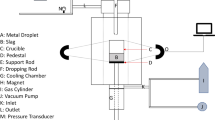
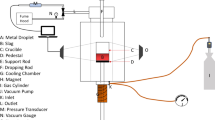
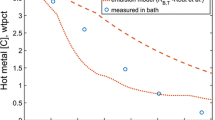
Abstract
A decarburization model has been developed for a Fe–C–S liquid droplet reacting in an oxidizing slag at high temperature (1580 °C to 1640 °C). The model incorporates the partitioning of oxygen at the slag/metal interface between decarburization at the slag/metal interface and transport into the droplet. The kinetics of nucleation and growth of CO bubbles within the liquid metal droplet have also been introduced to describe internal decarburization. The model parameters were determined using one set of experimental conditions and then used to predict behavior over a wide range of conditions. The prediction was validated for variation of, sulfur concentration, droplet mass, temperature, and droplet carbon concentration. Decarburization was found to proceed in three stages. The model was found to show good agreement for the initial two stages of decarburization: the incubation period and peak decarburization period. This observation suggested that the oxygen partitioning and nucleation kinetics had been incorporated properly. The model failed to predict the sudden shutdown of decarburization at the end stage of decarburization.














Similar content being viewed by others
Abbreviations
- [pct C]:
-
Concentration of dissolved carbon in liquid metal (wt pct)
- [pct O]:
-
Concentration of dissolved oxygen in liquid metal (wt pct)
- [pct Al2O3]:
-
Slag Al2O3 concentration (wt pct)
- [pct CaO]:
-
Slag CaO concentration (wt pct)
- [pct FeO]:
-
Slag FeO concentration (wt pct)
- [pct SiO2]:
-
Slag SiO2 concentration (wt pct)
- A :
-
Interfacial area (m2)
- \({A}_{\mathrm{g}/\mathrm{m}}^{\mathrm{gr}}\left(t\right)\) :
-
Total gas/metal surface area generated from the growth at time t (m2)
- \({A}_{\mathrm{g}/\mathrm{m}}^{\mathrm{nuc}}\left(t\right)\) :
-
Total gas/metal interfacial area generated due to nucleation at time step t (m2)
- J C :
-
Flux of C in the metal (mol/s)
- J FeO :
-
Flux of FeO in the slag (mol/s)
- J O :
-
Flux of O in the metal (mol/s)
- K CO :
-
Equilibrium constant of CO formation reaction (–)
- \({K}_{{\mathrm{FeO}}_{\mathrm{eq}}}\) :
-
Equilibrium constant of FeO dissociation reaction (–)
- K j :
-
Adsorption coefficient of species j, where j are [S] and [O] (–)
- \({M}_{\mathrm{C}}\), \({M}_{\mathrm{O}}\) :
-
Molecular weight of C and O (kg/mol)
- \({M}_{\mathrm{FeO}}\), \({M}_{\mathrm{CaO}}\), \({M}_{{\mathrm{SiO}}_{2}}\), \({M}_{{\mathrm{Al}}_{2}{\mathrm{O}}_{3}}\) :
-
Molecular weight of FeO, CaO, SiO2 and Al2O3, respectively (kg/mol)
- N 0 :
-
No of nucleation sites per unit volume (/m3)
- P L :
-
Liquid pressure (Pa)
- P ve :
-
Pressure of CO bubble at equilibrium (Pa)
- P CO :
-
Pressure of CO at the interface (Pa)
- R CO :
-
Rate of CO formation at the slag/metal interface (mol/s)
- R FeO :
-
Rate of FeO dissociation reaction at the slag/metal interface (mol/s)
