Abstract
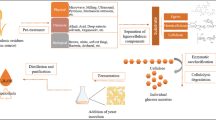
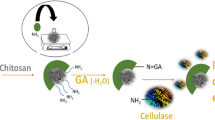
Enzymatic hydrolysis is a key process for lignocellulosic biomass conversion; a way to increase its efficiency and lessen the process cost is by targeting alternative strategies to improve the use of cellulolytic enzymes. This work addresses enzyme immobilization as a strategy to enhance lignocellulosic biomass hydrolysis by simplifying enzyme recovery and opening possibility for continuous processes. Magnetic nanoparticles hold the advantage of easy separation compared to other immobilization supports. Hence, a cellulase cocktail was successfully bonded onto chitosan coated magnetic nanoparticles reaching 65 mgprotein per g of support. The biocatalyst was stable at 4 °C after 30 days storage maintaining 80% of the initial activity and could be reused up to 13 cycles retaining 48.8% of the initial activity. Considering the information available on corn cob processing, it was used as model biomass for evaluating the efficiency of the system proposed. Corn cob was submitted to a hydrothermal pretreatment at 211 °C and non-isothermal regime for biomass fractionation. The pretreated corn cob solid rich in cellulose (61.17 g per 100 g of raw material) was used as substrate [5% (w/v) solid loading] to evaluate the hydrolysis potential of the immobilized cellulase, obtaining 21.84 g/L of glucose which corresponds to 64.45% conversion yield. Thus, this work validates the use of immobilized cellulase cocktails to effectively hydrolyze lignocellulosic substrates, a step-forward in lignocellulosic biorefineries and enzyme reusability. Moreover, the magnetic properties of the support make it a promising technique for eventual continuous operation, an important contribution for cost reduction of the process.
Graphical abstract







Similar content being viewed by others
Availability of Data and Materials
Not applicable.
References
Gomes D, Cunha J, Zanuso E, Teixeira J, Domingues L (2021) Strategies towards reduction of cellulases consumption: debottlenecking the economics of lignocellulosics valorization processes. Polysaccharides 2:287–310. https://doi.org/10.3390/polysaccharides2020020
Cunha JT, Soares PO, Baptista SL, Costa CE, Domingues L (2020) Engineered Saccharomyces cerevisiae for lignocellulosic valorization: a review and perspectives on bioethanol production. Bioengineered 11:883–903. https://doi.org/10.1080/21655979.2020.1801178
Baptista SL, Costa CE, Cunha JT, Soares PO, Domingues L (2021) Metabolic engineering of Saccharomyces cerevisiae for the production of top value chemicals from biorefinery carbohydrates. Biotechnol Adv 47:107697. https://doi.org/10.1016/j.biotechadv.2021.107697
Pino MS, Rodríguez-Jasso RM, Michelin M, Ruiz HA (2019) Enhancement and modeling of enzymatic hydrolysis on cellulose from agave bagasse hydrothermally pretreated in a horizontal bioreactor. Carbohydr Polym 211:349–359. https://doi.org/10.1016/j.carbpol.2019.01.111
Ruiz HA, Vicente AA, Teixeira JA (2012) Kinetic modeling of enzymatic saccharification using wheat straw pretreated under autohydrolysis and organosolv process. Ind Crops Prod 36:100–107. https://doi.org/10.1016/j.indcrop.2011.08.014
Barbosa FC, Silvello MA, Goldbeck R (2020) Cellulase and oxidative enzymes: new approaches, challenges and perspectives on cellulose degradation for bioethanol production. Biotechnol Lett 42:875–884. https://doi.org/10.1007/s10529-020-02875-4
Kumar B, Bhardwaj N, Agrawal K, Chaturvedi V, Verma P (2020) Current perspective on pretreatment technologies using lignocellulosic biomass: an emerging biorefinery concept. Fuel Process Technol 199:106244. https://doi.org/10.1016/j.fuproc.2019.106244
