Abstract
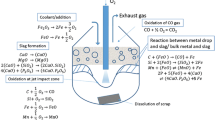
In a previous study by the authors (Rout et al. in Metall Mater Trans B 49:537–557, 2018), a dynamic model for the BOF, employing the concept of multizone kinetics was developed. In the current study, the kinetics of decarburization reaction is investigated. The jet impact and slag–metal emulsion zones were identified to be primary zones for carbon oxidation. The dynamic parameters in the rate equation of decarburization such as residence time of metal drops in the emulsion, interfacial area evolution, initial size, and the effects of surface-active oxides have been included in the kinetic rate equation of the metal droplet. A modified mass-transfer coefficient based on the ideal Langmuir adsorption equilibrium has been proposed to take into account the surface blockage effects of SiO2 and P2O5 in slag on the decarburization kinetics of a metal droplet in the emulsion. Further, a size distribution function has been included in the rate equation to evaluate the effect of droplet size on reaction kinetics. The mathematical simulation indicates that decarburization of the droplet in the emulsion is a strong function of the initial size and residence time. A modified droplet generation rate proposed previously by the authors has been used to estimate the total decarburization rate by slag–metal emulsion. The model’s prediction shows that about 76 pct of total carbon is removed by reactions in the emulsion, and the remaining is removed by reactions at the jet impact zone. The predicted bath carbon by the model has been found to be in good agreement with the industrially measured data.










Similar content being viewed by others
Abbreviations
- a i :
-
Thermodynamic activity (–)
- A app :
-
Apparent area of the droplet (m2)
- C b :
-
Carbon concentration in bulk metal (wt pct)
- C eq :
-
Equilibrium carbon concentration (wt pct)
- D c :
-
Diffusion coefficient of carbon (m2/s)
- d :
-
Diameter of droplet (m)
- dW C/dt :
-
Decarburization rate (kg/s)
- k d :
-
Mass-transfer coefficient of decarburization of a droplet (s−1)
- k eff :
-
Effective mass-transfer coefficient with surface blocking effect (s−1)
- \( K_{{{\text{SiO}}_{ 2} }} \) :
-
Adsorption coefficient of SiO2 (–)
- \( K_{{{\text{P}}_{ 2} {\text{O}}_{ 2} }} \) :
-
Adsorption coefficient of P2O5 (–)
- P amb :
-
Ambient pressure inside furnace (Pa)
- p :
-
Class in droplet size spectrum (–)
- r c :
-
Decarburization rate of droplet (wt pct/s)
- \( r_{\text{c}}^{*} \) :
-
Critical decarburization for bloating (wt pct/s)
- u :
-
Velocity of droplet (m/s)
- V app :
-
Apparent volume of the droplet (m3)
- W b :
-
Weight of bulk metal (kg)
- Wc:
-
Weight of carbon (kg)
- W sc :
-
Weight of scrap added (kg)
- \( W_{\text{m}}^{\text{sl}} \) :
-
Weight of metal in slag (kg)
- \( W_{\text{m}}^{\text{eject}} \) :
-
Weight of metal ejected (kg)
- \( W_{\text{m}}^{\text{return}} \) :
-
Weight of metal return to the bath (kg)
- W slag :
-
Weight of slag (kg)
- W lime :
-
Weight of lime dissolute into slag (kg)
- θ c :
-
Fraction of occupied sites due to surface-active oxides (–)
- ρ d :
-
Density of droplet (kg/m3)
- ρ d,0 :
-
Initial density of droplet (kg/m3)
- σ :
-
Surface tension (N/m)
- Γ0 :
-
Saturation concentration of adsorbed species (mole/cm2)
- η :
-
Efficiency of decarburization (–)
- em:
-
Emulsion zone
- iz:
-
Impact zone
- deC:
-
Decarburization
References
B. K. Rout, G. Brooks, M. A. Rhamdhani, Z. Li, F. Schrama, and J. Sun: Metall. and Mater. Trans. B, 2018, 49B, 537–557
P. Kozakevitch: Journal of Metals 1968, vol. 22, pp. 57-67.
H. W. Meyer, W. F. Porter, G. C. Smith and J. Szekely: J. Metals 1968, vol. 20, pp. 35-42.
C. Cicutti, M. Valdez, T. Pérez, J. Petroni, A. Gómez, R. Donayo and L. Ferro: In Sixth International Conference on Molten Slags, Fluxes and Salts, ISS, ed., Stockholm-Helsinki, Warrandale, PA, 2000.
