Abstract
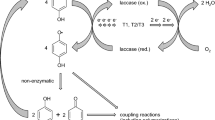
Laccases are able to mediate both cleavage and synthesis processes. The basis for this dual reaction capability lies in the property of the enzyme laccase to oxidize phenolic, and to some extent non-phenolic substances, to reactive radicals which can undergo on the one hand separations of small substitutents or large molecule parts from the parent compound and on the other hand coupling reactions with other radicals or molecules which are not themselves oxidizable by laccase. The cleavage of the non-phenolic compound 4-morpholinoaniline as well as the deamination of 4-aminophenol and the dechlorination of 4-chlorophenol resulted in the formation of 1,4-hydroquinone which is immediately oxidized by laccase to 1,4-benzoquinone. The formation of the 1,4-hydroquinone/1,4-benzoquinone is the rate limiting step for the synthesis of the heteromolecular dimers and trimers composed of 1,4-benzoquinone and one or two molecules of morpholine. In addition to the synthesis of new compounds from the cleavage products, 4-morpholinoaniline polymerized probably via azo groups and C-N bonds to a homomolecular dimer and trimer. Similarities and differences in cleavage and synthesis reactions catalyzed by the low redox potential laccase of Myceliophthora thermophila (0.46 V) and the high redox potential laccase of Pycnoporus cinnabarinus (0.79 V) were determined. In addition, the dependency of the cleavage and synthesis efficiencies on the (a) structure and redox potential of the laccase, (b) structure and redox potential of the substrate, (c) pH value of the buffer used, (d) incubation temperature, (e) solvent concentration, and (f) laccase activity is discussed in general.




Similar content being viewed by others
References
Agematu H, Tsuchida T, Kominato K, Shibamoto N, Yoshioka T, Nishida H, Okamoto R, Shin T, Murao S (1993) Enzymatic dimerization of penicillin X. J Antibiot 46:141–148
Berka RM, Schneider P, Golightly EJ, Brown SH, Madden M, Brown KM, Halkier T, Mondorf K, Xu F (1997) Characterization of the gene encoding an extracellular laccase of Myceliophthora thermophila and analysis of the recombinant enzyme expressed in Aspergillus oryzae. Appl Environ Microbiol 63:3151–3157
Bertrand T, Jolivalt C, Briozzo P, Caminade E, Joly N, Madzak C, Mougin C (2002) Crystal structure of a four-copper laccase complexed with an arylamine: insights into substrate recognition and correlation with kinetics. Biochemistry 41:7325–7333
Bourbonnais R, Paice MG (1990) Oxidation of non-phenolic substrates—an expanded role for laccase in lignin biodegradation. FEBS Lett 267:99–102
Cambria MT, Gullotto D, Garavaglia S, Cambria A (2012) In silico study of structural determinants modulating the redox potential of Rigidoporus lignosus and other fungal laccases. J Biomol Struct Dyn 30:89–101
Campos R, Kandelbauer A, Robra KH, Cavaco-Paulo A, Gübitz GM (2001) Indigo degradation with purified laccases from Trametes hirsuta and Sclerotium rolfsii. J Biotechnol 89:131–139
Chivukula M, Renganathan V (1995) Phenolic azo dye oxidation by laccase from Pyricularia oryzae. Appl Environ Microbiol 61:4374–4377
Claus H (2003) Laccases and their occurrence in prokaryotes. Arch Microbiol 179:145–150
Claus H (2004) Laccases: structure, reactions, distribution. Micron 35:93–96
Corbett JF (1969a) Benzoquinone imines. I. p-Phenylenediamine-ferricyanide and p-aminophenol-ferricyanide redox systems. J Chem Soc B 3:207–212
Corbett JF (1969b) Benzoquinone imines. 2. Hydrolysis of p-benzoquinone monoimine and p-benzoquinone di-imine. J Chem Soc B 3:213–216
d’Acunzo F, Barreca AM, Galli C (2004) Determination of the activity of laccase, and mediated oxidation of a lignin model compound, in aqueous-organic mixed solvents. J Mol Catal B: Enzym 31:25–30
d’Acunzo F, Galli C, Gentili P, Sergi F (2006) Mechanistic and steric issues in the oxidation of phenolic and non-phenolic compounds by laccase or laccase-mediator systems. The case of bifunctional substrates. New J Chem 30:583–591
Dawel G, Kästner M, Michels J, Poppitz W, Gunther W, Fritsche W (1997) Structure of a laccase-mediated product of coupling of 2,4- diamino-6-nitrotoluene to guajacol, a model for coupling of 2,4,6- trinitrotoluene metabolites to a humic organic soil matrix. Appl Environ Microbiol 63:2560–2565
Domagala S, Dziegiec J (1997) Oxidative cross-coupling of some 2,6- and N, N-disubstituted aniline derivatives with 4-aminophenol mediated by cerium(IV) ions in aqueous perchloric acid. Monatsh Chem 128:749–757
Eggert C, Temp U, Eriksson KEL (1996) The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol 62:1151–1158
Frasconi M, Favero G, Boer H, Koivula A, Mazzei F (2010) Kinetic and biochemical properties of high and low redox potential laccases from fungal and plant origin. Biochim Biophys Acta 1804:899–908
