Abstract
In this study, a magnetically separable graphene oxide support has been successfully synthesized and employed for immobilizing industrial cellulase (Cellic Ctec2) via physical adsorption. The support material has been thoroughly characterized using X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy, field-emission scanning electron microscopy (FE-SEM), and high-resolution transmission electron microscopy (HR-TEM). The research focuses on critical factors crucial for industrial applications, including enzyme loading capacity, the impact of substrate loading on glucose yield, and the reusability of the immobilized enzyme. The results demonstrate an impressive 270 mg/g enzyme loading capacity for the support material (FeGO-E). Additionally, 87%, 76%, 61%, and 53% cellulose conversion rates have been achieved at 5%, 7.5%, 10%, and 12.5% w/v substrate loadings, respectively. Stability tests conducted under varying pH (2.0 to 8.0) and temperature (30 to 80 °C) reveal that the microenvironment of the support significantly enhances the stability and recyclability of the immobilized enzyme, outperforming the free enzyme. The nanobiocatalyst demonstrates excellent stability and can be reused for up to six cycles. The adsorption isotherm analysis confirms that the enzyme adsorption onto the support material adheres to the Langmuir isotherm model. Furthermore, the kinetic studies indicate that first-order kinetics is more appropriate for our data. Based on the obtained product profile, a plausible mechanism for the enzymatic hydrolysis of cellulose is proposed.
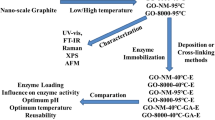
Graphical Abstract
Schematic representation of cellulase immobilization on magnetic graphene oxide for hydrolysis of cellulose









Similar content being viewed by others
Data availability
The manuscript and the supplementary material include all data obtained supporting the findings of this study. Raw data will be made available from the corresponding author upon reasonable request.
References
Reboredo FH, Lidon F, Pessoa F, Ramalho JC (2016) The fall of oil prices and the effects on biofuels. Trends Biotechnol 34:3–6. https://doi.org/10.1016/j.tibtech.2015.10.002
Satari B, Karimi K, Kumar R (2019) Cellulose solvent-based pretreatment for enhanced second-generation biofuel production: a review. Sustainable Energy Fuels 3:11–62. https://doi.org/10.1039/C8SE00287H
He R, Ma L, Li C et al (2014) Trpac1, a pH response transcription regulator, is involved in cellulase gene expression in Trichoderma reesei. Enzyme Microb Technol 67:17–26. https://doi.org/10.1016/j.enzmictec.2014.08.013
Srivastava N, Srivastava M, Mishra PK et al (2018) Applications of fungal cellulases in biofuel production: advances and limitations. Renew Sustain Energy Rev 82:2379–2386. https://doi.org/10.1016/j.rser.2017.08.074
Naik SN, Goud VV, Rout PK, Dalai AK (2010) Production of first and second generation biofuels: a comprehensive review. Renew Sustain Energy Rev 14:578–597. https://doi.org/10.1016/j.rser.2009.10.003
Antizar-Ladislao B, Turrion-Gomez JL (2008) Second-generation biofuels and local bioenergy systems. Biofuels Bioprod Bioref 2:455–469. https://doi.org/10.1002/bbb.97
Lynd LR, Cushman JH, Nichols RJ, Wyman CE (1991) Fuel ethanol from cellulosic biomass. Science 251:1318–1323. https://doi.org/10.1126/science.251.4999.1318
Khoshnevisan K, Bordbar A-K, Zare D et al (2011) Immobilization of cellulase enzyme on superparamagnetic nanoparticles and determination of its activity and stability. Chem Eng J 171:669–673. https://doi.org/10.1016/j.cej.2011.04.039
Suo H, Xu L, Xu C et al (2019) Graphene oxide nanosheets shielding of lipase immobilized on magnetic composites for the improvement of enzyme stability. ACS Sustainable Chem Eng 7:4486–4494. https://doi.org/10.1021/acssuschemeng.8b06542
Singhania RR, Sukumaran RK, Patel AK et al (2010) Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme Microb Technol 46:541–549. https://doi.org/10.1016/j.enzmictec.2010.03.010
