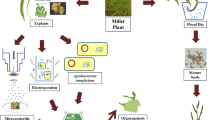
Abstract
Millets are a group of small seeded cereals and forage grasses grown in arid and semi-arid regions of Asia and Africa, where majority of cereals cannot be relied upon to provide sustainable yield. While major cereals such as wheat, rice and maize provide only food security, millets provide multiple securities, viz., food, fodder, health, nutrition, livelihood and ecological. Each of the millets is a reservoir of several nutrients in large quantity. They are nutritionally rich in minerals, dietary fiber, phenolics, vitamins, and are gluten-free. In the present review recent advances in tissue culture and genetic transformation studies conducted in millets to date have been summarized. Although there is a vast amount of literature available on millet tissue culture, only a limited number of transformation experiments have been conducted so far. Millets have been transformed primarily by particle bombardment, whereas Agrobacterium-mediated transformation is still lagging behind. Efforts need to be made to genetically improve millets by incorporating certain agronomically important traits, such as resistance to biotic and abiotic stresses, resistance to lodging, increased seed size and palatability along with softness of grain to make these crops more desirable for consumer.
Similar content being viewed by others
References
Almerei, A., Lane, S., & Fuller, M. P. (2014). Genetic transformation of immature zygotic embryos of maize genotypes via Agrobacterium tumefaciens. Life Science Journal, 11, 966–975.
Amali, P., Kingsley, S. J., & Ignacimuthu, S. (2014). Enhanced plant regeneration involving somatic embryogenesis from shoot tip explants of Sorghum bicolor (L. Moench). Asian Journal of Plant Science and Research, 4, 26–34.
Anjaneyulu, E., Hemalatha, S., Raj, S. B., & Balaji, M. (2011). Callus induction and plant regeneration in finger millet (Eleusine coracana L.). Libyan Agriculture Research Center Journal International, 2, 57–61.
Anju, C., Rabindran, R., Velazhahan, R., & Ravikesavan, R. (2016). Callusing and regeneration in finger millet [Eleusine coracana (L.) Gaertn.]. Research Journal of Agricultural Sciences, 7, 324–329.
Avila, P. O., Nava-Cedillo, A., Jofre-Garfias, A. E., & Cabrera-Ponce, J. L. (1995). Plant regeneration from shoot apex explants of foxtail millet. Plant Cell, Tissue and Organ Culture, 40, 33–35.
Bekele, E., Klock, G., & Zimmermann, U. (1995). Somatic embryogenesis and plant regeneration from leaf and root explants and from seeds of Eragrostis tef (Gramineae). Hereditas, 123, 183–189.
Benson, E. E. (2000). In vitro plant recalcitrance: An introduction. In Vitro Cellular and Developmental Biology-Plant, 36, 141–148.
Bhaskaran, S., & Smith, R. H. (1990). Regeneration in cereal tissue culture: A review. Crop Science, 30, 1328–1336.
Bregitzer, P., Dahleen, L. S., & Campbell, R. D. (1998). Enhancement of plant regeneration from embryogenic callus of commercial barley cultivars. Plant Cell Report, 17, 941–945.
Campos, J. M. S., Davide, L. C., Salgado, C. C., Santos, F. C., Costa, P. N., Silva, P. S., et al. (2009). In vitro induction of hexaploid plants from triploid hybrids of Pennisetum purpureum and Pennisetum glaucum. Plant Breeding, Berlin, 128, 101–104.
Ceasar, S. A., & Ignacimuthu, S. (2008). Efficient somatic embryogenesis and plant regeneration from shoot apex explants of different Indian genotypes of finger millet (Eleusine coracana (L) Gaertn). In Vitro Cellular and Developmental Biology-Plant, 44, 427–435.
Ceasar, S. A., & Ignacimuthu, S. (2010). Effects of cytokinins, carbohydrates and amino acids on induction and maturation of somatic embryos in kodo millet (Paspalum scorbiculatum Linn.). Plant Cell, Tissue and Organ Culture, 102, 153–162.
Ceasar, S. A., & Ignacimuthu, S. (2011). Agrobacterium mediated transformation of Finger millet (Eleusine coracana (L.) Gaertn.) using shoot apex explants. Plant Cell Report, 30, 1759–1770.