Zanuso E, Lara-Flores AA, Aguilar DL, Velazquez-Lucio J, Aguilar CN, Rodríguez-Jasso RM, Ruiz HA, (2017) Kinetic modeling, operational conditions, and biorefinery products from hemicellulose: depolymerization and solubilization during hydrothermal processing. In: Ruiz HA, Thomsen MH, Trajano HL (eds) Hydrothermal Processing in Biorefineries. Springer International Publishing, Switzerland, pp 141–160. https://doi.org/10.1007/978-3-319-56457-9_5
Ruiz HA, Conrad M, Sun S-N, Sanchez A, Rocha GJM, Romaní A, Castro E, Torres A, Rodríguez-Jasso RM, Andrade LP, Smirnova I, Sun R-C, Meyer AS (2020) Engineering aspects of hydrothermal pretreatment: From batch to continuous operation, scale-up and pilot reactor under biorefinery concept. Bioresour Technol 299:122685. https://doi.org/10.1016/j.biortech.2019.122685
Larnaudie V, Ferrari MD, Lareo C (2019) Techno-economic analysis of a liquid hot water pretreated switchgrass biorefinery: effect of solids loading and enzyme dosage on enzymatic hydrolysis. Biomass Bioenergy 130:105394. https://doi.org/10.1016/j.biombioe.2019.105394
Liu G, Zhang J, Bao J (2016) Cost evaluation of cellulase enzyme for industrial-scale cellulosic ethanol production based on rigorous Aspen Plus modeling. Bioprocess Biosyst Eng 39:133–140. https://doi.org/10.1007/s00449-015-1497-1
Van Rijn R, Nieves IU, Shanmugam KT, Ingram LO, Vermerris W (2018) Techno-Economic evaluation of cellulosic ethanol production based on pilot biorefinery data: a case study of sweet sorghum bagasse processed via L+SScF. Bioenergy Res 11:414–425. https://doi.org/10.1007/s12155-018-9906-3
Boakye-Boaten NA, Kurkalova L, Xiu S, Shahbazi A (2017) Techno-economic analysis for the biochemical conversion of Miscanthus x giganteus into bioethanol. Biomass Bioenergy 98:85–94. https://doi.org/10.1016/j.biombioe.2017.01.017
Papadaskalopoulou C, Sotiropoulos A, Novacovic J, Barabouti E, Mai S, Malamis D, Kekos D, Loizidou M (2019) Comparative life cycle assessment of a waste to ethanol biorefinery system versus conventional waste management methods. Resour Conserv Recycl 149:130–139. https://doi.org/10.1016/j.resconrec.2019.05.006
Gomes D, Rodrigues AC, Domingues L, Gama M (2015) Cellulase recycling in biorefineries—is it possible? Appl Microbiol Biotechnol 99:4131–4143. https://doi.org/10.1007/s00253-015-6535-z
Gomes DG, Serna-Loaiza S, Cardona C, Gama M, Domingues L (2018) Insights into the economic viability of cellulases recycling on bioethanol production from recycled paper sludgle. Bioresour Technol 264:347–355. https://doi.org/10.1016/j.biortech.2018.07.056
Oliveira C, Romaní A, Gomes D, Cunha JT, Gama FM, Domingues L (2018) Recombinant family 3 carbohydrate-binding module as a new additive for enhanced enzymatic saccharification of whole slurry from autohydrolyzed Eucalyptus globulus wood. Cellulose 25:2505–2514. https://doi.org/10.1007/s10570-018-1722-6
Aguilar DL, Rodríguez-Jasso RM, Zanuso E, Lara-Flores AA, Aguilar CN, Sánchez A, Ruiz HA (2018) Operational strategies for enzymatic hydrolysis in a biorefinery. In: Kumar S, Sani R (eds.) Biorefining Biomass to Biofuels – Opportunities and Perception. Springer, Cham, pp 223–248. https://doi.org/10.1007/978-3-319-67678-4_10
Pino MS, Rodríguez-Jasso RM, Michelin M, Flores-Gallegos AC, Morales-Rodriguez R, Teixeira JA, Ruiz HA (2018) Bioreactor design for enzymatic hydrolysis of biomass under the biorefinery concept. Chem Eng J 347:119–136. https://doi.org/10.1016/j.cej.2018.04.057
Singh A, Rodríguez-Jasso RM, Gonzalez-Gloria KD, Rosales M, BelmaresCerda R, Aguilar CN, Singhania RR, Ruiz HA (2019) The enzyme biorefinery platform for advanced biofuels production. Bioresour Technol Reports 7:100257. https://doi.org/10.1016/j.biteb.2019.100257
