Abstract
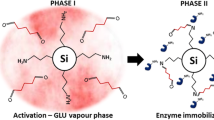
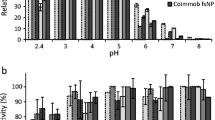
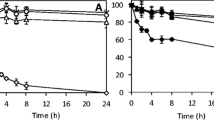
In this study, a tailor-made biocatalyst consisting of a co-immobilized lignolytic enzyme cascade on multi-functionalized magnetic silica microspheres (MSMS) was developed. Physical adsorption was the most promising strategy for the synthesis of individual immobilized laccase (IL), immobilized versatile peroxidase (IP), as well as co-immobilized laccase (Lac) and versatile peroxidase (VP) with an enzyme activity recovery of about 79, 93, 27, and 27.5%, respectively. Similarly, the biocatalytic load of 116, 183, 23.6, and 31 U/g was obtained for IL, IP, and co-immobilized Lac and VP, respectively. The co-immobilized enzyme system exhibited better pH stability than the free and individual immobilized system by retaining more than 100% residual activity at pH 7.0 after a 150-h incubation; whereas, the thermal stability and kinetics of the co-immobilized biocatalyst were not much improved. IL and IP could be recycled for 10 cycles after which they retained 31 and 44% of their initial activities. Co-immobilized Lac and VP were reused for ten consecutive cycles at the end of which Lac activity was depleted, and 37% of VP activity was left. Free enzymes, IL, IP, co-immobilized Lac, and VP were applied to biorefinery wastewater (BRW) in a batch study to investigate the transformation of phenolic contaminants over a period of 5 days. The major classes of phenolic constituents in terms of their order of removal in a Lac-VP system was phenol >2-chlorophenol > trichlorophenol > dichlorophenol > cresols > dimethylphenol >2 methyl- 4, 6-dinitrophenol > 4-nitrophenol > tetrachlorophenols > pentachlorophenol. The free enzymes and individually immobilized enzymes resulted in 80% dephenolization in 5 days. By contrast, the co-immobilized biocatalyst provided rapid dephenolization yielding the same 80% removal within 24 h and 96% removal of phenols in 60 h after which the system stabilized, which is the major advantage of the co-immobilized biocatalyst.

ᅟ
Graphical abstract







Similar content being viewed by others
References
Arca-Ramos A, Kumar V, Eibes G, Moreira M, Cabana H (2016) Recyclable cross-linked laccase aggregates coupled to magnetic silica microbeads for elimination of pharmaceuticals from municipal wastewater. Environ Sci Pollut Res 23:8929–8939
Balcázar-López E, Méndez-Lorenzo LH, Batista-García RA, Esquivel-Naranjo U, Ayala M, Kumar VV, Savary O, Cabana H, Herrera-Estrella A, Folch-Mallol JL (2016) Xenobiotic compounds degradation by heterologous expression of a Trametes sanguineus laccase in Trichoderma atroviride. PLoS One 11:e0147997
Barbosa O, Torres R, Ortiz C, Berenguer-Murcia Á, Rodrigues RC, Fernandez-Lafuente R (2013) Heterofunctional supports in enzyme immobilization: from traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 14:2433–2462
Barbosa O, Ortiz C, Berenguer-Murcia Á, Torres R, Rodrigues RC, Fernandez-Lafuente R (2015) Strategies for the one-step immobilization–purification of enzymes as industrial biocatalysts. Biotechnol Adv 33:435–456
Bautista LF, Morales G, Sanz R (2010) Immobilization strategies for laccase from Trametes versicolor on mesostructured silica materials and the application to the degradation of naphthalene. Bioresour Technol 101:8541–8548
Brady D, Jordaan J (2009) Advances in enzyme immobilisation. Biotechnol Lett 31:1639–1650
Camarero S, Sarkar S, Ruiz-Dueñas FJ, Martinez MJ, Martinez AT (1999) Description of a versatile peroxidase involved in the natural degradation of lignin that has both manganese peroxidase and lignin peroxidase substrate interaction sites. J Biol Chem 274:10324–10330
Chouyyok W, Panpranot J, Thanachayanant C, Prichanont S (2009) Effects of pH and pore characters of mesoporous silicas on horseradish peroxidase immobilization. J Mol Catal B Enzym 56:246–252