M.S. Millman, A. Kapilashrami, M. Bramming and D. Malmberg: European Union, Luxembourg, 2011.
C. L. Molloseau and R. J. Fruehan: Metall. Mater. Trans. B 2002, vol. 33, pp. 335-44.
G. Brooks, Y. Pan, D. Subagyo and K. Coley: Metall. and Mater. Trans. B 2005, vol. 36, pp. 525-35 .
N. Dogan, G. A. Brooks and M. A. Rhamdhani: ISIJ Int. 2011, vol. 51, pp. 1086-92.
N. Dogan, G. A. Brooks and M. A. Rhamdhani: ISIJ Int. 2011, vol. 51, pp. 1093-1101.
N. Dogan, G. A. Brooks and M. A. Rhamdhani: ISIJ Int. 2011, vol. 51, pp. 1102-1109.
R. Sarkar, P. Gupta, S. Basu and N. B. Ballal: Metall. and Mater. Trans. B 2015, vol. 46, pp. 961-976.
Subagyo, G. A. Brooks, K. S. Coley and G. A. Irons: ISIJ Int. 2003, vol. 43, pp. 983-989.
B. K. Rout, G. A. Brooks, M. A. Rhamdhani, Z Li: Metall. Mater. Trans. B 2016, vol 47, No. 6, pp. 3350-61.
S. C. Koria and K. W. Lange: Metall. Trans. B 1984, vol. 15, pp. 109-16.
Q. L. He and N. Standish: ISIJ Int., 1990, vol. 30, pp. 305-09.
H. Sun: ISIJ Int.,2006, vol. 46, pp. 1560-69.
E. Chen and K. S. Coley: Ironmak. & Steelmak. 2010, vol. 37, pp. 541-45.
E. W. Mulholland, G. S. F Hazeldea and M. W. Davies: J Iron Steel, 1973, vol. 211, pp. 632-39.
D.E. Woolley and U.B. Pal: In 58 th Ironmaking Conference, 1999, pp 413–29.
W. M. Kim, G. Granzdorffer and H. Fine: Steel Res. 1988, vol. 60, pp. 166-70.
W. Pan, M. Sano, M. Hirasawa and K. Mori: ISIJ Int. 1991, vol. 31, pp. 358-65.
R. D. Morales, H. Rodríguez-Hernández, P. Garnica-González and J. A. Romero-Serrano: ISIJ Int., 1997, vol. 37, pp. 1072-80.
D.J. Price and M.J. Jones: Process Engineering of Pyrometallurgy Symposium, 1974.
G.A. Brooks, M.A. Rhamdhani, K.S. Coley, D. Subagyo and Y. Pan: Metall. Mater. Trans. B 2008, vol. 40B, pp. 353-62.
Y. Chung and A. Cramb: Metall. and Mater. Trans B, 2000, vol. 31, pp. 957–71.
X. Hu, H. Matsuura and F. Tsukihashi: Metall. and Mater. Trans. B 2006, vol. 37, pp. 395-401.
K. Chou, U. B. Pal and R. G. Reddy: ISIJ Int., 1993, vol. 33, pp. 862-68.
ET Turkdogan: Fundamentals of steelmaking. (The Institute of Materials, London, 1996), pp. 182.
S. Kitamura, T. Kitamura, K. Shibata, Y. Mizukami, S. Mukawa and J. Nakagawa: ISIJ Int., 1991, vol. 31, pp. 1322-28.
Honglei Sun and Guangqing Zhang: ICS Proceeding, 2005, pp 257–68.
F. D. Richardson: Phys. Chem. Melts Metall., 1974 vol. 2, p. 441 .
Acknowledgment
The authors gratefully acknowledge Tata Steel, Netherlands for providing financial support for this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted June 23, 2016.
Appendices
Appendix A
A1 Calculation of Adsorption Coefficient \( K_{{{\text{SiO}}_{2} }} \)
Combining Gibbs isotherm with that of Langmuir, the surface tension of FeO-SiO2 slag system can be expressed by the following equation:
where \( \varGamma_{{{\text{SiO}}_{2} }}^{0} \) is the saturation concentration of adsorbed SiO2, \( K_{{{\text{SiO}}_{2} }} \) is the adsorption equilibrium constant, and \( a_{{{\text{SiO}}_{2} }} \) is the activity of SiO2. σ is the surface tension of the FeO-SiO2 melt, and σ0 is the surface tension of SiO2-free iron oxide melt. The above equation can be rearranged in the form of a linear equation as \( y = mx, \) where \( y = \exp \left( {\frac{{\sigma_{0} - \sigma }}{{RT\varGamma_{{{\text{SiO}}_{2} }}^{0} }}} \right) - 1 \) and \( x = a_{{{\text{SiO}}_{2} }} \). The plot of y vs x is shown in Figure A1. The saturation concentration \( \varGamma_{{{\text{SiO}}_{2} }}^{0} \) was taken to be 6.55 × 10−10 mole/cm2 from the surface tension data proposed by Richardson et al.[31] The value of \( K_{{{\text{SiO}}_{2} }} \) was calculated to be 8.86 from the slope of the plot in Figure A1. Similar value of adsorption coefficient of SiO2 was reported by Wei et al.[21] for FeO-SiO2 slag system.