Abraham RE, Puri M (2020) Nano-immobilized cellulases for biomass processing with application in biofuel production, In: Kumar, C.V. (ed) Methods in Enzymology. Elsevier Inc. Academic Press, pp 327–346. https://doi.org/10.1016/bs.mie.2019.09.006
Grewal J, Ahmad R, Khare SK (2017) Development of cellulase-nanoconjugates with enhanced ionic liquid and thermal stability for in situ lignocellulose saccharification. Bioresour Technol 242:236–243. https://doi.org/10.1016/j.biortech.2017.04.007
Qi B, Luo J, Wan Y (2018) Immobilization of cellulase on a core-shell structured metal-organic framework composites: better inhibitors tolerance and easier recycling. Bioresour Technol 268:577–582. https://doi.org/10.1016/j.biortech.2018.07.115
Zanuso E, Gomes DG, Ruiz HA, Teixeira JA, Domingues L (2021) Enzyme immobilization as a strategy towards efficient and sustainable lignocellulosic biomass conversion into chemicals and biofuels: current status and perspectives. Sustainable Energy Fuels 5:4233–4247. https://doi.org/10.1039/D1SE00747E
Paz-Cedeno FR, Carceller JM, Iborra S, Donato RK, Godoy AP, Veloso de Paula A, Monti R, Corma A, Masarin F (2021) Magnetic graphene oxide as a platform for the immobilization of cellulases and xylanases: ultrastructural characterization and assessment of lignocellulosic biomass hydrolysis. Renew Energy 164:491–501. https://doi.org/10.1016/j.renene.2020.09.059
Sillu D, Agnihotri S (2020) Cellulase immobilization onto magnetic halloysite nanotubes: enhanced enzyme activity and stability with high cellulose saccharification. ACS Sustain Chem Eng 8:900–913. https://doi.org/10.1021/acssuschemeng.9b05400
Mo H, Qiu J, Yang C, Zang L, Sakai E (2020) Preparation and characterization of magnetic polyporous biochar for cellulase immobilization by physical adsorption. Cellulose 27:4963–4973. https://doi.org/10.1007/s10570-020-03125-6
Zang L, Qiu J, Wu X, Zhang W, Sakai E, Wei Y (2014) Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization. Ind Eng Chem Res 53:3448–3454. https://doi.org/10.1021/ie404072s
Aguilar-Reynosa A, Romaní A, Rodríguez-Jasso RM, Aguilar CN, Garrote G, Ruiz HA (2017) Comparison of microwave and conduction-convection heating autohydrolysis pretreatment for bioethanol production. Bioresour Technol 243:273–283. https://doi.org/10.1016/j.biortech.2017.06.096
Costa CE, Romaní A, Cunha JT, Johansson B, Domingues L (2017) Integrated approach for selecting efficient Saccharomyces cerevisiae for industrial lignocellulosic fermentations: importance of yeast chassis linked to process conditions. Bioresour Technol 227:24–34. https://doi.org/10.1016/j.biortech.2016.12.016
Baptista SL, Cunha JT, Romaní A, Domingues L (2018) Xylitol production from lignocellulosic whole slurry corn cob by engineered industrial Saccharomyces cerevisiae PE-2. Bioresour Technol 267:481–491. https://doi.org/10.1016/j.biortech.2018.07.068
Baptista SL, Carvalho LC, Romaní A, Domingues L (2020) Development of a sustainable bioprocess based on green technologies for xylitol production from corn cob. Ind Crops Prod 156:112867. https://doi.org/10.1016/j.indcrop.2020.112867
Anukam AI, Goso BP, Okoh OO, Mamphweli SN (2017) Studies on characterization of corn cob for application in a gasification process for energy production. J Chem 2017:6478389. https://doi.org/10.1155/2017/6478389
Hoarau M, Badieyan S, Marsh ENG (2017) Immobilized enzymes: understanding enzyme-surface interactions at the molecular level. Org Biomol Chem 15:9539–9551. https://doi.org/10.1039/c7ob01880krsc.li/obc