Chauhan, M., & Kothari, S. L. (2004). Optimization of nutrient levels in the medium increases the efficiency of callus induction and plant regeneration in recalcitrant Indian barley (Hordeum vulgare L.) in vitro. In Vitro Cellular and Developmental Biology-Plant, 40, 520–527.
Dahleen, L. S. (1995). Improved plant regeneration from barley callus cultures by increased copper levels. Plant Cell, Tissue and Organ Culture, 43, 267–269.
Devi, P., & Sticklen, M. (2002). Culturing shoot-tip clumps of pearl millet (Pennisetum glaucum (L.) R. Br.) and optimal microprojectile bombardment parameters for transient expression. Euphytica, 125, 45–50.
Devi, P., Zhong, H., & Sticklen, M. B. (2000). In vitro morphogenesis of pearl millet (Pennisetum glaucum (L.) R.Br.): Efficient production of multiple shoots and inflorescences from shoot apices. Plant Cell Report, 19, 546–550.
Dhillon, N. K., & Gosal, S. S. (2013). Analysis of maize inbred lines for their response to somatic embryogenesis. Journal of Cell and Tissue Research, 13, 3557–3563.
Dosad, S., & Chawla, H. S. (2015a). In vitro plant regeneration from mature seeds of finger millet (Eleusine coracana) through somatic embryogenesis. Indian Journal of Plant Physiology, 20, 360–367.
Dosad, S., & Chawla, H. S. (2015b). Optimization of different doses of growth regulators for in vitro regeneration of Echinochloa frumentacea Roxb. from caryopsis. Indian Journal of Plant Physiology, 20, 339–344.
Eapen, S., & George, L. (1990). Influence of phytohormones, carbohydrates, aminoacids, growth supplements and antibiotics on somatic embryogenesis and plant differentiation in finger millet. Plant Cell, Tissue and Organ Culture, 22, 87–93.
Enriquez-Obregon, G. A., Prieto-Samsonov, D. L., de la Riva, G. A., Perez, M., Selman-Housein, G., & Vazquez-Padron, R. I. (1999). Agrobacterium-mediated Japonica rice transformation: A procedure assisted by an antinecrotic treatment. Plant Cell, Tissue and Organ Culture, 59, 159–168.
Faleiro, F. G., Kannan, B., & Altpeter, F. (2016). Regeneration of fertile, hexaploid, interspecific hybrids of elephantgrass and pearl millet following treatment of embryogenic calli with antimitotic agents. Plant Cell, Tissue and Organ Culture, 124, 57–67.
Fang, F. Q., Qian, Z., Guang, M. A. & Jing, J. Y. (2007). Co-suppression of Si401, a maize pollen speciWc Zm401 homologous gene, results in aberrant anther development in foxtail millet. Euphytica, 163, 103–111.
Fernandez, S., Michaux-Ferriere, N., & Coumans, M. (1999). The embryogenic response of immature embryo cultures of durum wheat (Triticum durum): Histology and improvement by AgNO3. Plant Growth Regulation, 28, 147–155.
George, L., & Eapen, S. (1990). High frequency plant regeneration through direct shoot development and somatic embryogenesis from immature inflorescence cultures of finger millet (Eleusine coracana Gaertn.). Euphytica, 48, 269–274.
Ghobeishavi, H., Uliaie, E. D., Alavikia, S. S., & Valizadeh, M. (2015). Study of factors influencing somatic embryogenesis in rice (Oryza Sativa L.). International Journal of Advanced Biological and Biomedical Research, 3, 43–50.
Girgi, M., O’Kennedy, M. M., Morgenstern, A., Mayer, G., Lorz, H., & Oldach, K. H. (2002). Transgenic and herbicide resistant pearl millet (Pennisetum glaucum L.) R.Br. via microprojectile bombardment of scutellar tissue. Molecular Breeding, 10, 243–252.
Goldman, J. J., Hanna, W. W., Fleming, G., & Ozias-Akins, P. (2003). Fertile transgenic pearl millet (Pennisetum glaucum (L.) R. Br.) plants recovered through microprojectile bombardment and phosphinothricin selection of apical meristem-, inflorescence and immature embryo derived embryogenic tissues. Plant Cell Report, 21, 999–1009.