Da Silva AS, Espinheira RP, Teixeira RSS et al (2020) Constraints and advances in high-solids enzymatic hydrolysis of lignocellulosic biomass: a critical review. Biotechnol Biofuels 13:58. https://doi.org/10.1186/s13068-020-01697-w
Chylenski P, Felby C, Østergaard Haven M et al (2012) Precipitation of Trichoderma reesei commercial cellulase preparations under standard enzymatic hydrolysis conditions for lignocelluloses. Biotechnol Lett 34:1475–1482. https://doi.org/10.1007/s10529-012-0916-5
Reese ET, Ryu DY (1980) Shear inactivation of cellulase of Trichoderma reesei. Enzyme Microb Technol 2:239–240. https://doi.org/10.1016/0141-0229(80)90054-X
Ganesh K, Joshi JB, Sawant SB (2000) Cellulase deactivation in a stirred reactor. Biochem Eng J 4:137–141. https://doi.org/10.1016/S1369-703X(99)00045-5
Ghadge RS, Patwardhan AW, Sawant SB, Joshi JB (2005) Effect of flow pattern on cellulase deactivation in stirred tank bioreactors. Chem Eng Sci 60:1067–1083. https://doi.org/10.1016/j.ces.2004.09.069
Haven MØ, Lindedam J, Jeppesen MD et al (2015) Continuous recycling of enzymes during production of lignocellulosic bioethanol in demonstration scale. Appl Energy 159:188–195. https://doi.org/10.1016/j.apenergy.2015.08.062
Barsberg S, Selig MJ, Felby C (2013) Impact of lignins isolated from pretreated lignocelluloses on enzymatic cellulose saccharification. Biotechnol Lett 35:189–195. https://doi.org/10.1007/s10529-012-1061-x
Kashyap P, Bhardwaj S, Chodimella VP, Sinha AK (2022) Carbon-based heterogeneous catalysts for conversion of cellulose and cellulosic feedstock. Biomass Conv Bioref. https://doi.org/10.1007/s13399-022-02675-y
Jesionowski T, Zdarta J, Krajewska B (2014) Enzyme immobilization by adsorption: a review. Adsorption 20:801–821. https://doi.org/10.1007/s10450-014-9623-y
Carvalho T, Pereira ADS, Bonomo RCF et al (2020) Simple physical adsorption technique to immobilize Yarrowia lipolytica lipase purified by different methods on magnetic nanoparticles: adsorption isotherms and thermodynamic approach. Int J Biol Macromol 160:889–902. https://doi.org/10.1016/j.ijbiomac.2020.05.174
Sillu D, Agnihotri S (2020) Cellulase immobilization onto magnetic halloysite nanotubes: enhanced enzyme activity and stability with high cellulose saccharification. ACS Sustain Chem Eng 8:900–913. https://doi.org/10.1021/acssuschemeng.9b05400
Zang L, Qiu J, Wu X et al (2014) Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization. Ind Eng Chem Res 53:3448–3454. https://doi.org/10.1021/ie404072s
Zanuso E, Ruiz HA, Domingues L, Teixeira JA (2022) Magnetic nanoparticles as support for cellulase immobilization strategy for enzymatic hydrolysis using hydrothermally pretreated corn cob biomass. Bioenerg Res 15:1946–1957. https://doi.org/10.1007/s12155-021-10384-z
Yasmin HAN, Naresh S, Kunasundari B, Shuit SH (2022) Immobilization of cellulase on magnetized multiwall carbon nanotubes (m-MWCNTs) synthesized via eco-friendly (water-based) method. Chem Pap 76:453–464. https://doi.org/10.1007/s11696-021-01874-7
Udomsin J, Prasannan A, Wang C-F et al (2021) Preparation of carbon nanotube/tannic acid/polyvinylpyrrolidone membranes with superwettability for highly efficient separation of crude oil-in-water emulsions. J Membr Sci 636:119568. https://doi.org/10.1016/j.memsci.2021.119568
Nayeri MD, Nikkhah H, Zilouei H, Bazarganipour M (2023) Immobilization of cellulase on graphene oxide coated with NiFe2O4 and Fe3O4 for hydrolysis of rice straw. Cellulose 30:5549–5571. https://doi.org/10.1007/s10570-023-05207-7
Ashwini John J, Samuel MS, Selvarajan E (2023) Immobilized cellulase on Fe3O4/GO/CS nanocomposite as a magnetically recyclable catalyst for biofuel application. Fuel 333:126364. https://doi.org/10.1016/j.fuel.2022.126364
Suganuma S, Nakajima K, Kitano M et al (2008) Hydrolysis of cellulose by amorphous carbon bearing SO3 H, COOH, and OH groups. J Am Chem Soc 130:12787–12793. https://doi.org/10.1021/ja803983h
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339–1339. https://doi.org/10.1021/ja01539a017