Crecchio C, Ruggiero P, Pizzigallo M, Curci M (1997) Potential utilization of phenoloxidases immobilized in organic gels for decontamination of polluted sites. Mod Agric Environ. Springer, pp. 545–552
Crestini C, Argyropoulos DS (1998) The early oxidative biodegradation steps of residual kraft lignin models with laccase. Bioorg Med Chem 6(11):2161–2169
Cun-guang Y (1998) Progress of optical determination for phenolic compounds in sewage. J Environ Sci (China) 10:76–86
Dannis M (1951) Determination of phenols by the amino-antipyrine method. Sewage Ind Wastes:1516–1522
DiCosimo R, McAuliffe J, Poulose AJ, Bohlmann G (2013) Industrial use of immobilized enzymes. Chem Soc Rev 42:6437–6474
Duran N, Esposito E (2000) Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: a review. Appl Catal B Environ 28:83–99
Durán N, Rosa MA, D’Annibale A, Gianfreda L (2002) Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: a review. Enzym Microb Technol 31:907–931
Fernandez-Lafuente R (2009) Stabilization of multimeric enzymes: strategies to prevent subunit dissociation. Enzym Microb Technol 45:405–418
Galliker P, Hommes G, Schlosser D, Corvini PF-X, Shahgaldian P (2010) Laccase-modified silica nanoparticles efficiently catalyze the transformation of phenolic compounds. J Colloid Interface Sci 349:98–105
Gambhir A, Gerard M, Mulchandani A, Malhotra B (2001) Coimmobilization of urease and glutamate dehydrogenase in electrochemically prepared polypyrrole-polyvinyl sulfonate films. Appl Biochem Biotechnol 96:249–257
Garcia-Galan C, Berenguer-Murcia Á, Fernandez-Lafuente R, Rodrigues RC (2011) Potential of different enzyme immobilization strategies to improve enzyme performance. Adv Synth Catal 353:2885–2904
Ghoul M, Chebil L (2012) Enzymatic polymerization of phenolic compounds by oxidoreductases. Springer, pp. 1–46
Gianfreda L, Xu F, Bollag J-M (1999) Laccases: a useful group of oxidoreductive enzymes. Bioremediat J 3:1–26
González-Pombo P, Fariña L, Carrau F, Batista-Viera F, Brena BM (2014) Aroma enhancement in wines using co-immobilized Aspergillus niger glycosidases. Food Chem 143:185–191
Gopinath S, Sugunan S (2004) Leaching studies over immobilized a-amylase. Importance of the nature of enzyme attachment. React Kinet Catal Lett 83:79–83
Guerra R (2001) Ecotoxicological and chemical evaluation of phenolic compounds in industrial effluents. Chemosphere 44:1737–1747
Guerriero G, Hausman JF, Strauss J, Ertan H, Siddiqui KS (2016) Lignocellulosic biomass: biosynthesis, degradation, and industrial utilization. Eng Life Sci 16:1–16
Gupta A, Kumar V, Dubey A, Verma A (2014): Kinetic characterization and effect of immobilized thermostable β-glucosidase in alginate gel beads on sugarcane juice. ISRN biochemistry 2014
Guzik U, Hupert-Kocurek K, Wojcieszyńska D (2014) Immobilization as a strategy for improving enzyme properties-application to oxidoreductases. Molecules 19:8995–9018
Hanefeld U, Gardossi L, Magner E (2009) Understanding enzyme immobilisation. Chem Soc Rev 38:453–468
Hernandez K, Fernandez-Lafuente R (2011) Control of protein immobilization: coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzym Microb Technol 48:107–122
Hernandez K, Berenguer-Murcia A, Rodrigues RC, Fernandez-Lafuente R (2012) Hydrogen peroxide in biocatalysis. A dangerous liaison. Curr Org Chem 16:2652–2672
Huang Y, Xi Y, Yang Y, Chen C, Yuan H, Liu X (2014) Degradation of 2, 4-dichlorophenol catalyzed by the immobilized laccase with the carrier of Fe3O4@ MSS–NH2. Chin Sci Bull 59:509–520
Hwang ET, Gu MB (2013) Enzyme stabilization by nano/microsized hybrid materials. Eng Life Sci 13:49–61
Insin N, Tracy JB, Lee H, Zimmer JP, Westervelt RM, Bawendi MG (2008) Incorporation of iron oxide nanoparticles and quantum dots into silica microspheres. ACS Nano 2:197–202
Jesionowski T, Zdarta J, Krajewska B (2014) Enzyme immobilization by adsorption: a review. Adsorption 20:801–821
Jiang D-S, Long S-Y, Huang J, Xiao H-Y, Zhou J-Y (2005) Immobilization of Pycnoporus sanguineus laccase on magnetic chitosan microspheres. Biochem Eng J 25:15–23