- \({V}_{\mathrm{CO}}{\left(t\right)}^{\mathrm{nuc}}\), \({V}_{\mathrm{CO}}{\left(t\right)}^{\mathrm{gr}}\) :
-
Volume of CO bubbles which are generated from nucleation and growth, respectively, at time t (m3)
- \({V}_{\mathrm{CO}}\left(t\right)\) :
-
Volume of the CO bubbles remaining in the droplet at time t (m3)
- \({V}^{\mathrm{esc}}(t)\) :
-
Volume of bubbles which are escaping from the bloated droplet at time t (m3)
- \({V}_{\mathrm{m}}\) :
-
Volume of liquid metal (m3)
- \({W}_{\mathrm{slag}}^{\mathrm{dense}}\) :
-
Mass of dense slag (kg)
- \({W}_{\mathrm{slag}}^{\mathrm{foamy}}\) :
-
Mass of foamy slag (kg)
- \({W}_{\mathrm{slag}}^{\mathrm{tot}}\) :
-
Total slag mass (kg)
- X FeO, \({X}_{Si{O}_{2}}\), X CaO :
-
Mole fraction of FeO, SiO2 and CaO (–)
- \({a}_{\mathrm{j}}\) :
-
Activity of species j, where j can be [O], [C], [S] in metal or (FeO) in slag (–)
- f C :
-
Henrian activity coefficient of carbon (–)
- f O :
-
Henrian activity coefficient of oxygen (–)
- \(\overrightarrow{k}\) :
-
Forward reaction rate constant of FeO dissociation (mol/m2 s)
- K O :
-
Overall growth rate constant (mol/m2 s)
- \({k}_{\mathrm{eff}}\) :
-
Effective slag mass transfer coefficient (m/s)
- \({k}_{\mathrm{m}}\) :
-
Mass transfer coefficient of species in metal (m/s)
- \({k}_{\mathrm{r}}\) :
-
Forward reaction rate constant of CO formation (kg/m2 s)
- \({k}_{\mathrm{s}}\) :
-
Slag Mass transfer coefficient (m/s)
- \({n}_{\mathrm{CO}}\) :
-
No of moles of CO in a bubble of radius r (mol)
- \({r}^{\mathrm{crit}}\) :
-
Critical radius of CO bubble in liquid metal (m)
- \({r}_{t}\) :
-
Radius of a bubble at t times step (m)
- \({y}_{t}^{\mathrm{Model}}\) :
-
Model predicted data point at time t (mol)
- \({y}_{t}^{\mathrm{exp}}\) :
-
Experimentally found data point at time t (mol)
- γFeO :
-
Activity coefficient of FeO of the bulk slag concentration (–)
- \({\theta }_{\mathrm{j}}\) :
-
Fraction of surface area blocked due to poisoning (–)
- \({\rho }_{\mathrm{d}}\left(t\right)\) :
-
Density of droplet at time t (kg/m3)
- \({\rho }_{\mathrm{m}}\) :
-
Density of metal (kg/m3)
- \({\rho }_{\mathrm{s}}\) :
-
Density of slag (kg/m3)
- \({\sigma }_{0}\) :
-
Surface tension of the metal (N/m)
- \(\Delta t\) :
-
Time step
- R :
-
Universal gas constant (J/mol K)
- \(J\) :
-
Rate of nucleation (number of nuclei/m3 s)
- \(N\) :
-
No of bubble at any time t (–)
- \(N^{\prime}\) :
-
No of data points (–)
- \(T\) :
-
Temperature (K)
- \(k\) :
-
Boltzmann constant (m2 kg s−2 K−1)
- \(m\) :
-
Mass of one CO molecule (kg)
- \(n\) :
-
Coefficient of mass transfer model
- \(\eta \) :
-
Slag viscosity (Pa s)
- \(\psi \) :
-
Surface tension modifying parameter (–)
- \(\mathrm{i}\) :
-
At the slag/metal interface
- \(\mathrm{b}\) :
-
Within the bulk
- \(\mathrm{s}/\mathrm{m}\) :
-
Slag/metal interface
- \(\mathrm{g}/\mathrm{m}\) :
-
Gas/metal interface
References
Subagyo, G.A. Brooks, and K.S. Coley: Can. Metall. Q., 2005, vol. 44, pp. 119–30.
