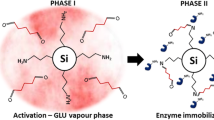
Abstract
Enzymes immobilization is a useful way to allow enzyme reuse and increase their stability. A high redox potential laccase from Trametes versicolor (TvL) and a low redox potential, but commercially available low-cost laccase from Myceliophthora thermophila (MtL), were successfully immobilized and co-immobilized onto fumed silica nanoparticles (fsNP). Enzyme loads of 1.78 ± 0.07, 0.69 ± 0.03, and 1.10 ± 0.01 U/mg fsNP were attained for the optimal doses of TvL, MtL, and co-immobilized laccases, respectively. In general, the laccase-fsNP conjugates showed a higher resistance against an acidic pH value (i.e., pH 3), and a higher storage stability than free enzymes. In addition, immobilized enzymes exhibited a superior long-term stability than free laccases when incubated in a secondary effluent from a municipal wastewater treatment plant (WWTP). For instance, the residual activity after 2 weeks for the co-immobilized laccases and the mixture of free laccases were 40.2 ± 2.5 % and 16.8 ± 1.0 %, respectively. The ability of the laccase-fsNP to remove a mixture of 14C-bisphenol A (BPA) and 14C-sodium diclofenac (DCF) from spiked secondary effluents was assessed in batch experiments. The catalytic efficiency was highly dependent on both the microbial source and state of the biocatalyst. The high redox potential TvL in free form attained a four-fold higher percentage of BPA transformation than the free MtL. Compared to free laccases, immobilized enzymes led to much slower rates of BPA transformation. For instance, after 24 h, the percentages of BPA transformation by 1000 U/L of a mixture of free laccases or co-immobilized enzymes were 67.8 ± 5.2 and 27.0 ± 3.9 %, respectively. Nevertheless, the use of 8000 U/L of co-immobilized laccase led to a nearly complete removal of BPA, despite the unfavorable conditions for laccase catalysis (pH ~ 8.4). DCF transformation was not observed for any of the enzymatic systems, showing that this compound is highly recalcitrant toward laccase oxidation under realistic conditions.







Similar content being viewed by others
References
Arca-Ramos A, Eibes G, Feijoo G, Lema JM, Moreira MT (2015) Potentiality of a ceramic membrane reactor for the laccase-catalyzed removal of bisphenol A from secondary effluents. Appl Microbiol Biotechnol 1–10 doi:10.1007/s00253-015-6826-4
Ba S, Arsenault A, Hassani T, Jones JP, Cabana H (2013) Laccase immobilization and insolubilization: from fundamentals to applications for the elimination of emerging contaminants in wastewater treatment. Crit Rev Biotechnol 33:404–418
Babot ED, Rico A, Rencoret J, Kalum L, Lund H, Romero J, del Río JC, Martínez ÁT, Gutiérrez A (2011) Towards industrially-feasible delignification and pitch removal by treating paper pulp with myceliophthora thermophila laccase and a phenolic mediator. Bioresour Technol 102:6717–6722
Cabana H, Jiwan J-LH, Rozenberg R, Elisashvili V, Penninckx M, Agathos SN, Jones JP (2007) Elimination of endocrine disrupting chemicals nonylphenol and bisphenol a and personal care product ingredient triclosan using enzyme preparation from the white rot fungus coriolopsis polyzona. Chemosphere 67:770–778
Cabana H, Alexandre C, Agathos SN, Jones JP (2009) Immobilization of laccase from the white rot fungus coriolopsis polyzona and use of the immobilized biocatalyst for the continuous elimination of endocrine disrupting chemicals. Bioresour Technol 100:3447–3458
Cabana H, Ahamed A, Leduc R (2011) Conjugation of laccase from the white rot fungus Trametes versicolor to chitosan and its utilization for the elimination of triclosan. Bioresour Technol 102:1656–1662
Cao L (2005) Carrier-bound immobilized enzymes: principles, application and design. Wiley–VCH, Weinheim
Cipolatti EP, Silva MJA, Klein M, Feddern V, Feltes MMC, Oliveira JV, Ninow JL, de Oliveira D (2014) Current status and trends in enzymatic nanoimmobilization. J Mol Catal B Enzym 99:56–67
Cruz J, Würges K, Kramer M, Pfromm P, Rezac M, Czermak P (2011) Immobilization of enzymes on fumed silica nanoparticles for applications in nonaqueous media. In: Wang P (ed) Nanoscale Biocatalysis. Methods in molecular biology. Humana Press, 147–160
Deblonde T, Cossu-Leguille C, Hartemann P (2011) Emerging pollutants in wastewater: a review of the literature. J Hyg Environ Health 214:442–448