Kim J, Grate JW, Wang P (2006) Nanostructures for enzyme stabilization. Chem Eng Sci 61:1017–1026
Krastanov A (2000) Removal of phenols from mixtures by co-immobilized laccase/tyrosinase and polyclar adsorption. J Ind Microbiol Biotechnol 24:383–388
Kumar VV, Sivanesan S, Cabana H (2014) Magnetic cross-linked laccase aggregates—bioremediation tool for decolorization of distinct classes of recalcitrant dyes. Sci Total Environ 487:830–839
Kumar VV, Cabana H (2016) Towards high potential magnetic biocatalysts for on-demand elimination of pharmaceuticals. Bioresour Technol 200:81–89
Li Y, Wang Z, Xu X, Jin L (2015) A Ca-alginate particle co-immobilized with Phanerochaete chrysosporium cells and the combined cross-linked enzyme aggregates from Trametes versicolor. Bioresour Technol 198:464–469
Liese A, Hilterhaus L (2013) Evaluation of immobilized enzymes for industrial applications. Chem Soc Rev 42:6236–6249
Marco-Urrea E, Pérez-Trujillo M, Vicent T, Caminal G (2009) Ability of white-rot fungi to remove selected pharmaceuticals and identification of degradation products of ibuprofen by Trametes versicolor. Chemosphere 74:765–772
Martínková L, Kotik M, Marková E, Homolka L (2016) Biodegradation of phenolic compounds by Basidiomycota and its phenol oxidases: a review. Chemosphere 149:373–382
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym Microb Technol 40:1451–1463
Michałowicz J, Duda W (2007) Phenols—sources and toxicity. Pol J Environ Stud 16:347–362
Min K, Yoo YJ (2014) Recent progress in nanobiocatalysis for enzyme immobilization and its application. Biotechnol Bioprocess Eng 19:553–567
Moreira PR, Duez C, Dehareng D, Antunes A, Almeida-Vara E, Frère J-M, Malcata FX, Duarte J (2005) Molecular characterisation of a versatile peroxidase from a Bjerkandera strain. J Biotechnol 118:339–352
Nicolucci C, Rossi S, Menale C, Godjevargova T, Ivanov Y, Bianco M, Mita L, Bencivenga U, Mita DG, Diano N (2011) Biodegradation of bisphenols with immobilized laccase or tyrosinase on polyacrylonitrile beads. Biodegradation 22:673–683
Pal A, Khanum F (2012) Covalent immobilization of xylanase on the surface of alginate glutaraldehyde beads decreases the ‘catalytic efficiency’ but provides ‘low temperature stabilization’ effect. J Biochem Technol 3:409–413
Peirce S, Virgen-Ortíz JJ, Tacias-Pascacio VG, Rueda N, Bartolome-Cabrero R, Fernandez-Lopez L, Russo ME, Marzocchella A, Fernandez-Lafuente R (2016) Development of simple protocols to solve the problems of enzyme coimmobilization. Application to coimmobilize a lipase and a β-galactosidase. RSC Adv 6:61707–61715
Rodrigues RC, Ortiz C, Berenguer-Murcia Á, Torres R, Fernández-Lafuente R (2013) Modifying enzyme activity and selectivity by immobilization. Chem Soc Rev 42:6290–6307
Salis A, Pisano M, Monduzzi M, Solinas V, Sanjust E (2009) Laccase from Pleurotus sajor-caju on functionalised SBA-15 mesoporous silica: immobilisation and use for the oxidation of phenolic compounds. J Mol Catal B Enzym 58:175–180
Sheldon RA, van Pelt S (2013) Enzyme immobilisation in biocatalysis: why, what and how. Chem Soc Rev 42:6223–6235
Shen Y, Zhang Y, Zhang X, Zhou X, Teng X, Yan M, Bi H (2015) Horseradish peroxidase-immobilized magnetic mesoporous silica nanoparticles as a potential candidate to eliminate intracellular reactive oxygen species. Nano 7:2941–2950
Shuttleworth KL, Bollag J-M (1986) Soluble and immobilized laccase as catalysts for the transformation of substituted phenols. Enzym Microb Technol 8:171–177
Singh RK, Tiwari MK, Singh R, Lee J-K (2013) From protein engineering to immobilization: promising strategies for the upgrade of industrial enzymes. Int J Mol Sci 14:1232–1277
Sizer IW (2006) Effects of temperature on enzyme kinetics. Adv Enzymol Relat Areas Mol Biol 3:35–62
Strong P, Claus H (2011) Laccase: a review of its past and its future in bioremediation. Crit Rev Environ Sci Technol 41:373–434
