxetic cardiac patches with tunable mechanical and conductive properties toward treating myocardial infarction. Adv Funct Mater 2018;28:1800618.
Zhao G, Zhang X, Lu TJ, Xu F. Recent advances in electrospun nanofibrous scaffolds for cardiac tissue engineering. Adv Funct Mater 2015;25:5726.
Hsiao CW, Bai MY, Chang Y, Chung MF, Lee TY, Wu CT, Maiti B, Liao ZX, Li RK, Sung HW. Electrical coupling of isolated cardiomyocyte clusters grown on aligned conductive nanofibrous meshes for their synchronized beating. Biomaterials 2013;34:1063.
Bouma BJ, Mulder BJM. Changing landscape of congenital heart disease. Circ Res 2017;120:908.
Zhang J, Zhu W, Radisic M, Vunjak-Novakovic G. Can we engineer a human cardiac patch for therapy? Circ Res 2018;123:244.
Agarwal U, Smith AW, French KM, Boopathy AV, George A, Trac D, Brown ME, Shen M, Jiang R, Fernandez JD, Kogon BE, Kanter KR, Alsoufi B, Wagner MB, Platt MO, Davis ME. Age-dependent effect of pediatric cardiac progenitor cells after juvenile heart failure. Stem Cells Transl Med 2016;5:883.
Streeter BW, Xue J, Xia Y, Davis ME. Electrospun nanofiber-based patches for the delivery of cardiac progenitor cells. ACS Appl Mater Interfaces 2019;11:18242.
Streeter BW, Brown ME, Shakya P, Park HJ, Qiu J, Xia Y, Davis ME. Using computational methods to design patient-specific electrospun cardiac patches for pediatric heart failure. Biomaterials 2022;283:121421.
Zheng J, Rahman N, Li LF, Zhang JS, Tan HZ, Xue Y, Zhao Y, Zhai JL, Zhao NN, Xu FJ, Zhang LQ, Shi R, Lvov Y, Xue JJ. Biofunctionalization of electrospun fiber membranes by LbL-collagen/chondroitin sulfate nanocoating followed by mineralization for bone regeneration. Mat Sci Eng C-Mater 2021;128:13.
Zhang XD, Koo S, Kim JH, Huang XG, Kong N, Zhang LQ, Zhou J, Xue JJ, Harris MB, Tao W, Kim JS. Nanoscale materials-based platforms for the treatment of bone-related diseases. Matter 2021;4:2727.
Shi R, Zhang JS, Niu K, Li WY, Jiang N, Li JL, Yu QS, Wu CA. Electrospun artificial periosteum loaded with DFO contributes to osteogenesis via the TGF-beta 1/Smad2 pathway. Biomater Sci 2021;9:2090.
Ren S, Zhou Y, Zheng K, Xu X, Yang J, Wang X, Miao L, Wei H, Xu Y. Cerium oxide nanoparticles loaded nanofibrous membranes promote bone regeneration for periodontal tissue engineering. Bioact Mater 2022;7:242.
Cheng X, Cheng G, Xing X, Yin CC, Cheng Y, Zhou X, Jiang S, Tao FH, Deng HB, Li ZB. Controlled release of adenosine from core-shell nanofibers to promote bone regeneration through STAT3 signaling pathway. J Control Release 2020;319:234.
Bhattarai DP, Kim MH, Park H, Park WH, Kim BS, Kim CS. Coaxially fabricated polylactic acid electrospun nanofibrous scaffold for sequential release of tauroursodeoxycholic acid and bone morphogenic protein2 to stimulate angiogenesis and bone regeneration. Chem Eng J 2020;389:123470.
Cheng G, Yin CC, Tu H, Jiang S, Wang Q, Zhou X, Xing X, Xie CY, Shi XW, Du YM, Deng HB, Li ZB. Controlled co-delivery of growth factors through layer-by-layer assembly of core-shell nanofibers for improving bone regeneration. ACS Nano 2019;13:6372.
Wu ZZ, Bao CY, Zhou SB, Yang T, Wang L, Li MZ, Li L, Luo E, Yu YJ, Wang YS, Guo XD, Liu X. The synergetic effect of bioactive molecule-loaded electrospun core-shell fibres for reconstruction of critical-sized calvarial bone defect-The effect of synergetic release on bone Formation. Cell Prolif 2020;53:e12796.
