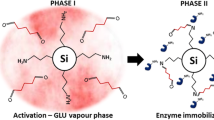
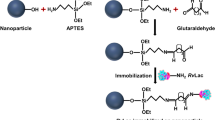
Abstract
Enyzme immobilization on solid surfaces is one of the most relevant methods to improve enzyme activity and stability under harsh conditions over extended periods. A typically interesting application is the immobilization of laccases, multicopper enzymes oxidizing aromatic compounds, to solid surfaces in order to develop valuable tools for the elimination of micropollutants in wastewater. Laccase of the white-rot fungus Coriolopsis polyzona has been successfully immobilized on fumed silica nanoparticles using a novel method. It consists in the sorption of the enzyme to amino-modified silica nanoparticles and the subsequent covalent cross-linking using glutaraldehyde as a homobifunctional linker. The so-produced nanoparticulate material has been characterized by means of scanning electron microscopy and Brunauer–Emmett–Teller surface area analysis revealing modifications of the surface structure and area during the coupling procedure. Laccase immobilization on spherical nanoparticles produced according to the method of Stöber has been shown to be much less efficient than on fumed silica nanoparticles. Long-term stability assays revealed that the novel developed method allows a drastic stabilization of the enzyme. In real wastewater, 77% of the laccase activity remained on the nanoparticles over 1 month, whereas the activity of free laccase dropped to 2.5%. The activity loss on the nanoparticles resulted from partial inactivation of the immobilized enzymes and additional release into the surrounding solution with subsequent fast inactivation of the free enzymes, since almost no activity was found in the supernatants.





Similar content being viewed by others
References
Auriol M, Filali-Meknassi Y, Tyagi RD, Adams CD (2007) Laccase-catalyzed conversion of natural and synthetic hormones from a municipal wastewater. Water Res 41:3281–3288
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 30:215–242
Brady D, Jordaan J (2009) Advances in enzyme immobilisation. Biotechnol Lett 31:1639–1650
Cabana H, Jiwan JLH, Rozenberg R, Elisashvili V, Penninckx M, Agathos SN, Jones JP (2007a) Elimination of endocrine disrupting chemicals nonylphenol and bisphenol A and personal care product ingredient triclosan using enzyme preparation from the white rot fungus Coriolopsis polyzona. Chemosphere 67:770–778
Cabana H, Jones JP, Agathos SN (2007b) Elimination of endocrine disrupting chemicals using white rot fungi and their lignin modifying enzymes: a review. Eng Life Sci 7:429–456
Cabana H, Jones JP, Agathos SN (2007c) Preparation and characterization of cross-linked laccase aggregates and their application to the elimination of endocrine disrupting chemicals. J Biotechnol 132:23–31
Cabana H, Alexandre C, Agathos SN, Jones JP (2009a) Immobilization of laccase from the white rot fungus Coriolopsis polyzona and use of the immobilized biocatalyst for the continuous elimination of endocrine disrupting chemicals. Bioresour Technol 100:3447–3458
Cabana H, Jones JP, Agathos SN (2009b) Utilization of cross-linked laccase aggregates in a perfusion basket reactor for the continuous elimination of endocrine-disrupting chemicals. Biotechnol Bioeng 102:1582–1592
Cao LQ, van Langen L, Sheldon RA (2003) Immobilised enzymes: carrier-bound or carrier-free? Curr Opin Biotechnol 14:387–394
Childs RE, Bardsley WG (1975) The steady-state kinetics of peroxidase with 2,2′-azino-di-(3-ethylbenzthiazoline-6-sulphonic acid) as chromogen. Biochem J 145:93–103
Cirja M, Ivashechkin P, Schäffer AI, Corvini PFX (2008) Factors affecting the removal of organic micropollutants from wastewater in conventional treatment plants (CTP) and membrane bioreactors (MBR). Rev Environ Sci Bio/Technol 7:61–78
Corvini PFX, Shahgaldian P (2010) LANCE: laccase-nanoparticle conjugates for the elimination of micropollutants (endocrine disrupting chemicals) from wastewater in bioreactors. Rev Environ Sci Bio/Technol 9:23–27
Durán N, Rosa MA, D'Annibale A, Gianfreda L (2002) Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: a review. Enzyme Microb Technol 31:907–931
Galliker P, Hommes G, Schlosser D, Corvini PFX, Shahgaldian P (2010) Laccase-modified silica nanoparticles efficiently catalyze the transformation of phenolic compounds. J Colloid Interface Sci 349:98–105
Garoma T, Matsumoto SA, Wu Y, Klinger R (2010) Removal of bisphenol A and its reaction-intermediates from aqueous solution by ozonation. Ozone: Sci Eng 32:338–343
Hildén K, Hakala TK, Lundell T (2009) Thermotolerant and thermostable laccases. Biotechnol Lett 31:1117–1128
Husain Q, Ulber R (2011) Immobilized peroxidase as a valuable tool in the remediation of aromatic pollutants and xenobiotic compounds: a review. Crit Rev Environ Sci Tech 41:770–804