L.A. Baker and R.G. Ward: J. Iron Steel Inst., 1967, vol. 205, pp. 714–17.
G.G.K. Murthy, Y. Sawada, and J.F. Elliot: Ironmak. Steelmak., 1993, vol. 20, pp. 179–200.
T. Gare and G.S.F. Hazeldean: Ironmak. Steelmak., 1981, vol. 8, pp. 169–81.
K. Gao, V. Sahajwalla, H. Sun, C. Wheatley, and R. Dry: ISIJ Int., 2000, vol. 40, pp. 301–8.
J.H. Zong and J.K. Yoon: Metall. Trans. B., 1990, vol. 21B, pp. 49–57.
D. Widlund, D.S. Sarma, and P.G. Jönsson: ISIJ Int., 2006, vol. 46, pp. 1149–57.
N. Simento, H. LEE, and P. Hayes: ISIJ Int., 1999, vol. 39, pp. 1217–23.
E. Chen and K.S. Coley: Ironmak. Steelmak., 2010, vol. 37, pp. 541–5.
E.W. Mulholland, G.S.F. Hazeldean, and M. Davies: J. Iron Steel Inst., 1973, vol. 211, pp. 632–9.
L.A. Baker, N.A. Warner, and A.E. Jenkins: Trans. Metall. Soc., 1967, vol. 239, pp. 857–64.
R.S. Kaplan and W.O. Philbrook: Metall. Trans., 1972, vol. 3, pp. 487–91.
D.J. Min and R.J. Fruehan: Metall. Trans. B., 1992, vol. 23B, pp. 29–37.
N.H. El Kaddah and D.G.C. Robertson: Metall. Mater. Trans. B., 1988, vol. 19B, pp. 831–7.
N.H. El Kaddah and D.G.C. Robertson: J. Colloid Interface Sci., 1977, vol. 60, pp. 349–60.
K. Gu, N. Dogan, and K.S. Coley: Metall. Mater. Trans. B Process Metall. Mater. Process. Sci., 2017, vol. 48B, pp. 2343–53.
E. Chen and K.S. Coley: McMaster University, 2010.
G.G.K. Murthy, A. Hasham, and U.B. Pal: Ironmak. Steelmak., 1993, vol. 20, pp. 191–200.
C.L. Molloseau and R.J. Fruehan: Metall. Mater. Trans. B Process Metall. Mater. Process. Sci., 2002, vol. 33B, pp. 335–44.
G. Brooks, Y. Pan, and K.S. Coley: Metall. Mater. Trans. B., 2005, vol. 36B, pp. 525–35.
N. Dogan, G.A. Brooks, and M.A. Rhamdhani: ISIJ Int., 2011, vol. 51, pp. 1093–101.
A. Kadrolkar and N. Dogan: Metall. Mater. Trans. B Process Metall. Mater. Process. Sci., 2019, vol. 50B, pp. 2912–29.
B.K. Rout, G. Brooks, M.A. Rhamdhani, Z. Li, F.N.H. Schrama, and A. Overbosch: Metall. Mater. Trans. B., 2018, vol. 49B, pp. 1022–33.
H.S. Levine: Met. Trans., 1973, vol. 4, pp. 777–82.
H. Sun: ISIJ Int., 2006, vol. 46, pp. 1560–9.
C. Kattenbelt and B. Roffel: Metall. Mater. Trans. B., 2008, vol. 39B, pp. 764–9.
M.D. Pomeroy: McMaster University, 2011.
R. Sarkar, P. Gupta, S. Basu, and N.B. Ballal: Metall. Mater. Trans. B., 2015, vol. 46B, pp. 961–76.
W. van der Knoop, B. Deo, A.B. Snoeijer, G. van Unen, and R. Boom: Proc. 4th Int. Conf. Molten Slags Fluxes, 1992, pp. 302–307.