Zhang Q, Ji YJ, Zheng WP, Yan MZ, Wang DY, Li M, Chen JB, Yan X, Zhang Q, Yuan X, Zhou QH. Electrospun nanofibers containing strontium for bone tissue engineering. J Nanomater 2020;2020:1257646.
Ji HZ, Wang YY, Liu HH, Liu Y, Zhang XH, Xu JZ, Li ZM, Luo E. Programmed core-shell electrospun nanofibers to sequentially regulate osteogenesis-osteoclastogenesis balance for promoting immediate implant osseointegration. Acta Biomater 2021;135:274.
Maharjan B, Kaliannagounder VK, Jang SR, Awasthi GP, Bhattarai DP, Choukrani G, Park CH, Kim CS. In-situ polymerized polypyrrole nanoparticles immobilized poly(epsilon-caprolactone) electrospun conductive scaffolds for bone tissue engineering. Mat Sci Eng C-Mater 2020;114:111056.
Nekounam H, Allahyari Z, Gholizadeh S, Mirzaei E, Shokrgozar MA, Faridi-Majidi R. Simple and robust fabrication and characterization of conductive carbonized nanofibers loaded with gold nanoparticles for bone tissue engineering applications. Mat Sci Eng C-Mater 2020;117:111226.
Tong LP, Liao Q, Zhao YT, Huang H, Gao A, Zhang W, Gao XY, Wei W, Guan M, Chu PK, Wang HY. Near-infrared light control of bone regeneration with biodegradable photothermal osteoimplant. Biomaterials 2019;193:1.
Ma K, Liao CA, Huang LL, Liang RM, Zhao JM, Zheng L, Su W. Electrospun PCL/MoS2 nanofiber membranes combined with NIR-triggered photothermal therapy to accelerate bone regeneration. Small 2021;17:15.
Lu Y, Wan Y, Gan D, Zhang Q, Luo H, Deng X, Li Z, Yang Z. Enwrapping polydopamine on doxorubicin-loaded lamellar hydroxyapatite/poly(lactic-co-glycolic acid) composite fibers for inhibiting bone tumor recurrence and enhancing bone regeneration. ACS Appl Bio Mater 2021;4:6036.
He X, Feng B, Huang C, Wang H, Ge Y, Hu R, Yin M, Xu Z, Wang W, Fu W, Zheng J. Electrospun gelatin/polycaprolactone nanofibrous membranes combined with a coculture of bone marrow stromal cells and chondrocytes for cartilage engineering. Int J Nanomed 2015;10:2089.
Zhao Y, Wei C, Chen X, Liu J, Yu Q, Liu Y, Liu J. Drug delivery system based on near-infrared light-responsive molybdenum disulfide nanosheets controls the high-efficiency release of dexamethasone to inhibit inflammation and treat osteoarthritis. ACS Appl Mater Interfaces 2019;11:11587.
Kishan AP, Cosgriff-Hernandez EM. Recent advancements in electrospinning design for tissue engineering applications: a review. J Biomed Mater Res A 2017;105:2892.
Mirzaei S, Karkhaneh A, Soleimani M, Ardeshirylajimi A, Seyyed Zonouzi H, Hanaee-Ahvaz H. Enhanced chondrogenic differentiation of stem cells using an optimized electrospun nanofibrous PLLA/PEG scaffolds loaded with glucosamine. J Biomed Mater Res A 2017;105:2461.
Zhao W, Du Z, Fang J, Fu L, Zhang X, Cai Q, Yang X. Synthetic/natural blended polymer fibrous meshes composed of polylactide, gelatin and glycosaminoglycan for cartilage repair. J Biomater Sci Polym Ed 2020;31:1437.
Wang C, Hou W, Guo X, Li J, Hu T, Qiu M, Liu S, Mo X, Liu X. Two-phase electrospinning to incorporate growth factors loaded chitosan nanoparticles into electrospun fibrous scaffolds for bioactivity retention and cartilage regeneration. Mat Sci Eng C-Mater 2017;79:507.
Kim C, Shores L, Guo Q, Aly A, Jeon OH, Kim DH, Bernstein N, Bhattacharya R, Chae JJ, Yarema KJ, Elisseeff JH. Electrospun microfiber scaffolds with anti-Inflammatory tributanoylated N-acetyl-d-glucosamine promote cartilage regeneration. Tissue Eng Part A 2016;22:689.