Johannes C, Majcherczyk A (2000) Laccase activity tests and laccase inhibitors. J Biotechnol 78:193–199
Kadima TA, Pickard MA (1990) Immobilization of chloroperoxidase on aminopropyl-glass. Appl Environ Microbiol 56:3473–3477
Krasnoslobodtsev AV, Smirnov SN (2002) Effect of water on silanization of silica by trimethoxysilanes. Langmuir 18:3181–3184
Lleu PL, Rebel G (1991) Interference of good buffers and other biological buffers with protein determination. Anal Biochem 192:215–218
López-Gallego F, Betancor L, Mateo C, Hidalgo A, Alonso-Morales N, Dellamora-Ortiz G, Guisán JM, Fernández-Lafuente R (2005) Enzyme stabilization by glutaraldehyde crosslinking of adsorbed proteins on aminated supports. J Biotechnol 119:70–75
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Majeau JA, Brar SK, Tyagi RD (2010) Laccases for removal of recalcitrant and emerging pollutants. Bioresour Technol 101:2331–2350
Martin C, Moeder M, Daniel X, Krauss G, Schlosser D (2007) Biotransformation of the polycyclic musks HHCB and AHTN and metabolite formation by fungi occurring in freshwater environments. Environ Sci Technol 41:5395–5402
Modaressi K, Taylor KE, Bewtra JK, Biswas N (2005) Laccase-catalyzed removal of bisphenol-A from water: protective effect of PEG on enzyme activity. Water Res 39:4309–4316
Nicell JA, Bewtra JK, Biswas N, Taylor E (1993) Reactor development for peroxidase catalyzed polymerization and precipitation of phenols from wastewater. Water Res 27:1629–1639
Pratsinis SE (1998) Flame aerosol synthesis of ceramic powders. Progr Energ Combust Sci 24:197–219
Qiu HJ, Xu CX, Huang XR, Ding Y, Qu YB, Gao PJ (2009) Immobilization of laccase on nanoporous gold: comparative studies on the immobilization strategies and the particle size effects. J Phys Chem 113:2521–2525
Rekuć A, Bryjak J, Szymańska K, Jarzębski AB (2009) Laccase immobilization on mesostructured cellular foams affords preparations with ultra high activity. Process Biochem 44:191–198
Rekuć A, Bryjak J, Szymańska K, Jarzębski AB (2010) Very stable silica-gel-bound laccase biocatalysts for the selective oxidation in continuous systems. Bioresour Technol 101:2076–2083
Rodríguez-Vico F, Martínez-Cayuela M, García-Peregrín E, Ramírez H (1989) A procedure for eliminating interferences in the Lowry method of protein determination. Anal Biochem 183:275–278
Sangeetha K, Abraham TE (2008) Preparation and characterization of cross-linked enzyme aggregates (CLEA) of Subtilisin for controlled release applications. Int J Biol Macromol 43:314–319
Schäfer AI, Fane AG, Waite TD (2001) Cost factors and chemical pretreatment effects in the membrane filtration of waters containing natural organic matter. Water Res 35:1509–1517
Sheldon RA (2007) Cross-linked enzyme aggregates (CLEA®s): stable and recyclable biocatalysts. Biochem Soc Trans 35:1583–1587
Sheldon RA, Schoevaart R, Van Langen LM (2005) Cross-linked enzyme aggregates (CLEAs): a novel and versatile method for enzyme immobilization (a review). Biocatal Biotransform 23:141–147
Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in micron size range. J Colloid Interface Sci 26:62–69
Wandrey C, Liese A, Kihumbu D (2000) Industrial biocatalysis: past, present, and future. Org Process Res Dev 4:286–290
Wang F, Guo C, Yang LR, Liu CZ (2010) Magnetic mesoporous silica nanoparticles: fabrication and their laccase immobilization performance. Bioresour Technol 101:8931–8935
Winters AL, Minchin FR (2005) Modification of the Lowry assay to measure proteins and phenols in covalently bound complexes. Anal Biochem 346:43–48
Zhao M, Wang Y, Liu ZJ, Cui DZ, Bian XJ (2011) Properties of immobilized laccase on mesostructured cellular foam silica and its use in dye decolorization. J Macromol Sci Pure Appl Chem 48:447–453
Zhu Y, Kaskel S, Shi J, Wage T, van Pée KH (2007) Immobilization of Trametes versicolor laccase on magnetically separable mesoporous silica spheres. Chem Mater 19:6408–6413
Acknowledgments
The authors thank Dr. D. Schlosser (Helmholtz Centre for Environmental Research, UFZ) for his valuable comments on this study and Wetlands Engineering SPRL for the supply of laccase. The support of the Commission for Technology and Innovation of the Swiss Federal Office for Professional Education and Technology (Grant PFNM-NM 9632.1) and the European Commission within the Seventh Framework Program under grant agreement 265946 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zimmermann, YS., Shahgaldian, P., Corvini, P.F.X. et al. Sorption-assisted surface conjugation: a way to stabilize laccase enzyme. Appl Microbiol Biotechnol 92, 169–178 (2011). https://doi.org/10.1007/s00253-011-3534-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3534-6