Verma D, Tiwari R, Sinha AK (2013) Depolymerization of cellulosic feedstocks using magnetically separable functionalized graphene oxide. RSC Adv 3:13265. https://doi.org/10.1039/c3ra41025k
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Ameer S, Gul IH (2016) Influence of reduced graphene oxide on effective absorption bandwidth shift of hybrid absorbers. PLoS ONE 11:e0153544. https://doi.org/10.1371/journal.pone.0153544
Huang NM, Lim HN, Chia CH et al (2011) Simple room-temperature preparation of high-yield large-area graphene oxide. IJN 6:3443–3448. https://doi.org/10.2147/IJN.S26812
Marcano DC, Kosynkin DV, Berlin JM et al (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814. https://doi.org/10.1021/nn1006368
Çiplak Z, Yildiz N, Çalimli A (2015) Investigation of graphene/Ag nanocomposites synthesis parameters for two different synthesis methods. Fullerenes Nanotubes Carbon Nanostruct 23:361–370. https://doi.org/10.1080/1536383X.2014.894025
He D, Peng Z, Gong W et al (2015) Mechanism of a green graphene oxide reduction with reusable potassium carbonate. RSC Adv 5:11966–11972. https://doi.org/10.1039/C4RA14511A
Ahangaran F, Hassanzadeh A, Nouri S (2013) Surface modification of Fe3O4@SiO2 microsphere by silane coupling agent. Int Nano Lett 3:23. https://doi.org/10.1186/2228-5326-3-23
Verma D, Verma S, Sinha AK, Jain SL (2013) Iron nanoparticles supported on graphene oxide: a robust, magnetically separable heterogeneous catalyst for the oxidative cyanation of tertiary amines. ChemPlusChem 78:860–865. https://doi.org/10.1002/cplu.201300196
De Andrade ST, Keijok WJ, Guimarães MCC et al (2022) Impact of immobilization strategies on the activity and recyclability of lipases in nanomagnetic supports. Sci Rep 12:6815. https://doi.org/10.1038/s41598-022-10721-y
Scardaci V, Compagnini G (2021) Raman spectroscopy investigation of graphene oxide reduction by laser scribing. C 7:48. https://doi.org/10.3390/c7020048
Schönfelder R, Rümmeli MH, Gruner W et al (2007) Purification-induced sidewall functionalization of magnetically pure single-walled carbon nanotubes. Nanotechnology 18:375601. https://doi.org/10.1088/0957-4484/18/37/375601
Johra FT, Lee J-W, Jung W-G (2014) Facile and safe graphene preparation on solution based platform. J Ind Eng Chem 20:2883–2887. https://doi.org/10.1016/j.jiec.2013.11.022
Kim, Seon-Guk, 박옥경, Lee, Joong Hee, Ku, Bon-Cheol (2013) Layer-by-layer assembled graphene oxide films and barrier properties of thermally reduced graphene oxide membranes. Carbon Lett 14:247–250. https://doi.org/10.5714/CL.2013.14.4.247
Kumar A, Sadanandhan AM, Jain SL (2019) Silver doped reduced graphene oxide as a promising plasmonic photocatalyst for oxidative coupling of benzylamines under visible light irradiation. New J Chem 43:9116–9122. https://doi.org/10.1039/C9NJ00852G
Marycz K, Sobierajska P, Roecken M et al (2020) Iron oxides nanoparticles (IOs) exposed to magnetic field promote expression of osteogenic markers in osteoblasts through integrin alpha-3 (INTa-3) activation, inhibits osteoclasts activity and exerts anti-inflammatory action. J Nanobiotechnol 18:33. https://doi.org/10.1186/s12951-020-00590-w
Maiti NC, Apetri MM, Zagorski MG et al (2004) Raman spectroscopic characterization of secondary structure in natively unfolded proteins: α-Synuclein. J Am Chem Soc 126:2399–2408. https://doi.org/10.1021/ja0356176
Wang G, Yang J, Park J et al (2008) Facile synthesis and characterization of graphene nanosheets. J Phys Chem C 112:8192–8195. https://doi.org/10.1021/jp710931h
Xu W, Sun Z, Meng H et al (2018) Immobilization of cellulase proteins on zeolitic imidazolate framework (ZIF-8)/polyvinylidene fluoride hybrid membranes. New J Chem 42:17429–17438. https://doi.org/10.1039/C8NJ03366H
Raza S, Yong X, Deng J (2019) Immobilizing cellulase on multi-layered magnetic hollow particles: preparation, bio-catalysis and adsorption performances. Microporous Mesoporous Mater 285:112–119. https://doi.org/10.1016/j.micromeso.2019.05.011
Buntić A, Pavlović M, Antonović D et al (2018) Customizing the spent coffee for Trichoderma reesei cellulase immobilization by modification with activating agents. Int J Biol Macromol 107:1856–1863. https://doi.org/10.1016/j.ijbiomac.2017.10.060