Wang Z, Wang Y, Zhang P, Chen X. Methylsulfonylmethane-loaded electrospun poly(lactide-co-glycolide) mats for cartilage tissue engineering. RSC Adv 2015;5:96725.
Feng B, Tu H, Yuan H, Peng H, Zhang Y. Acetic-acid-mediated miscibility toward electrospinning homogeneous composite nanofibers of GT/PCL. Biomacromol 2012;13:3917.
Zheng R, Duan H, Xue J, Liu Y, Feng B, Zhao S, Zhu Y, Liu Y, He A, Zhang W, Liu W, Cao Y, Zhou G. The influence of gelatin/PCL ratio and 3-D construct shape of electrospun membranes on cartilage regeneration. Biomaterials 2014;35:152.
Semitela A, Ramalho G, Capitao A, Sousa C, Mendes AF, Aap Marques P, Completo A. Bio-electrospraying assessment toward in situ chondrocyte-laden electrospun scaffold fabrication. J Tissue Eng 2022;13:20417314211069344.
Sadeghi D, Karbasi S, Razavi S, Mohammadi S, Shokrgozar MA, Bonakdar S. Electrospun poly(hydroxybutyrate)/chitosan blend fibrous scaffolds for cartilage tissue engineering. J Appl Polym Sci 2016;133:44171.
Li Y, Liu Y, Xun X, Zhang W, Xu Y, Gu D. Three-dimensional porous scaffolds with biomimetic microarchitecture and bioactivity for cartilage tissue engineering. ACS Appl Mater Interfaces 2019;11:36359.
Dalle Carbonare L, Bertacco J, Marchetto G, Cheri S, Deiana M, Minoia A, Tiso N, Mottes M, Valenti MT. Methylsulfonylmethane enhances MSC chondrogenic commitment and promotes pre-osteoblasts formation. Stem Cell Res Ther 2021;12:326.
Venugopal E, Sahanand KS, Bhattacharyya A, Rajendran S. Electrospun PCL nanofibers blended with Wattakaka volubilis active phytochemicals for bone and cartilage tissue engineering. Nanomedicine 2019;21:102044.
Li X, Ding J, Zhang Z, Yang M, Yu J, Wang J, Chang F, Chen X. Kartogenin-incorporated thermogel supports stem cells for significant cartilage regeneration. ACS Appl Mater Interfaces 2016;8:5148.
Shi D, Xu X, Ye Y, Song K, Cheng Y, Di J, Hu Q, Li J, Ju H, Jiang Q, Gu Z. Photo-cross-linked scaffold with kartogenin-encapsulated nanoparticles for cartilage regeneration. ACS Nano 2016;10:1292.
Silva JC, Udangawa RN, Chen J, Mancinelli CD, Garrudo FFF, Mikael PE, Cabral JMS, Ferreira FC, Linhardt RJ. Kartogenin-loaded coaxial PGS/PCL aligned nanofibers for cartilage tissue engineering. Mat Sci Eng C-Mater 2020;107:110291.
Gupta N, Kamath SM, Rao SK, Patil DJS, Gupta N, Arunachalam KD. Kaempferol loaded albumin nanoparticles and dexamethasone encapsulation into electrospun polycaprolactone fibrous mat—concurrent release for cartilage regeneration. J Drug Deliv Sci Technol 2021;64:102666.
Martin AR, Patel JM, Locke RC, Eby MR, Saleh KS, Davidson MD, Sennett ML, Zlotnick HM, Chang AH, Carey JL, Burdick JA, Mauck RL. Nanofibrous hyaluronic acid scaffolds delivering TGF-beta3 and SDF-1alpha for articular cartilage repair in a large animal model. Acta Biomater 2021;126:170.
Feng B, Ji T, Wang X, Fu W, Ye L, Zhang H, Li F. Engineering cartilage tissue based on cartilage-derived extracellular matrix cECM/PCL hybrid nanofibrous scaffold. Mater Des 2020;193:108773.
Formica FA, Ozturk E, Hess SC, Stark WJ, Maniura-Weber K, Rottmar M, Zenobi-Wong M. A bioinspired ultraporous nanofiber-hydrogel mimic of the cartilage extracellular matrix. Adv Healthc Mater 2016;5:3129.