Demarche P, Junghanns C, Nair RR, Agathos SN (2012) Harnessing the power of enzymes for environmental stewardship. Biotechnol Adv 30:933–953
Dodor DE, Hwang H-M, Ekunwe SIN (2004) Oxidation of anthracene and benzo[a]pyrene by immobilized laccase from Trametes versicolor. Enzym Microb Technol 35:210–217
Fernández-Fernández M, Sanromán MÁ, Moldes D (2013) Recent developments and applications of immobilized laccase. Biotechnol Adv 31:1808–1825
Fukuda T, Uchida H, Suzuki M, Miyamoto H, Morinaga H, Nawata H, Uwajima T (2004) Transformation products of bisphenol a by a recombinant Trametes villosa laccase and their estrogenic activity. J Chem Technol Biotechnol 79:1212–1218
Galliker P, Hommes G, Schlosser D, Corvini PFX, Shahgaldian P (2010) Laccase-modified silica nanoparticles efficiently catalyze the transformation of phenolic compounds. J Colloid Interface Sci 349:98–105
Gasser C, Ammann E, Shahgaldian P, Corvini PX (2014a) Laccases to take on the challenge of emerging organic contaminants in wastewater. Appl Microbiol Biotechnol 98:9931–9952
Gasser C, Yu L, Svojitka J, Wintgens T, Ammann E, Shahgaldian P, Corvini PX, Hommes G (2014b) Advanced enzymatic elimination of phenolic contaminants in wastewater: a nano approach at field scale. Appl Microbiol Biotechnol 98:3305–3316
Hahn V, Mikolasch A, Schauer F (2014) Cleavage and synthesis function of high and low redox potential laccases towards 4-morpholinoaniline and aminated as well as chlorinated phenols. Appl Microbiol Biotechnol 98:1609–1620
Han M, Choi H, Song H (2006) Purification and characterization of laccase from the white rot fungus Trametes versicolor. J Microbiol 43:555–560
Hommes G, Gasser CA, Howald CBC, Goers R, Schlosser D, Shahgaldian P, Corvini PFX (2012) Production of a robust nanobiocatalyst for municipal wastewater treatment. Bioresour Technol 115:8–15
Hou J, Dong G, Ye Y, Chen V (2014) Laccase immobilization on titania nanoparticles and titania-functionalized membranes. J Membr Sci 452:229–240
Kim Y-J, Nicell JA (2006) Impact of reaction conditions on the laccase-catalyzed conversion of bisphenol a. Bioresour Technol 97:1431–1442
Kulikova NA, Davidchik VN, Tsvetkova EA, Koroleva OV (2013) Interaction of coal humic acids with fungal laccase. Adv Microbiol 3:145–153
Kunamneni A, Ballesteros A, Plou FJ, Alcalde M (2007) Fungal laccase - a versatile enzyme for biotechnological applications. In: Mendez-Vilas, A (Ed), Communicating current research and educational topics and trends in applied microbiology. Formatex 233–245
Kunamneni A, Camarero S, Garcia-Burgos C, Plou F, Ballesteros A, Alcalde M (2008a) Engineering and applications of fungal laccases for organic synthesis. Microb Cell Factories 7:32
Kunamneni A, Ghazi I, Camarero S, Ballesteros A, Plou FJ, Alcalde M (2008b) Decolorization of synthetic dyes by laccase immobilized on epoxy-activated carriers. Process Biochem 43:169–178
Kurniawati S, Nicell J (2008) Characterization of Trametes versicolor laccase for the transformation of aqueous phenol. Bioresour Technol 99:7825–7834
Li K, Xu F, Eriksson K-EL (1999) Comparison of fungal laccases and redox mediators in oxidation of a nonphenolic lignin model compound. Appl Environ Microbiol 65:2654–2660
Lloret L, Hollmann F, Eibes G, Feijoo G, Moreira MT, Lema JM (2012) Immobilisation of laccase on eupergit supports and its application for the removal of endocrine disrupting chemicals in a packed-bed reactor. Biodegradation 23:373–386
Lloret L, Eibes G, Moreira MT, Feijoo G, Lema JM (2013) On the use of a high-redox potential laccase as an alternative for the transformation of non-steroidal anti-inflammatory drugs (NSAIDs). J Mol Catal B Enzym 97:233–242
Luo Y, Guo W, Ngo HH, Nghiem LD, Hai FI, Zhang J, Liang S, Wang XC (2014) A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci Total Environ 473–474:619–641
Madhavi V, Lele SS (2009) Laccase: properties and applications. BioResources 4
Majeau J-A, Brar SK, Tyagi RD (2010) Laccases for removal of recalcitrant and emerging pollutants. Bioresour Technol 101:2331–2350
Marco-Urrea E, Pérez-Trujillo M, Cruz-Morató C, Caminal G, Vicent T (2010) Degradation of the drug sodium diclofenac by Trametes versicolor pellets and identification of some intermediates by NMR. J Hazard Mater 176:836–842