Azevedo MA, Cerqueira MA, Fuciños P, Silva BFB, Teixeira JA, Pastrana L (2020) Rhamnolipids-based nanostructured lipid carriers: effect of lipid phase on physicochemical properties and stability. Food Chem 128670. https://doi.org/10.1016/j.foodchem.2020.128670
Tan L, Tan Z, Feng H, Qiu J (2018) Cellulose as a template to fabricate a cellulase-immobilized composite with high bioactivity and reusability. New J Chem 42:1665–1672. https://doi.org/10.1039/C7NJ03271D
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Asar MF, Ahmad N, Husain Q (2020) Chitosan modified Fe3O4/graphene oxide nanocomposite as a support for high yield and stable immobilization of cellulase: its application in the saccharification of microcrystalline cellulose. Prep Biochem Biotechnol 50:460–467. https://doi.org/10.1080/10826068.2019.1706562
Cunha JT, Romaní A, Inokuma K, Johansson B, Hasunuma T, Kondo A, Domingues L (2020) Consolidated bioprocessing of corn cob-derived hemicellulose: engineered industrial Saccharomyces cerevisiae as efficient whole cell biocatalysts. Biotechnol Biofuels 13:1–15. https://doi.org/10.1186/s13068-020-01780-2
Overend RP, Chornet E (1987) Fractionation of lignocellulosics by steam-aqueous pretreatment. Philos Trans R Soc London 321:523–536. https://doi.org/10.1098/rsta.1987.0029
Sluiter A, Hames R, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and ligning in biomass – NREL/TP-510–42618. NREL Analytical procedure, National Renewable Energy Laboratory
Li GY, Jiang YR, Huang KL, Ding P, Chen J (2008) Preparation and properties of magnetic Fe3O4-chitosan nanoparticles. J Alloys Compd 466:451–456. https://doi.org/10.1016/j.jallcom.2007.11.100
Wu Q, Lin D, Yao S (2014) Design of chitosan and its water soluble derivatives-based drug carriers with polyelectrolyte complexes. Mar Drugs 12:6236–6253. https://doi.org/10.3390/md12126236
Boudrant J, Woodley JM, Fernandez-Lafuente R (2020) Parameters necessary to define an immobilized enzyme preparation. Process Biochem 90:66–80. https://doi.org/10.1016/j.procbio.2019.11.026
Xu J, Liu Y, Hsu SH (2019) Hydrogels based on Schiff base linkages for biomedical applications. Molecules 24:1–21. https://doi.org/10.3390/molecules24163005
Hosseini SH, Hosseini SA, Zohreh N, Yaghoubi M, Pourjavadi A (2018) Covalent immobilization of cellulase using magnetic poly (ionic liquid) support: improvement of the enzyme activity and stability. J Agric Food Chem 66:789–798. https://doi.org/10.1021/acs.jafc.7b03922
Wang D, Jiang W (2019) Preparation of chitosan-based nanoparticles for enzyme immobilization. Int J Biol Macromol 126:1125–1132. https://doi.org/10.1016/j.ijbiomac.2018.12.243
Alftrén J, Hobley TJ (2014) Immobilization of cellulase mixtures on magnetic particles for hydrolysis of lignocellulose and ease of recycling. Biomass Bioenergy 65:72–78. https://doi.org/10.1016/j.biombioe.2014.03.0090961-9534
Schnell F, Kube M, Berensmeier S, Schwaminger SP (2019) Magnetic recovery of cellulase from cellulose substrates with bare iron oxide nanoparticles. ChemNanoMat 5:422–426. https://doi.org/10.1002/cnma.201800658
Sigurdardóttir SB, Lehmann J, Grivel J, Zhang W, Kaiser A, Pinelo M (2019) Alcohol dehydrogenase on inorganic powders: zeta potential and particle agglomeration as main factors determining activity during immobilization. Colloids Surf B 175:136–142. https://doi.org/10.1016/j.colsurfb.2018.11.080
Sigurdardóttir SB, Lehmann J, Ovtar S, Grivel J-C, Della Negra M, Kaiser A, Pinelo M (2018) Enzyme immobilization on inorganic surfaces for membrane reactor applications: mass transfer challenges, enzyme leakage and reuse of materials. Adv Synth Catal 360:2578–2607. https://doi.org/10.1002/adsc.201800307