Chen Y, Xu W, Shafiq M, Tang J, Hao J, Xie X, Yuan Z, Xiao X, Liu Y, Mo X. Three-dimensional porous gas-foamed electrospun nanofiber scaffold for cartilage regeneration. J Colloid Interface Sci 2021;603:94.
Chen Y, Xu W, Shafiq M, Song D, Xie X, Yuan Z, El-Newehy M, El-Hamshary H, Morsi Y, Liu Y, Mo X. Chondroitin sulfate cross-linked three-dimensional tailored electrospun scaffolds for cartilage regeneration. Mat Sci Eng C-Mater 2022;1:12643.
Chen T, Bai J, Tian J, Huang P, Zheng H, Wang J. A single integrated osteochondral in situ composite scaffold with a multi-layered functional structure. Colloids Surf B 2018;167:354.
Chen W, Chen S, Morsi Y, El-Hamshary H, El-Newhy M, Fan C, Mo X. Superabsorbent 3D scaffold based on electrospun nanofibers for cartilage tissue engineering. ACS Appl Mater Interfaces 2016;8:24415.
Chen S, Chen W, Chen Y, Mo X, Fan C. Chondroitin sulfate modified 3D porous electrospun nanofiber scaffolds promote cartilage regeneration. Mat Sci Eng C-Mater 2021;118:111312.
Rampichova M, Kost’akova Kuzelova E, Filova E, Chvojka J, Safka J, Pelcl M, Dankova J, Prosecka E, Buzgo M, Plencner M, Lukas D, Amler E. Composite 3D printed scaffold with structured electrospun nanofibers promotes chondrocyte adhesion and infiltration. Cell Adh Migr 2018;12:271.
Chen W, Xu Y, Liu Y, Wang Z, Li Y, Jiang G, Mo X, Zhou G. Three-dimensional printed electrospun fiber-based scaffold for cartilage regeneration. Mater Des 2019;179:107886.
Liang R, Zhao J, Li B, Cai P, Loh XJ, Xu C, Chen P, Kai D, Zheng L. Implantable and degradable antioxidant poly(epsilon-caprolactone)-lignin nanofiber membrane for effective osteoarthritis treatment. Biomaterials 2020;230:119601.
Nazarnezhad S, Baino F, Kim HW, Webster TJ, Kargozar S. Electrospun nanofibers for improved angiogenesis: Promises for tissue engineering applications. Nanomaterials 2020;10:1609.
Wang YN, Ma BX, Yin AL, Zhang B, Luo RF, Pan JQ, Wang YB. Polycaprolactone vascular graft with epigallocatechin gallate embedded sandwiched layer-by-layer functionalization for enhanced antithrombogenicity and anti-inflammation. J Control Release 2020;320:226.
Xiang ZH, Chen RH, Ma ZF, Shi Q, Ataullakhanov FI, Panteleev M, Yin JH. A dynamic remodeling bio-mimic extracellular matrix to reduce thrombotic and inflammatory complications of vascular implants. Biomater Sci 2020;8:6025.
Yang FH, Wang JX, Li XY, Jia ZZ, Wang Q, Yu DZ, Li JY, Niu XF. Electrospinning of a sandwich-structured membrane with sustained release capability and long-term anti-inflammatory effects for dental pulp regeneration. Bio-Des Manuf 2021;5:305.
Boda SK, Almoshari Y, Wang HJ, Wang XY, Reinhardt RA, Duan B, Wang D, Xie JW. Mineralized nanofiber segments coupled with calcium-binding BMP-2 peptides for alveolar bone regeneration. Acta Biomater 2019;85:282.
Qian YZ, Zhou XF, Zhang FM, Diekwisch TGH, Luan XH, Yang JX. Triple PLGA/PCL scaffold modification including silver impregnation, collagen coating, and electrospinning significantly improve biocompatibility, antimicrobial, and osteogenic properties for orofacial tissue regeneration. ACS Appl Mater Interfaces 2019;11:37381.
Edmans JG, Clitherow KH, Murdoch C, Hatton PV, Spain SG, Colley HE. Mucoadhesive electrospun fibre-based technologies for oral medicine. Pharmaceutics 2020;12:504.