Gorji, A. H., Zolnoori, M., Jamasbi, A., & Zolnoori, Z. (2011). In vitro plant generation of tropical maize genotypes. International Conference on Environmental, Biomedical and Biotechnology, 16, 52–59.
Grando, M. F., Varnier, M. L., Silva, M. R., Emydio, B. M., Pereira, L. R., & Suzin, M. (2013). Immature tassels as alternative explants in somatic embryogenesis and plant regeneration in south Brazilian maize genotypes. Acta Scientiarum, 35, 39–47.
Gupta, P., Raghuvanshi, S., & Tyagi, A. K. (2001). Assessment of the efficiency of various gene promoters via biolistics in leaf and regenerating seed callus of millets, Eleusine coracana and Echinochloa crusgalli. Plant Biotechnology, 18, 275–282.
Haque, M., Siddique, A. B., & Islam, S. M. S. (2015). Effect of silver nitrate and amino acids on high frequency plants regeneration in Barley (Hordeum vulgare L.). Plant Tissue Culture and Biotechnology, 25, 37–50.
Hauptmann, R. M., Ozias-Akins, P., Vasil, V., Tabaeizadeh, Z., Rogers, S. G., Horsch, R. B., et al. (1987). Transient expression of electroporated DNA in monocotyledonous and dicotyledonous species. Plant Cell Reports, 6, 265–270.
Hema, R., Vemanna, R. S., Sreeramulu, S., Reddy, C. P., Kumar, M. S., & Udayakumar, M. (2014). Stable expression of mtlD gene imparts multiple stress tolerance in Finger millet. PLoS One. doi:10.1371/journal.pone.0099110.
Ignacimuthu, S., & Ceasar, S. A. (2012). Development of transgenic finger millet (Eleusine coracana (L.) Gaertn.) resistant to leaf blast disease. Journal of Biosciences, 37, 135–147.
Ignacimuthu, S., & Kannan, P. (2013). Agrobacterium-mediated transformation of pearl millet (Pennisetum typhoides (L.) R. Br.) for fungal rust. Asian Journal of Plant Sciences, 112, 97–108.
Jagga-Chugh, S., Kachhwaha, S., Sharma, M., Kothari-Chajer, A., & Kothari, S. L. (2012). Optimization of factors influencing microprojectile bombardment-mediated genetic transformation of seed-derived callus and regeneration of transgenic plants in Eleusine coracana (L.) Gaertn. Plant Cell Tissue and Organ Culture. doi:10.1007/s1124001101047.
Jalaja, N., Maheshwari, P., Naidu, K. R., & Kavikishor, P. B. (2016). In vitro regeneration and optimization of conditions for transformation methods in Pearl millet, Pennisetum glaucum (L.). International Journal of Clinical and Biological Sciences, 1, 34–52.
Jayasudha, B. G., Sushma, A. M., Prashantkumar, H. S., & Sashidhar, V. R. (2014). An efficient in vitro agrobacterium –mediated transformation protocol for raising salinity tolerant transgenic plants in fingermillet [Eleusine coracana (L.) Gaertn.]. Plant Archives, 14, 823–829.
Jha, P., Shashi, Rustagi, A., Agnihotri, P. K., Kulkarni, V. M., & Bhat, V. (2011). Efficient Agrobacterium-mediated transformation of Pennisetum glaucum (L.) R. Br. using shoot apices as explant source. Plant Cell, Tissue and Organ Culture, 107, 501–512.
Jha, P., Yadav, C. B., Anjaiah, V., & Bhat, V. (2009). In vitro plant regeneration through somatic embryogenesis and direct shoot organogenesis in Pennisetum glaucum (L.) R. Br. In Vitro Cellular and Developmental Biology-Plant, 45, 145–154.
Joshi, A., & Kothari, S. L. (2007). High cooper levels in the medium improves shoot bud differentiation and elongation from the cultured cotyledons of Capsicum annuum L. Plant Cell, Tissue and Organ Culture, 88, 127–133.
Joshi, J. B., Yathish, K. R., Joel, A. J., Kumar, K. K., Kokiladevi, E., Arul, L., et al. (2014). A high-throughput regeneration protocol for recalcitrant tropical Indian maize (Zea mays L) inbreds. Maydica, 59, 211–216.
