Abstract
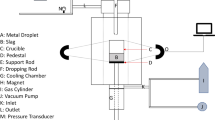
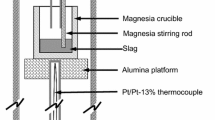
The rate of reduction of FeO in the slag by carbon in iron droplets (2.9 wt pct C, 0.01 wt pct S) was studied for CaO-SiO2-MgO slags containing between 3 and 35 wt pct FeO and temperatures ranging from 1643 to 1763 K. The effects of Fe2O3 additions to the slag and sulfur variations in the metal on the reaction rate were also studied. It was found that the behavior of the metal droplets in the slag, as observed by X-ray fluoroscopy, changed significantly with FeO content in the slag. Below 10 wt pct FeO, the droplet remained intact while reacting with the slag; however, above this FeO concentration, the droplet became emulsified within the slag. The large increase in surface area of the metal droplet due to emulsification caused the rate of reaction to be one to two orders of magnitude faster than for droplets that did not become emulsified. It was suggested that when the droplet is emulsified, the surface area and reaction kinetics are greatly increased, and the rate becomes controlled by mass transfer of FeO as Fe2+ and O2− ions in the slag to the emulsified droplet. At low FeO contents for which the droplet does not emulsify, the rate is controlled by dissociation of CO2 on the metal. It was also found that a critical temperature exists for a given FeO content at which point the rate of CO evolution increases dramatically. Additions of Fe2O3 to the slag and sulfur to the metal caused significant changes to the rate of reaction possibly by affecting the emulsification behavior of the droplet.
Similar content being viewed by others
References
E.W. Mulholland, G.S.F. Hazeldean, and M.W. Davies: J. Iron Steel Inst., 1973, vol. 9, pp. 632–39.
H. Gaye and P.V. Riboud: Metall. Trans. B, 1977, vol. 8B, pp. 409–15.
T. Gare and G.S.F. Hazeldean: Ironmaking and Steelmaking, 1981, vol. 8 (4), pp. 168–81.
D.J. Min and R.J. Fruehan: Metall. Trans. B, 1992, vol. 23B, pp. 29–37.
G.G. Krishna Murthy, Y. Sawada, and J.F. Elliott: Ironmaking and Steelmaking, 1993, vol. 20 (3), pp. 179–90.
G.G. Krishna Murthy, A. Hasham, and U.B. Pal: Iron Steel Inst. Jpn. Int., 1994, vol. 34 (5), pp. 408–13.
G.G. Krishna Murthy, A. Hasham, and U.B. Pal: Ironmaking and Steelmaking, 1993, vol. 20 (3), pp. 191–200.
R.J. Fruehan, editor, The Making, Shaping and Treating of Steel, 11th Ed., Steelmaking and Refining Volume, The AISE Steel Foundation, Pittsburgh, PA, 1998, pp. 119 and 134.
D.R. Sain and G.R. Belton: Metall. Trans. B, 1978, vol. 9B, pp. 403–07.
D.G.C. Robertson and A.E. Jenkins: Heterogeneous Kinetics at Elevated Temperatures, Plenum Press, New York, NY, 1970, pp. 393–408.
J.H. Swisher and E.T. Turkdogan: Trans. AIME, 1967, vol. 239, pp. 602–10.
L.A. Baker, N.A. Warner, and A.E. Jenkins: Trans. AIME, 1967, vol. 239 (6), pp. 857–64.
L.A. Baker and R.G. Ward: J. Iron Steel Inst., 1967, vol. 205 (7), pp. 714–17.
P.A. Distin, G.D. Hallett, and F.D. Richardson: J. Iron Steel Inst., 1968, vol. 206 (8), pp. 821–33.
K. Gao, V. Sahajwalla, and R. Dry: 1998 ICSTI/Ironmaking Conf. Proc., Iron and Steel Society, Toronto, Canada, Apr. 1998, pp. 1489–99.
N. Ibl and J. Venczel: Metalloberflache, 1970, vol. 24, pp. 365–74.
D.P. Agarwal and D.R. Gaskell: Metall. Trans. B, 1975, vol. 6B, pp. 263–67.
B. Sarma: Ph.D Thesis, Carnegie Mellon University, Pittsburgh, PA, 1992, pp. 30–31 and 149.
D.R. Sain and G.R. Belton: Metall. Trans. B, 1976, vol. 7B, pp. 235–44.
F.J. Mannion and R.J. Fruehan: Metall. Trans. B, 1989, vol. 20B, pp. 853–61.
G.K. Sigworth and J.F. Elliot: Met. Sci., 1974, vol. 8, pp. 298–310.
A.W. Cramb and I. Jimbo: 1994 Turkdogan Symp. Proc., Iron and Steel Society, Pittsburgh, PA, pp. 195–206.
R.A. Berryman and I.D. Sommerville: Metall. Trans. B, 1992, vol. 23B, pp. 223–27.
T.B. Massalski: Binary Alloy Phase Diagrams, 2nd Ed., ASM INTERNATIONAL, Materials Park, OH, 1990, vol. 1, p. 843.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Molloseau, C.L., Fruehan, R.J. The reaction behavior of Fe-C-S droplets in CaO-SiO2-MgO-FeO slags. Metall Mater Trans B 33, 335–344 (2002). https://doi.org/10.1007/s11663-002-0045-y
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11663-002-0045-y