Xue JJ, He M, Liang YZ, Crawford A, Coates P, Chen DF, Shi R, Zhang LQ. Fabrication and evaluation of electrospun PCL-gelatin micro-/nanofiber membranes for anti-infective GTR implants. J Mater Chem B 2014;2:6867.
Xue JJ, He M, Liu H, Niu YZ, Crawford A, Coates PD, Chen DF, Shi R, Zhang LQ. Drug loaded homogeneous electrospun PCL/gelatin hybrid nanofiber structures for anti-infective tissue regeneration membranes. Biomaterials 2014;35:9395.
He M, Wang Q, Xie L, Wu H, Zhao WF, Tian WD. Hierarchically multi-functionalized graded membrane with enhanced bone regeneration and self-defensive antibacterial characteristics for guided bone regeneration. Chem Eng J 2020;398:125542.
Wang Y, Jiang YX, Zhang YF, Wen SZ, Wang YG, Zhang HY. Dual functional electrospun core-shell nanofibers for anti-infective guided bone regeneration membranes. Mat Sci Eng C-Mater 2019;98:134.
Abdelaziz D, Hefnawy A, Al-Wakeel E, El-Fallal A, El-Sherbiny IM. New biodegradable nanoparticles-in-nanofibers based membranes for guided periodontal tissue and bone regeneration with enhanced antibacterial activity. J Adv Res 2021;28:51.
Nasajpour A, Ansari S, Rinoldi C, Rad AS, Aghaloo T, Shin SR, Mishra YK, Adelung R, Swieszkowski W, Annabi N, Khademhosseini A, Moshaverinia A, Tamayol A. A multifunctional polymeric periodontal membrane with osteogenic and antibacterial characteristics. Adv Funct Mater 2018;28:1703437.
Colley HE, Said Z, Santocildes-Romero ME, Baker SR, D’Apice K, Hansen J, Madsen LS, Thornhill MH, Hatton PV, Murdoch C. Pre-clinical evaluation of novel mucoadhesive bilayer patches for local delivery of clobetasol-17-propionate to the oral mucosa. Biomaterials 2018;178:134.
Pardo-Figuerez M, Teno J, Lafraya A, Prieto C, Lagaron JM. Development of an electrospun patch platform technology for the delivery of carvedilol in the oral mucosa. Nanomaterials 2022;12:438.
Edmans JG, Murdoch C, Santocildes-Romero ME, Hatton PV, Colley HE, Spain SG. Incorporation of lysozyme into a mucoadhesive electrospun patch for rapid protein delivery to the oral mucosa. Mat Sci Eng C-Mater 2020;112:110917.
Clitherow KH, Binaljadm TM, Hansen J, Spain SG, Hatton PV, Murdoch C. Medium-chain fatty acids released from polymeric electrospun patches inhibit Candida albicans growth and reduce the biofilm viability. ACS Biomater Sci Eng 2020;6:4087.
Alimohammadi M, Aghli Y, Fakhraei O, Moradi A, Passandideh-Fard M, Ebrahimzadeh MH, Khademhosseini A, Tamayol A, Shaegh SAM. Electrospun nanofibrous membranes for preventing tendon adhesion. ACS Biomater Sci Eng 2020;6:4356.
Wu SH, Zhou R, Zhou F, Streubel PN, Chen SJ, Duan B. Electrospun thymosin Beta-4 loaded PLGA/PLA nanofiber/ microfiber hybrid yarns for tendon tissue engineering application. Mat Sci Eng C-Mater 2020;106:110268.
Huang K, Su W, Zhang XC, Chen CA, Zhao S, Yan XY, Jiang J, Zhu TH, Zhao JZ. Cowpea-like bi-lineage nanofiber mat for repairing chronic rotator cuff tear and inhibiting fatty infiltration. Chem Eng J 2020;392:123671.
Song Y, Li LH, Zhao WK, Qian YN, Dong LL, Fang YN, Yang L, Fan YB. Surface modification of electrospun fibers with mechano-growth factor for mitigating the foreign-body reaction. Bioact Mater 2021;6:2983.
Zhao X, Jiang SC, Liu S, Chen S, Lin ZY, Pan GQ, He F, Li FF, Fan CY, Cui WG. Optimization of intrinsic and extrinsic tendon healing through controllable water-soluble mitomycin-C release from electrospun fibers by mediating adhesion-related gene expression. Biomaterials 2015;61:61.