Kaur, P., & Kothari, S. L. (2004). In vitro culture of kodo millet: Influence of 2,4-D and picloram in combination with kinetin on callus initiation and regeneration. Plant Cell, Tissue and Organ Culture, 77, 73–79.
Kishore, N. S., Visarada, K. B. R. S., Lakshmi, Y. A., Pashupatinath, E., Rao, S. V., & Seetharama, N. (2006). In vitro culture methods in sorghum with shoot tip as the explants material. Plant Cell Report, 25, 174–182.
Kothari, S. L., Agarwal, K., & Kumar, S. (2004). Inorganic nutrient manipulation for highly improved in vitro plant regeneration in finger millet- Eleusine coracana (L.) Gaertn. In Vitro Cellular and Developmental Biology-Plant, 40, 515–519.
Kothari, S. L., Kumar, S., Vishnoi, R. K., Kothari, A., & Watanabe, K. N. (2005). Applications of biotechnology for improvement of millet crops: Review of progress and future prospects. Plant Biotechnology, 22, 81–88.
Kothari-Chajer, A., Sharma, M., Kachhwaha, S., & Kothari, S. L. (2008). Micronutrient optimization results into highly improved in vitro plant regeneration in kodo (Paspalum Scrobiculatum L.) and finger (Eleusine coracana (L.) Gaertn.) millets. Plant Cell, Tissue and Organ Culture, 94, 105–112.
Krishania, S., & Agarwal, K. (2012). Effects of heavy metals on Eleusine coracana (L.) Gaertn. Research in Plant Biology, 2, 43–54.
Kumar, S., Agarwal, K., & Kothari, S. L. (2001). In vitro induction and enlargement of apical domes and formation of multiple shoots in finger millet, Eleusine coracana (L.) Gaertn and crowfoot grass, Eleusine indica (L.) Gaertn. Current Science, 81, 1482–1485.
Kumar, V., & Parvatam, G. (2009). AgNO3—A potent growth regulator of ethylene activity and plant growth modulator. Electronic Journal of Biotechnology, 12, 1–15.
Lambe, P., Dinant, M., & Matagneb, R. F. (1995). Differential long-term expression and methylation of the hygromycin phosphotransferase (hph) and P-glucuronidase (GUS) genes in transgenic pearl millet (Pennisetum glaucum) callus. Plant Science, 108, 51–62.
Lambe, P., Mutambel, H. S. N., Deltour, R., & Dinant, M. (1999). Somatic embryogenesis in pearl millet (Pennisetum glaucum): Strategies to reduce genotype limitation and to maintain long-term totipotency. Plant Cell, Tissue and Organ Culture, 55, 23–29.
Latha, A. M., Rao, K. V., & Reddy, V. D. (2005). Production of transgenic plants resistant to leaf blast disease in finger millet (Eleusine coracana (L.) Gaertn). Plant Science, 169, 657–667.
Latha, A. M., Rao, K. V., Reddy, T. P., & Reddy, V. D. (2006). Development of transgenic pearl millet (Pennisetum glaucum (L.) R. Br.) plants resistant to downy mildew. Plant Cell Report, 25, 927–935.
Liu, G., & Godwin, I. D. (2012). Highly efficient sorghum transformation. Plant Cell Report, 31, 999–1007.
Liu, Y., Yu, J., Zhao, Q., Zhu, D. & Ao, G. (2005) Genetic transformation of millet (Setaria italica) by Agrobacterium-mediated. Chinese Journal of Agricultural Biotechnology, 13, 32–37.
Liu, G. Q., Gilding, E. K., & Godwin, I. D. (2013). Additive effects of three auxins and copper on sorghum in vitro root induction. In Vitro Cellular and Developmental Biology-Plant, 49, 191–197.
Liu, G., Gilding, E. K., & Godwin, I. D. (2015). A robust tissue culture system for sorghum [Sorghumbicolor (L.) Moench]. South African Journal of Botany, 98, 157–160.
Maksymiec, W. (1997). Effect of copper on cellular processes in higher plants. Photosynthetica, 34, 321–342.