K. Gu, N. Dogan, and K.S. Coley: Metall. Mater. Trans. B Process Metall. Mater. Process. Sci., 2017, vol. 48B, pp. 2984–3001.
I. Langmuir: J. Am. Chem. Soc., 1918, vol. 40, pp. 1361–403.
S. Basu, A.K. Lahiri, and S. Seetharaman: Metall. Mater. Trans. B Process Metall. Mater. Process. Sci., 2010, vol. 41B, pp. 414–9.
M. Hino and K. Ito: Thermodynamic Data for Steelmaking. 2010th ed. KONNO Printing Co. Ltd, Sendai, 2010.
M. Blander and J.L. Katz: AIChE J., 1975, vol. 21, pp. 833–48.
A. Attar: AIChE J., 1978, vol. 24, pp. 106–15.
S.D. Lubetkin: Langmuir Am. Chem. Soc., 2003, vol. 19, pp. 2575–87.
E.N. Harvey, A. Whiteley, W. McElroy, D. Pease, and D. Barnes: J. Cell. Comp. Physiol., 1944, vol. 24, pp. 23–34.
P.G. Bowers, K. Bar-Eli, and R.M. Noyes: J. Chem. Soc. Faraday Trans., 1996, vol. 92, pp. 2843–9. .
R.C. Tolman: J. Chem. Phys., 1949, vol. 17, pp. 333–7. .
Y. Chung and A.W. Cramb: Metall. Mater. Trans. B., 2000, vol. 31B, pp. 957–71. .
B. von Szyszkowski: Z. Phys. Chem., 1908, vol. 64, pp. 385–414. .
G.R. Belton: Metall. Trans. B., 1976, vol. 7B, pp. 35–42. .
T.X. Zhu: Bubble Escape Model, Private Communication, Hamilton, 2018.
K. Gu, N. Dogan, and K.S. Coley: Metall. Mater. Trans. B Process Metall. Mater. Process. Sci., 2017, vol. 48B, pp. 2595–606. .
J. Biswas, K. Gu, and K.S. Coley: Decarburization of Bloated Droplets: An Experimental Study to Understand the Kinetics of Decarburization of Metallic Iron Droplets in FeO Containing CaO-SiO2 Slags. McMaster University, Unpublished Research, 2020.
P.A.A. Distin, G.D.D. Hallett, and F.. D. Richardson: J. Iron Steel Inst., 1968, vol. August, pp. 821–33.
J.B. See and N.A. Warner: J. Iron Steel Inst., 1973, vol. 211, pp. 44–52. .
H. Sun, K. Gao, V. Sahajwalla, K. Mori, and R.D. Pehlke: ISIJ Int., 1999, vol. 39, pp. 1125–33. .
K. Ito and K. Sano: Tetsu-to-Hagane., 1965, vol. 51, pp. 1252–9. .
H. Gaye and P.V. Riboud: Metall. Trans. B., 1977, vol. 8B, pp. 409–15. .
U.B. Pal, S.A. Macdonald, D.W. Woolley, and A.C. Powell: Metall. Mater. Trans. B., 2005, vol. 36B, pp. 209–18. .
D.E. Woolley and U.B. Pal: Ironmak. Steelmak., 2002, vol. 29, pp. 125–32. .
K. Gu, N. Dogan, and K.S. Coley: Metall. Mater. Trans. B., 2018, vol. 49B, pp. 1119–35. .
Acknowledgments
The authors wish to thank McMaster Steel Research Center and Natural Science and Engineering Research Council of Canada for funding this project.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted April 4, 2021; accepted August 13, 2021.
Rights and permissions
About this article
Cite this article
Biswas, J., Gu, K. & Coley, K.S. A Decarburization Model for a Fe–C Droplet Reacting in Oxidizing Slag. Metall Mater Trans B 52, 3888–3906 (2021). https://doi.org/10.1007/s11663-021-02303-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02303-6