Li JN, Xu WG, Chen JJ, Li D, Zhang K, Liu TJ, Ding JX, Chen XS. Highly bioadhesive polymer membrane continuously releases cytostatic and anti-inflammatory drugs for peritoneal adhesion prevention. ACS Biomater Sci Eng 2018;4:2026.
Tavare AN, Perry NJS, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: Direct and indirect effects of anesthetic agents*. Int J Cancer 2012;130:1237.
Li X, Pan J, Li Y, Xu F, Hou J, Yang G, Zhou S. Development of a localized drug delivery system with a step-by-step cell internalization capacity for cancer immunotherapy. ACS Nano 2022;16:5778.
Kim Y-J, Park MR, Kim MS, Kwon OH. Polyphenol-loaded polycaprolactone nanofibers for effective growth inhibition of human cancer cells. Mater Chem Phys 2012;133:674.
Merkow RP, Bilimoria KY, Tomlinson JS, Paruch JL, Fleming JB, Talamonti MS, Ko CY, Bentrem DJ. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg 2014;260:372.
Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L, Glynne-Jones R, Counsell N, Bastiaannet E, van den Broek CB, Liefers GJ, Putter H, van de Velde CJ. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 2015;16:200.
Zhao X, Yuan Z, Yildirimer L, Zhao J, Lin ZY, Cao Z, Pan G, Cui W. Tumor-triggered controlled drug release from electrospun fibers using inorganic caps for inhibiting cancer relapse. Small 2015;11:4284.
Yang G, Wang J, Wang Y, Li L, Guo X, Zhou S. An implantable active-targeting micelle-in-nanofiber device for efficient and safe cancer therapy. ACS Nano 2015;9:1161.
Chen S, Boda SK, Batra SK, Li X, Xie J. Emerging roles of electrospun nanofibers in cancer research. Adv Healthc Mater 2018;7:1701024.
Mu W, Chu Q, Liu Y, Zhang N. A review on nano-based drug delivery system for cancer chemoimmunotherapy. Nanomicro Lett 2020;12:142.
Xie D, Ma P, Ding X, Yang X, Duan L, Xiao B, Yi S. Pluronic F127-modified electrospun fibrous meshes for synergistic combination chemotherapy of colon cancer. Front Bioeng Biotechnol 2020;8:618516.
D’Arpa P, Beardmore C, Liu LF. Involvement of nucleic acid synthesis in cell killing mechanisms of topoisomerase poisons. Cancer Res 1990;50:6919.
Baliga MS, Joseph N, Venkataranganna MV, Saxena A, Ponemone V, Fayad R. Curcumin, an active component of turmeric in the prevention and treatment of ulcerative colitis: preclinical and clinical observations. Food Funct 2012;3:1109.
Li X, He Y, Hou J, Yang G, Zhou S. A time-programmed release of dual drugs from an implantable trilayer structured fiber device for synergistic treatment of breast cancer. Small 2020;16:1902262.
Qi F, Chang Y, Zheng R, Wu X, Wu Y, Li B, Sun T, Wang P, Zhang H, Zhang H. Copper phosphide nanoparticles used for combined photothermal and photodynamic tumor therapy. ACS Biomater Sci Eng 2021;7:2745.
Yang Y, Zhu D, Liu Y, Jiang B, Jiang W, Yan X, Fan K. Platinum-carbon-integrated nanozymes for enhanced tumor photodynamic and photothermal therapy. Nanoscale 2020;12:13548.
Hou X, Tao Y, Pang Y, Li X, Jiang G, Liu Y. Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment. Int J Cancer 2018;143:3050.
Xiao J, Cheng L, Fang T, Zhang Y, Zhou J, Cheng R, Tang W, Zhong X, Lu Y, Deng L, Cheng Y, Zhu Y, Liu Z, Cui W. Nanoparticle-embedded electrospun fiber-covered stent to assist intraluminal photodynamic treatment of oesophageal cancer. Small 2019;15:1904979.
Liu X, Zhang H, Cheng R, Gu Y, Yin Y, Sun Z, Pan G, Deng Z, Yang H, Deng L, Cui W, Santos HA, Shi Q. An immunological electrospun scaffold for tumor cell killing and healthy tissue regeneration. Mater Horiz 2018;5:1082.