Mandour, H. M., Soliman, S. S. A., Abd El-Hady, M. S., Mahmoud, A. A., & EI Naggar, H. M. (2015). In vitro selection for drought tolerance in wheat (Triticum aestivum L.). International Journal of ChemTech Research, 8, 318–333.
Martins, P. K., Nakayama, T. J., Ribeiro, A. P., Cunha, B. A. D. B. D., Nepomuceno, A. L., Harmon, F. G., et al. (2015). Setaria viridis floral-dip: A simple and rapid Agrobacterium-mediated transformation method. Biotechnology Reports, 6, 61–63.
Mohanty, B. D., Gupta, S. D., & Ghosh, P. D. (1985). Callus initiation and plant regeneration in ragi (Eleusine coracana Gaertn). Plant Cell, Tissue and Organ Culture, 5, 147–150.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassay for tobacco tissue cultures. Physiologia Plantarum, 15, 473–497.
Mythili, P. K., Satyavathi, V., Pavankumar, G., Rao, M. V. S., & Manga, V. (1997). Genetic analysis of short term callus culture and morphogenesis in pearl millet, Pennisetum glaucum. Plant Cell, Tissue and Organ Culture, 50, 171–178.
Nethra, N., Gowda, R., & Gowda, P. H. R. (2009). Influence of culture medium on callus proliferation and morphogenesis in finger millet. In: Tadele, Z. (Ed.) New approaches to plant breeding of orphan crops in Africa. In Proceedings of an International Conference, September 19–21, 2007. Bern, Switzerland. Univ. Bern. pp. 167–178.
Niedz, R. P., & Evens, T. J. (2007). Regulating plant tissue growth by mineral nutrition. In Vitro Cellular and Developmental Biology-Plant, 43, 370–381.
Nirwan, R. S., & Kothari, S. L. (2003). High copper levels improve callus induction and plant regeneration in Sorghum bicolor (L.) Moench. In Vitro Cellular and Developmental Biology-Plant, 39, 161–164.
O’Kennedy, M. M., Burger, J. T., & Botha, F. C. (2004). Pearl millet transformation system using the positive selectable marker gene phosphomannose isomerase. Plant Cell Report, 22, 684–690.
O’Kennedy, M. M., Crampton, B. G., Lorito, M., Chakauya, E., Breese, W. A., Burger, J. T., et al. (2011). Expression of a β-1,3-glucanase from a biocontrol fungus in transgenic pearl millet. South African Journal of Botany, 77, 335–345.
Oldach, K. H., Morgenstern, A., Rother, S., Girgi, M., O’Kennedy, M., & Lorz, H. (2001). Efficient in vitro plant regeneration from immature zygotic embryos of pearl millet [Pennisetum glaucum (L.) R. Br.] and Sorghum bicolor (L.) Moench. Plant Cell Report, 20, 416–421.
Pande, A., Dosad, S., Chawla, H. S., & Arora, S. (2015). In vitro organogenesis and plant regeneration from seed-derived callus cultures of finger millet (Eleusine coracana). Brazilian Journal of Botany, 38, 19–23.
Patil, S. M., Sawardekar, S. V., Bhave, S. G., Sawant, S. S., Jambhale, N. D., & Gokhale, N. B. (2009). Development of somaclones and their genetic diversity analysis through RAPD in Finger millet (Eleusine coracana L. Gaertn.). Indian Journal of Genetics, 69, 132–139.
Pius, J., Eapen, S., George, L., Rao, P. S., & Raut, R. S. (1999). Performance of plants regenerated through somatic embryogenesis in finger millet (Eleusine coracana Gaertn.). Tropical Agricultural Research and Extention, 2, 87–90.
Pius, J., George, L., Eapen, S., & Rao, P. S. (1994). Influence of genotype and phytohormones on somatic embryogenesis and plant regeneration in Finger millet. Proceedings of the Indian natninal Science Academy, 60, 53–56.
Plaza-Wuthrich, S., & Tadele, Z. (2012). Millet improvement through regeneration and transformation. Biotechnology and Molecular Biology Review, 7, 48–61.
Poddar, K., Vishnoi, R. K., & Kothari, S. L. (1997). Plant regeneration from embryogenic callus of finger millet Eleusine coracana (L) Gaertn. On higher concentrations of NH4NO3 as a replacement of NAA in the medium. Plant Science, 129, 101–106.