Daoud FB-O, Kaddour S, Sadoun T (2010) Adsorption of cellulase Aspergillus niger on a commercial activated carbon: kinetics and equilibrium studies. Colloids Surf B 75:93–99. https://doi.org/10.1016/j.colsurfb.2009.08.019
Singh R, Tiwari M, Singh R, Lee J-K (2013) From protein engineering to immobilization: promising strategies for the upgrade of industrial enzymes. IJMS 14:1232–1277. https://doi.org/10.3390/ijms14011232
Klibanov AM (1983) Stabilization of enzymes against thermal inactivation. In: Advances in applied microbiology. Elsevier, pp 1–28. https://doi.org/10.1016/S0065-2164(08)70352-6
Zhu C, Fang Z, Su T et al (2018) Cellulase immobilized on mesoporous biochar synthesized by ionothermal carbonization of cellulose. Cellulose 25:2473–2485. https://doi.org/10.1007/s10570-018-1704-8
Taqieddin E, Amiji M (2004) Enzyme immobilization in novel alginate–chitosan core-shell microcapsules. Biomaterials 25:1937–1945. https://doi.org/10.1016/j.biomaterials.2003.08.034
Sheldon RA (2007) Enzyme immobilization: the quest for optimum performance. Adv Synth Catal 349:1289–1307. https://doi.org/10.1002/adsc.200700082
Bolivar JM, Woodley JM, Fernandez-Lafuente R (2022) Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem Soc Rev 51:6251–6290. https://doi.org/10.1039/D2CS00083K
Ariaeenejad S, Motamedi E, Hosseini Salekdeh G (2020) Stable cellulase immobilized on graphene oxide@CMC-g-poly(AMPS-co-AAm) hydrogel for enhanced enzymatic hydrolysis of lignocellulosic biomass. Carbohyd Polym 230:115661. https://doi.org/10.1016/j.carbpol.2019.115661
Mo H, Qiu J (2020) Preparation of chitosan/magnetic porous biochar as support for cellulase immobilization by using glutaraldehyde. Polymers 12:2672. https://doi.org/10.3390/polym12112672
Paz-Cedeno FR, Carceller JM, Iborra S et al (2021) Magnetic graphene oxide as a platform for the immobilization of cellulases and xylanases: ultrastructural characterization and assessment of lignocellulosic biomass hydrolysis. Renewable Energy 164:491–501. https://doi.org/10.1016/j.renene.2020.09.059
Kristensen JB, Felby C, Jørgensen H (2009) Yield-determining factors in high-solids enzymatic hydrolysis of lignocellulose. Biotechnol Biofuels 2:11. https://doi.org/10.1186/1754-6834-2-11
Mo H, Qiu J, Yang C et al (2020) Preparation and characterization of magnetic polyporous biochar for cellulase immobilization by physical adsorption. Cellulose 27:4963–4973. https://doi.org/10.1007/s10570-020-03125-6
Gokhale AA, Lu J, Lee I (2013) Immobilization of cellulase on magnetoresponsive graphene nano-supports. J Mol Catal B Enzym 90:76–86. https://doi.org/10.1016/j.molcatb.2013.01.025
Papadopoulou A, Zarafeta D, Galanopoulou AP, Stamatis H (2019) Enhanced catalytic performance of Trichoderma reesei cellulase immobilized on magnetic hierarchical porous carb on nanoparticles. Protein J 38:640–648. https://doi.org/10.1007/s10930-019-09869-w
Acknowledgements
SB thanks to Department of Biotechnology (DBT) for the fellowship. All the authors are pleased to acknowledge the Director, CSIR-Indian Institute of Petroleum, Dehradun, for his kind support in obtaining these results. Thanks to all the lab members of the Analytical Division, Bio Fuels Division, and Material Resource Efficiency Division for their kind support throughout the study.
Author information
Authors and Affiliations
Contributions
SB: investigation, methodology, formal analysis, writing—original draft; DA: writing—review and editing, methodology, resources; DG: writing—review and editing, supervision, resources, data curation; AKS: conceptualization, supervision, resources, data curation, writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhardwaj, S., Agrawal, D., Ghosh, D. et al. Magnetically separable graphene oxide as a promising matrix for cellulase immobilization: enhanced activity and stability with high glucose yield. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-05055-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-05055-2