Yu Q, Han Y, Wang X, Qin C, Zhai D, Yi Z, Chang J, Xiao Y, Wu C. Copper silicate hollow microspheres-incorporated scaffolds for chemo-photothermal therapy of melanoma and tissue healing. ACS Nano 2018;12:2695.
Plaks V, Koopman CD, Werb Z. Cancer. Circulating tumor cells. Science 2013;341:1186.
Hou S, Zhao L, Shen Q, Yu J, Ng C, Kong X, Wu D, Song M, Shi X, Xu X, OuYang WH, He R, Zhao XZ, Lee T, Brunicardi FC, Garcia MA, Ribas A, Lo RS, Tseng HR. Polymer nanofiber-embedded microchips for detection, isolation, and molecular analysis of single circulating melanoma cells. Angew Chem Int Ed 2013;52:3379.
Xiao Y, Fan Y, Tu W, Ning Y, Zhu M, Liu Y, Shi X. Multifunctional PLGA microfibrous rings enable MR imaging-guided tumor chemotherapy and metastasis inhibition through prevention of circulating tumor cell shedding. Nano Today 2021;38:101123.
Wu T, Li HX, Xue JJ, Mo XM, Xia YN. Photothermal welding, melting, and patterned expansion of nonwoven mats of polymer nanofibers for biomedical and printing applications. Angew Chem Int Ed 2019;58:16416.
Chen Y, Dong X, Shafiq M, Myles G, Radacsi N, Mo X. Recent advancements on three-dimensional electrospun nanofiber scaffolds for tissue engineering. Adv Fiber Mater 2022. https://doi.org/10.1007/s42765-022-00170-7 .
Schilling K, El Khatib M, Plunkett S, Xue JJ, Xia YN, Vinogradov SA, Brown E, Zhang XP. Electrospun fiber mesh for high-resolution measurements of oxygen tension in cranial bone defect repair. ACS Appl Mater Interfaces 2019;11:33548.
He H, Zhang X, Du L, Ye M, Lu Y, Xue J, Wu J, Shuai X. Molecular imaging nanoprobes for theranostic applications. Adv Drug Deliv Rev 2022;186:114320.
Gong BW, Zhang XD, Zahrani AA, Gao WW, Ma GL, Zhang LQ, Xue JJ. Neural tissue engineering: From bioactive scaffolds and in situ monitoring to regeneration. Exploration 2022;2:20210035.
Wang S, Xu QC, Sun H. Functionalization of fiber devices: Materials, preparations and applications. Adv Fiber Mater 2022;4:324.
Cai LJ, Xu DY, Chen HX, Wang L, Zhao YJ. Designing bioactive micro-/nanomotors for engineered regeneration. Eng Regener 2021;2:109.
Feiner R, Wertheim L, Gazit D, Kalish O, Mishal G, Shapira A, Dvir T. A stretchable and flexible cardiac tissue-electronics hybrid enabling multiple drug release, sensing, and stimulation. Small 2019;15:1805526.
Wan X, Zhao Y, Li Z, Li L. Emerging polymeric electrospun fibers: from structural diversity to application in flexible bioelectronics and tissue engineering. Exploration 2022;2:20210029.
Yan W, Noel G, Loke G, Meiklejohn E, Khudiyev T, Marion J, Rui G, Lin J, Cherston J, Sahasrabudhe A, Wilbert J, Wicaksono I, Hoyt RW, Missakian A, Zhu L, Ma C, Joannopoulos J, Fink Y. Single fibre enables acoustic fabrics via nanometre-scale vibrations. Nature 2022;603:616.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant No. 52073014 and 82002049; to J. Xue), Special Funds for Fundamental Scientific Research Expenses of Central Universities (buctrc202020; to J. Xue), Key Program of Beijing Natural Science Foundation (Grant No. Z200025; to J. Xue). This work is also supported by the opening Foundation of State Key Laboratory of Organic-Inorganic Composites, Beijing University of Chemical Technology (oic-202201004; to Y. Zhao and J. Xue).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, L., Hao, R., Qin, J. et al. Electrospun Fibers Control Drug Delivery for Tissue Regeneration and Cancer Therapy. Adv. Fiber Mater. 4, 1375–1413 (2022). https://doi.org/10.1007/s42765-022-00198-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42765-022-00198-9