Popelka, J. E., & Altpeter, F. (2001). Interactions between genotypes and culture media components for improved in vitro response of rye (Secale cereale L.) inbred lines. Plant Cell Report, 20, 575–582.
Purnhauser, L. (1991). Stimulation of shoot and root regeneration in wheat (Triticum aestivum) callus cultures by copper. Cereal Research Communications, 19, 419–423.
Ramadevi, R., Rao, K. V., & Reddy, V. D. (2014). Agrobacterium tumefaciens-mediated genetic transformation and production of stable transgenic pearl millet (Pennisetum glaucum [L.] R. Br.). In Vitro Cellular and Developmental Biology-Plant, 50, 392–400.
Ramegowda, Y., Venkategowda, R., Jagadish, P., Govind, G., Hanumanthareddy, R. R., Makarla, U., et al. (2013). Expression of a rice Zn transporter, OsZIP1, increases Zn concentration in tobacco and finger millet transgenic plants. Plant Biotechnology Reports, 7, 309–319.
Rangan, T. S. (1974). Morphogenic investigations on tissue cultures of Panicum miliaceum. Zeitschrift für Pflanzenphysiologie, 72, 456–459.
Rangan, T. S. (1976). Growth and plantlet regeneration in tissue cultures of some Indian millets: Paspalum scrobiculatum L., Eleusine coracana GAERTN. and Pennisetum typhoideum PERS. Zeitschrift fur Pflanzenphysiologie, 78, 208–216.
Rao, A. M., Kavi-Kishore, P. B., Reddy, L. A., & Vaidyanath, K. (1988). Callus induction and high frequency plant regeneration in Italian millet (Setaria italica). Plant Cell Report, 7, 557–559.
Reddy, L. A., & Vaidyanath, K. (1990). Callus formation and regeneration in two induced mutants of foxtail millet (Setaria italica). Journal of Genetics and Breeding, 44, 133–138.
Rout, G. R., Samantaray, S., & Das, P. (1997). Regeneration of a metal tolerant grass Echinochloa colona via somatic embryogenesis from suspension culture. Biologia Plantarum, 40, 17–23.
Rout, G. R., Samantaray, S., & Das, P. (1998). In vitro selection and characterization of Ni-tolerant callus lines of Setaria italica L. Acta Physiologiae Plantaru, 20, 269–275.
Sah, S. K., Kaur, A., & Sandhu, J. S. (2014). High frequency embryogenic callus induction and whole plant regeneration in Japonica rice cv. kitaake. Rice Research, 2, 125. doi:10.4172/jrr.1000125.
Saha, P., & Blumwald, E. (2016). Spike-dip transformation of Setaria viridis. The Plant Journal, 86, 89–101.
Sahrawat, A. K., & Chand, S. (1999). Stimulatory effect of copper on plant regeneration in indica rice (Oryza sativa L.). Journal of Plant Physiology, 154, 517–522.
Samantaray, S., Rout, G. R., & Das, P. (1995). In vitro plant regeneration from leaf base and mesocotyl cultures of Echinochloa colona. Plant Cell, Tissue and Organ Culture, 40, 37–41.
Samantaray, S., Rout, G. R., & Das, P. (1997). Regeneration of plants via somatic embryogenesis from leaf base and leaf tip segments of Echinochloa colona. Plant Cell, Tissue and Organ Culture, 47, 119–125.
Samantaray, S., Rout, G. R., & Das, P. (2001). Induction, selection and characterization of Cr and Ni-tolerant cell lines of Echinochloa colona (L.) Link in vitro. Journal of Plant Physiology, 158, 1281–1290.
Sankhla, A., Davis, T. M., Sankhla, D., Sankhla, N., Upadhyayan, A., & Joshi, S. (1992). Influence of growth regulators on somatic embryogenesis, plantlet regeneration and post transplant survival of echinochloa fumentacea. Plant Cell Report, 11, 368–371.
Satish, L., Ceasar, S. A., Shilpha, J., Rency, A. S., Rathinapriya, P., & Ramesh, M. (2015). Direct plant regeneration from in vitro-derived shoot apical meristems of finger millet (Eleusine coracana (L.) Gaertn.). In Vitro Cellular and Developmental Biology-Plant, 51, 192–200.