Sun Q, Chen W-J, Pang B, Sun Z, Lam SS, Sonne C, Yuan T-Q (2021) Ultrastrucutral change in lignocellulosic biomass during hydrothermal pretreatment. Bioresour Technol 341:125807. https://doi.org/10.1016/j.biortech.2021.125807
Song B, Lin R, Lam CH, Wu H, Tsui T-H, Yu Y (2021) Recent advances and challenges of inter-disciplinary biomass valorization by integrating hydrothermal and biological techniques. Renewable Sustainable Energy Rev 135:110370. https://doi.org/10.1016/j.rser.2020.110370
Kaur P, Taggar MS, Kalia A (2020) Characterization of magnetic nanoparticle–immobilized cellulases for enzymatic saccharification of rice straw. Biomass Convers Biorefin 11:955–969. https://doi.org/10.1007/s13399-020-00628-x
Sánchez-Ramírez J, Martínez-Hernández JL, Segura-Ceniceros P, López G, Saade H, Medina-Morales MA, Ramos-González R, Aguilar CN, Ilyina A (2017) Cellulases immobilization on chitosan-coated magnetic nanoparticles: application for Agave Atrovirens lignocellulosic biomass hydrolysis. Bioprocess Biosyst Eng 40:9–22. https://doi.org/10.1007/s00449-016-1670-1
Jia J, Zhang W, Yang Z, Yang X, Wang N, Yu X (2017) Novel magnetic cross-linked cellulase aggregates with a potential application in lignocellulosic biomass bioconversion. Molecules 22. https://doi.org/10.3390/molecules22020269
Zheng P, Wang J, Lu C, Xu Y, Sun Z (2013) Immobilized β-glucosidase on magnetic chitosan microspheres for hydrolysis of straw cellulose. Process Biochem 48:683–687. https://doi.org/10.1016/j.procbio.2013.02.027
Rahikainen JL, Martin-Sampedro R, Heikkinen H, Rovio S, Marjamaa K, Tamminen T, Rojas OJ, Kruus K (2013) Inhibitory effect of lignin during cellulose bioconversion: the effect of lignin chemistry on non-productive enzyme adsorption. Bioresour Technol 133:270–278. https://doi.org/10.1016/j.biortech.2013.01.075
Singh A, Rodríguez-Jasso RM, Saxena R, Belmares Cerda R, Singhania RR, Ruiz HA (2021) Subcritical water pretreatment for agave bagasse fractionation from tequila production and enzymatic susceptibility. Bioresour Technol 338:125536. https://doi.org/10.1016/j.biortech.2021.125536
Kumar A, Singh S, Tiwari R, Goel R, Nain L (2017) Immobilization of indigenous holocellulase on iron oxide (Fe2O3) nanoparticles enhanced hydrolysis of alkali pretreated paddy straw. Int J Biol Macromol 96:538–548. https://doi.org/10.1016/j.ijbiomac.2016.11.109
Funding
This study was supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UIDB/04469/2020 unit. Elisa Zanuso is recipient of a PhD fellowship supported by the Mexican Science and Technology Council—Energy Sustainability Fund of Secretary of Energy Mexico (CONACY-SENER) (CONACYT ID 639021/495314).
Author information
Authors and Affiliations
Contributions
Elisa Zanuso: Methodology, investigation, writing-original draft preparation, writing-reviewing and editing; Héctor A. Ruiz: Conceptualization, writing-reviewing and editing, visualization, supervision; José A. Teixeira: Conceptualization, resources, writing-reviewing and editing, supervision; Lucília Domingues: Conceptualization, resources, writing-reviewing and editing, supervision.
Corresponding author
Ethics declarations
Ethics Approval
This article does not involve any studies with human participants or animals.
Consent to Participate
Not applicable.
Consent for Publication
All authors read and approved the final manuscript for publication.
Conflict of Interest
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zanuso, E., Ruiz, H.A., Domingues, L. et al. Magnetic Nanoparticles as Support for Cellulase Immobilization Strategy for Enzymatic Hydrolysis Using Hydrothermally Pretreated Corn Cob Biomass. Bioenerg. Res. 15, 1946–1957 (2022). https://doi.org/10.1007/s12155-021-10384-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10384-z