Margot J, Bennati-Granier C, Maillard J, Blanquez P, Barry D, Holliger C (2013a) Bacterial versus fungal laccase: potential for micropollutant degradation. AMB Expr 3:1–14
Margot J, Maillard J, Rossi L, Barry D, Holliger C (2013b) Influence of treatment conditions on the oxidation of micropollutants by Trametes versicolor laccase. New Biotechnol 30:803–813
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym Microb Technol 40:1451–1463
Matijošytė I, Arends IWCE, de Vries S, Sheldon RA (2010) Preparation and use of cross-linked enzyme aggregates (CLEAs) of laccases. J Mol Catal B Enzym 62:142–148
Mohidem NA, Mat H (2009) The catalytic activity of laccase immobilized in sol–gel silica. J Appl Sci 9:3141–3145
Morozova OV, Shumakovich GP, Gorbacheva MA, Shleev SV, Yaropolov AI (2007) “Blue” laccases. Biochem Mosc 72:1136–1150
Nair RR, Demarche P, Agathos SN (2013) Formulation and characterization of an immobilized laccase biocatalyst and its application to eliminate organic micropollutants in wastewater. New Biotechnol 30:814–823
Nicolucci C, Rossi S, Menale C, Godjevargova T, Ivanov Y, Bianco M, Mita L, Bencivenga U, Mita D, Diano N (2011) Biodegradation of bisphenols with immobilized laccase or tyrosinase on polyacrylonitrile beads. Biodegradation 22:673–683
Pang R, Li M, Zhang C (2015) Degradation of phenolic compounds by laccase immobilized on carbon nanomaterials: diffusional limitation investigation. Talanta 131:38–45
Patel S, Kalia V, Choi J, Haw J, Kim I, Lee J (2014) Immobilization of laccase on SiO2 nanocarriers improves its stability and reusability. J Microbiol Biotechnol 24:639–647
Pratsinis SE (1998) Flame aerosol synthesis of ceramic powders. Prog Energy Combust 24:197–219
Rekuć A, Bryjak J, Szymańska K, Jarzębski AB (2009) Laccase immobilization on mesostructured cellular foams affords preparations with ultra high activity. Process Biochem 44:191–1988
Schäfer AI, Fane AG, Waite TD (2001) Cost factors and chemical pretreatment effects in the membrane filtration of waters containing natural organic matter. Water Res 35:1509–1517
Singh RK, Tiwari MK, Singh R, Lee J-K (2013) From protein engineering to immobilization: promising strategies for the upgrade of industrial enzymes. Int J Mol Sci 14:1232–1277
Tavares APM, Rodríguez O, Fernández-Fernández M, Domínguez A, Moldes D, Sanromán MA, Macedo EA (2013) Immobilization of laccase on modified silica: stabilization, thermal inactivation and kinetic behaviour in 1-ethyl-3-methylimidazolium ethylsulfate ionic liquid. Bioresour Technol 131:405–412
Torres-Duarte C, Viana M, Vazquez-Duhalt R (2012) Laccase-mediated transformations of endocrine disrupting chemicals abolish binding affinities to estrogen receptors and their estrogenic activity in zebrafish. Appl Biochem Biotechnol 168:864–876
Xu F, Shin W, Brown S, Wahleithner J, Sundaram U, Solomon E (1996) A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim Biophys Acta 1292:303–311
Zhuravlev LT (2000) The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf A Physicochem Eng Asp 173(1–3):1–38
Zimmermann Y-S, Shahgaldian P, Corvini PX, Hommes G (2011) Sorption-assisted surface conjugation: a way to stabilize laccase enzyme. Appl Microbiol Biotechnol 92:169–178
Acknowledgments
Adriana Arca would like to thank the Spanish Ministry of Education for the FPU grant AP2010-2086. Gemma Eibes would like to thank the Xunta de Galicia for her postdoctoral grant (I2C Program). The Spanish Ministry of Science and Innovation (MICINN, CTQ2013-44762-R), and the European Commission within the 7th framework program under grant agreement FP7-KBBE-2012-6-311933 (Water4Crops) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing of interests.
Human and animal rights and informed consent
The current research has not involved human participants or animals.
Additional information
Responsible editor: Gerald Thouand
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 135 kb)
Rights and permissions
About this article
Cite this article
Arca-Ramos, A., Ammann, E.M., Gasser, C.A. et al. Assessing the use of nanoimmobilized laccases to remove micropollutants from wastewater. Environ Sci Pollut Res 23, 3217–3228 (2016). https://doi.org/10.1007/s11356-015-5564-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5564-6