Satish, L., Rathinapriya, P., Ceasar, S. A., Rency, A. S., Pandian, S., Rameshkumar, R., et al. (2016a). Effects of cefotaxime, amino acids and carbon source on somatic embryogenesis and plant regeneration in four Indian genotypes of foxtail millet (Setaria italica L.). In Vitro Cellular and Developmental Biology-Plant, 52, 140–153.
Satish, L., Rency, A. S., Rathinapriya, P., Ceasar, S. A., Pandian, S., Rameshkumar, R., et al. (2016b). Influence of plant growth regulators and spermidine on somatic embryogenesis and plant regeneration in four Indian genotypes of finger millet (Eleusine coracana (L.) Gaertn). Plant Cell, Tissue and Organ Culture, 124, 15–31.
Satyavathi, V., Rao, S. M. V., Manga, V., & Chittibabu, M. (2006). Genetics of some in vitro characters in pearl millet. Euphytica, 148, 243–249.
Sharma, M., Kothari-Chajer, A., Jagga-Chugh, S., & Kothari, S. L. (2011). Factors influencing Agrobacterium tumefaciens-mediated genetic transformation of (Eleusine coracana (L.) Gaertn). Plant Cell, Tissue and Organ Culture, 105, 93–104.
Shrivastava, S., Singh, N., & Chawla, H. S. (2000). Role of growth regulators and glutamine for enhancing regeneration response in wheat (Triticum aestivum L.). Journal of Genetics and Breeding, 54, 71–75.
Sivadas, P., Kothari, S. L., & Chandra, N. (1990). High frequency embryoid and plantlet formation from tissue cultures of the Finger millet-Eleusine coracana (L.) Gaertn. Plant Cell Report, 9, 93–96.
Skoog, F., & Miller, C. O. (1957). Chemical regulation of growth and organ formation in plant tissue cultured. In Vitro Symposia of the Society for Experimental Biology, 11, 118–130.
Tahiliani, S., & Kothari, S. L. (2004). Increased copper content of the medium improves plant regeneration from immature embryo derived callus of wheat (Triticum aestivum). Journal of Plant Biochemistry and Biotechnology, 13, 85–88.
Talwar, M., & Rashid, A. (1989). Somatic embryo formation from unemerged inflorescences and immature embryos of a graminaceous crop Echinochloa. Annals of Botany, 64, 195–199.
Taylor, M. G., Vasil, V., & Vasil, I. K. (1991). Histology of, and physical factors affecting, transient GUS expression in pearl millet (Pennisetum glaucum (L.) R. Br.) embryosfollowing microprojectile bombardment. Plant Cell Report, 10, 120–125.
Tiecoura, K., Kouassi, A. B., Oulo, N., Gonedele Bi, S., Dinant, M., & Ledou, L. (2015). In vitro transformation of pearl millet (Pennisetum glaucum (L). R. BR.): Selection of chlorsulfuron-resistant plants and long term expression of the gus gene under the control of the emu promoter. African Journal of Biotechnology, 14, 3112–3123.
Tiwari, A. K., Shamim, Md, Saxena, R. P., & Singh, K. D. N. (2012). Plant regeneration efficiency of two scented Indica rice varieties Pusa Basmati 1 and Kalanamak. Plant Tissue Culture and Biotechnology, 22, 163–169.
Vasil, I. K. (1982). Plant cell culture and somatic cell genetics of cereals and grasses. In I. K. Vasil, W. R. Scowcroft, & K. J. Frey (Eds.), Plant improvement and somatic cell genet (pp. 179–203). New York: Academic Press.
Vikrant, A., & Rashid, A. (2001). Direct as well as indirect somatic embryogenesis from immature (unemerged) inflorescence of a minor millet Paspalum scrobiculatum L. Euphytica, 120, 167–172.
Vikrant, A., & Rashid, A. (2002a). Induction of multiple-shoots from thidiazuron on culture of mature caryopses of a minor millet Paspalum scrobiculatum L. and its effect on regeneration of embryogenic cultures. Plant Cell Report, 21, 9–13.
Vikrant, A., & Rashid, A. (2002b). Somatic embryogenesis from immature and mature embryos of a minor millet Paspalum scrobiculatum L. Plant Cell, Tissue and Organ Culture, 69, 71–77.