Touahar IE, Haroune L, Ba S, Bellenger J-P, Cabana H (2014) Characterization of combined cross-linked enzyme aggregates from laccase, versatile peroxidase and glucose oxidase, and their utilization for the elimination of pharmaceuticals. Sci Total Environ 481:90–99
Tramper J, Müller F, Van Der Plas HC (1978) Immobilized xanthine oxidase: kinetics,(in) stability, and stabilization by coimmobilization with superoxide dismutase and catalase. Biotechnol Bioeng 20:1507–1522
Van Aken B, Ledent P, Naveau H, Agathos SN (2000) Co-immobilization of manganese peroxidase from Phlebia radiata and glucose oxidase from Aspergillus niger on porous silica beads. Biotechnol Lett 22:641–646
Voss R, Brook MA, Thompson J, Chen Y, Pelton RH, Brennan JD (2007) Non-destructive horseradish peroxidase immobilization in porous silica nanoparticles. J Mater Chem 17:4854–4863
Wang F, Guo C, Yang L-r, Liu C-Z (2010) Magnetic mesoporous silica nanoparticles: fabrication and their laccase immobilization performance. Bioresour Technol 101:8931–8935
Wang F, Hu Y, Guo C, Huang W, Liu C-Z (2012) Enhanced phenol degradation in coking wastewater by immobilized laccase on magnetic mesoporous silica nanoparticles in a magnetically stabilized fluidized bed. Bioresour Technol 110:120–124
Xu D-Y, Yang Y, Yang Z (2011) Activity and stability of cross-linked tyrosinase aggregates in aqueous and nonaqueous media. J Biotechnol 152:30–36
Yang H-H, Zhang S-Q, Chen X-L, Zhuang Z-X, Xu J-G, Wang X-R (2004) Magnetite-containing spherical silica nanoparticles for biocatalysis and bioseparations. Anal Chem 76:1316–1321
Yu L, Cen G, Feng W (2008) Preparation of magnetic silica nanoparticles and their application in laccase immobilization. Chin J Process Eng 8:583–589
Zhai R, Zhang B, Wan Y, Li C, Wang J, Liu J (2013) Chitosan–halloysite hybrid-nanotubes: horseradish peroxidase immobilization and applications in phenol removal. Chem Eng J 214:304–309
Zhang F, Zheng B, Zhang J, Huang X, Liu H, Guo S, Zhang J (2010) Horseradish peroxidase immobilized on graphene oxide: physical properties and applications in phenolic compound removal. J Phys Chem C 114:8469–8473
Zhang J, Liu X, Xu Z, Chen H, Yang Y (2008) Degradation of chlorophenols catalyzed by laccase. Int Biodeterior Biodegrad 61:351–356
Zheng X, Wang Q, Jiang Y, Gao J (2012) Biomimetic synthesis of magnetic composite particles for laccase immobilization. Ind Eng Chem Res 51:10140–10146
Zhu Y, Kaskel S, Shi J, Wage T, van Pée K-H (2007) Immobilization of Trametes versicolor laccase on magnetically separable mesoporous silica spheres. Chem Mater 19:6408–6413
Acknowledgements
The authors gratefully acknowledge financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC) and SRM University. Vaidyanathan Vinoth Kumar would like to thank SRM University, Chennai, India for their extensive support to carry out this research under “Faculty Abroad Programme”. Vaidyanathan Vinoth Kumar also acknowledges the FRQNT-Excellence Program Scholarships for Foreign Students 2015–16. The authors are also thankful to the members of the Bioprocess Engineering Laboratory and Environmental Engineering Laboratory for their assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All precautionary measures were implemented while working with the BRW and its safe disposal was ensured.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Gerald Thouand
Highlights
• Laccase and versatile peroxidase were successfully immobilized on magnetic particles.
• The immobilized enzymes displayed enhanced operational behaviors compared to the free enzymes.
• The treatment of biorefinery wastewaters showed high transformation of the phenols.
Rights and permissions
About this article
Cite this article
Vishnu, D., Neeraj, G., Swaroopini, R. et al. Synergetic integration of laccase and versatile peroxidase with magnetic silica microspheres towards remediation of biorefinery wastewater. Environ Sci Pollut Res 24, 17993–18009 (2017). https://doi.org/10.1007/s11356-017-9318-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9318-5