A2 Calculation of Adsorption Coefficient \( K_{{{\text{P}}_{2} {\text{O}}_{5} }} \)
The slag containing P2O5 can interfere with the FeO adsorption on the slag–metal interface and thus can retard the decarburization rate. Hu et al.[26] reported that the for FeO x -P2O5 melts, the activity of P2O5 follows Henry’s law when the concentration of P2O5 falls below 8 mole pct. The fraction of occupied sites of P2O5 at the interface according to Hu et al.[26] is defined as
From Eq. [A2], the value of \( K_{{{\text{P}}_{2} {\text{O}}_{5} }} a_{{{\text{P}}_{2} {\text{O}}_{5} }} \) is assumed to be \( 0.65\left( {{\text{mole}} \;{\text{pct}}\;{\text{P}}_{2} {\text{O}}_{5} } \right) \) in the current study.
Appendix B
B1 Effect of S on Decarburization Rate
Several studies indicated that the presence of S in the iron droplet influences the rate of decarburization by FeO in the slag. Sulfur is a highly surface-active element in liquid iron, and it can affect the decarburization rate in two ways: (i) segregating at the interface and limiting the interfacial area by blocking the reaction sites; and (ii) decreasing the slag–metal interfacial tension and making it easier for emulsification (enhances the interfacial area). Since the rate of reaction is directly proportional to the reaction area, the competition between the positive effect of emulsion formation and the negative effect of surface blockage has been observed. Molloseau et al.[6] observed that decarburization reaches a maximum value when S = 0.011 wt pct and any further increase in S decreases the reaction rate. Similar observation was made by Chen et al.,[17] and this value has been reported to be 0.013 wt pct. The total interfacial area evolution for Fe-C-S droplets in slag is a combination of both bloating due to CO nucleation and emulsification due to the presence of S.
Modeling of interfacial reaction area due to the effect of S in the iron droplet is complex. The decrease in surface area due to adsorption of S at the interface can be described by means of surface blockage mechanism. However, it is difficult to distinguish between the surface area change due to CO nucleation and emulsification by surface-active elements such as S. In the model proposed by Brooks et al.,[7] the total change in interfacial area of the metal droplet has been empirically correlated with decarburization rate and slag FeO. The correlation was developed on the basis of Fe-C-0.011S data, where the rate of decarburization is observed to be at peak value. This method of calculation of interfacial area can be considered as standard state, and for high concentration of sulfur (S > 0.011 wt pct), decarburization rate can be calculated by the correcting the interfacial area with respect to the standard state. For application of Brooks et al.’s model for high S concentration, we defined a rate constant modified as
where φ = 1 means the rate of decarburization reaches the maximum value due to large increase in surface area caused by S. At higher S level, φ is less than 1 due to the surface blocking effect of S on reaction interface.
At high S level in the steel, the decarburization rate must be multiplied by the rate constant-modified parameter to get the actual rate due to the effect of sulfur. The experimental data by Molloseau et al. and Chen et al. have been used, and φ as a function of S in the droplet is plotted in Figure B1.
For S > 0.011 and < 0.3 wt pct
It should be noted that due to lack of experimental data, the empirical equation derived for φ is simplistic in nature, and it has been derived at constant C, slag FeO, and temperature. In Cicutti’s heat data used for the present model’s calculation, S concentration is about 0.015 wt pct. The modified rate parameter φ corresponds to this S level which is estimated to be 0.96 in the current study.
Appendix C
C1 Activity Coefficient of C
The activity coefficient of C was calculated by the following relationships[27]:
Rights and permissions
About this article
Cite this article
Rout, B.K., Brooks, G., Akbar Rhamdhani, M. et al. Dynamic Model of Basic Oxygen Steelmaking Process Based on Multizone Reaction Kinetics: Modeling of Decarburization. Metall Mater Trans B 49, 1022–1033 (2018). https://doi.org/10.1007/s11663-018-1244-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-018-1244-5