Vikrant, A., & Rashid, A. (2003). Somatic embryogenesis from mesocotyl and leaf base segments of Paspalum scrobiculatum L., minor millet. In Vitro Cellular and Developmental Biology-Plant, 39, 485–489.
Vishnoi, R. K., & Kothari, S. L. (1996). Somatic embryogenesis and efficient plant regeneration in immature inflorescence culture of Setaria italic (L.) Beauv. Cereal Research Communications, 24, 291–297.
Wang, J., Nie, J., Pattanaik, S., & Yuan, L. (2016). Efficient Agrobacterium-mediated transformation of Artemisia annua L. using young inflorescence. In Vitro Cellular and Developmental Biology-Plant, 52, 204–211.
Wang, M., Pan, Y., Li, C., Liu, C., Zhao, Q., Ao, G., et al. (2011). Culturing of immature inflorescences and Agrobacterium-mediated transformation of foxtail millet (Setaria italica). African Journal of Biotechnology, 10, 16466–16479.
Wakizuka, T., & Yamaguchi, T. (1987) The induction of enlarged apical domes in vitro and multi-shoot formation from finger millet (Eleusine coracana). Annals of Botany, 60, 331–336.
Wani, S. H., Sanghera, G. S., & Gosal, S. S. (2011). An efficient and reproducible method for regeneration of whole plants from mature seeds of a high yielding Indica rice (Oryza sativa L.) variety PAU 201. New Biotechnology, 28, 418–422.
Wojnarowiez, G., Jacquard, C., Devaux, P., Sangwan, R. S., & Clement, C. (2002). Influence of copper sulfate on anther culture in barley (Hordeum vulgare L.). Plant Science, 162, 843–847.
Wu, L. M., Wei, Y. M., & Zhang, Y. L. (2006). Effect of silver nitrate on the tissue culture of immature wheat embryos. Russian Journal of Plant Physiology, 53, 530–534.
Xu, Z., Wang, D., Yang, L., & Wei, Z. (1984). Somatic embryogenesis and plant regeneration in callus cultured immature inflorescence of Setaria italica. Plant Cell Report, 3, 149–150.
Yadav, R., & Chawla, H. S. (2002). role of genotypes, growth regulators and amino acids on callus induction and plant regeneration from different developmental stages of inflorescence in wheat. Indian Journal of Genetics, 62, 55–60.
Yadav, T., Kothari, S. L., & Kachhwaha, S. (2011). Evaluation of regeneration potential of mature embryo derived callus in Indian cultivars of barley (Hordeum vulgare L.). Journal of Plant Biochemistry and Biotechnology, 20, 166–172.
Yang, L., & Xu, Z. (1985). Somatic embryogenesis and plant regeneration from cell suspension culture of Stearia italica (L) Beauv. Acta Biologiae Experimentalis Sinica, 18, 493–498.
Yang, Y. S., Zheng, Y. D., Chen, Y. L., & Jain, Y. Y. (1999). Improvement of plant regeneration from long term cultured calluses of Tipei-309, a model rice variety in in vitro studies. Plant Cell, Tissue and Organ Culture, 57, 199–206.
Yemets, A. I., Bayer, G. Y., & Blume, Y. B. (2013). An effective procedure for in vitro culture of Eleusine coracana (L.) and its application. ISRN Botany. doi:10.1155/2013/853121.
Yemets, A., Radchuk, V., Bayer, O., Bayer, G., Pakhomov, A., Baird, W. V., et al. (2008). The development of transformation vectors based upon a modified plant α-tubulin gene as the selectable marker. Cell Biology International, 32, 566–570.
Zhang, S., Cho, M. J., Koprek, T., Yun, R., Bregitzer, P., & Lemaux, P. G. (1999). Genetic transformation of commercial cultivars of oat and barley using in vitro shoot meristematic cultures derived from germinating seedlings. Plant Cell Report, 18, 959–966.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dosad, S., Chawla, H.S. In vitro plant regeneration and transformation studies in millets: current status and future prospects. Ind J Plant Physiol. 21, 239–254 (2016). https://doi.org/10.1007/s40502-016-0240-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-016-0240-5