Galli C, Gentili P, Jolivalt C, Madzak C, Vadalà R (2011) How is the reactivity of laccase affected by single-point mutations? Engineering laccase for improved activity towards sterically demanding substrates. Appl Microbiol Biotechnol 91:123–131
Garzillo AMV, Colao MC, Caruso C, Caporale C, Celletti D, Buonocore V (1998) Laccase from the white-rot fungus Trametes trogii. Appl Microbiol Biotechnol 49:545–551
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2010) Laccases: a never-ending story. Cell Mol Life Sci 67:369–385
Hahn V, Mikolasch A, Manda K, Gördes D, Thurow K, Schauer F (2009a) Laccase-catalyzed carbon–nitrogen bond formation: coupling and derivatization of unprotected L-phenylalanine with different para-hydroquinones. Amino Acids 37:315–321
Hahn V, Mikolasch A, Wende K, Bartrow H, Lindequist U, Schauer F (2009b) Synthesis of model morpholine derivatives with biological activities by laccase-catalyzed reactions. Biotechnol Appl Biochem 54:187–195
Hahn V, Mikolasch A, Wende K, Bartrow H, Lindequist U, Schauer F (2010) Derivatization of 1-aminobenzotriazole using laccase of Pycnoporus cinnabarinus and Myceliophthora thermophila influenced by methanol and biological evaluation of the derivatives. Biotechnol Appl Biochem 56:43–48
Hashemi MM, Akhbari M (2005) Efficient solvent-free oxidation of phenols to p-quinones with iodic acid on the surface of K10 montmorillonite. Russ J Org Chem 41:935–936
Hashemi MM, Beni YA (1999) Sodium hypochlorite/dowex 1X8-200: an effective oxidant for the oxidation of aromatic amines to quinones. J Chem Res (S) 11:672–673
Hildén K, Hakala TK, Lundell T (2009) Thermotolerant and thermostable laccases. Biotechnol Lett 31:1117–1128
Hoff T, Liu SY, Bollag J-M (1985) Transformation of halogen-, alkyl-, and alkoxy-substituted anilines by a laccase of Trametes versicolor. Appl Environ Microbiol 49:1040–1045
Iimura Y, Hartikainen P, Tatsumi K (1996) Dechlorination of tetrachloroguaiacol by laccase of the white-rot basidiomycete Coriolus versicolor. Appl Microbiol Biotechnol 45:434–439
Jonas U, Hammer E, Schauer F, Bollag J-M (1997) Transformation of 2-hydroxydibenzofuran by laccases of white rot fungi Trametes versicolor and Pycnoporus cinnabarinus and characterization of oligomerization products. Biodegradation 8:321–328
Kawai S, Umezawa T, Higuchi T (1988) Degradation mechanisms of phenolic β-1 lignin substructure model compounds by laccase of Coriolus versicolor. Arch Biochem Biophys 262:99–110
Kordon K, Mikolasch A, Schauer F (2010) Oxidative dehalogenation of chlorinated hydroxybiphenyls by laccases of white-rot fungi. Int Biodeterior Biodegrad 64:203–209
Kunamneni A, Camarero S, García-Burgos C, Plou FJ, Ballesteros A, Alcalde M (2008) Engineering and applications of fungal laccases for organic synthesis. Microb Cell Fact 7:32. doi:10.1186/1475-2859-7-32
Leonowicz A, Cho N-S, Luterek J, Wilkolazka A, Wojtas-Wasilewska M, Matuszewska A, Hofrichter M, Wesenberg D, Rogalski J (2001) Fungal laccase: properties and activity on lignin. J Basic Microbiol 41(3-4):185–227
Leontievsky AA, Myasoedova NM, Baskunov BP, Golovleva LA, Bucke C, Evans CS (2001) Transformation of 2,4,6-trichlorophenol by free and immobilized fungal laccase. Appl Microbiol Biotechnol 57:85–91
Lerner L (2011) Identity of a purple dye formed by peroxidic oxidation of p-aminophenol at low pH. J Phys Chem A 115:9901–9910
Li KC, Xu F, Eriksson KEL (1999) Comparison of fungal laccases and redox mediators in oxidation of a nonphenolic lignin model compound. Appl Environ Microbiol 65:2654–2660
Lugaro G, Carrea G, Cremonesi P, Casellato MM, Antonini E (1973) The oxidation of steroid hormones by fungal laccase in emulsion of water and organic solvents. Arch Biochem Biophys 159:1–6
Luňák S Jr, Nepraš M, Hrdina R, Mustroph H (1994) Excited states of azo compounds. II. Vibrational structure of the electronic absorption spectra of 4,4’-di-substituted azobenzene derivatives. Chem Phys 184:255–260
Mai C, Schormann W, Hüttermann A (2001) Chemo-enzymatically induced copolymerization of phenolics with acrylate compounds. Appl Microbiol Biotechnol 55:177–186
Maijala P, Mattinen ML, Nousiainen P, Kontro J, Asikkala J, Sipilä J, Viikari L (2012) Action of fungal laccases on lignin model compounds in organic solvents. J Mol Catal B: Enzym 76:59–67
Manda K, Hammer E, Mikolasch A, Gördes D, Thurow K, Schauer F (2006) Laccase-induced derivatization of unprotected amino acid L-tryptophan by coupling with p-hydroquinone 2,5-dihydroxy-N-(2-hydroxyethyl)-benzamide. Amino Acids 31:409–419
Mikolasch A, Niedermeyer THJ, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Gesell M, Hessel S, Jülich W-D, Lindequist U (2006) Novel penicillins synthesized by biotransformation using laccase from Trametes spec. Chem Pharm Bull 54:632–638
Mikolasch A, Matthies A, Lalk M, Schauer F (2008) Laccase-induced C-N coupling of substituted p-hydroquinones with p-aminobenzoic acid in comparison with known chemical routes. Appl Microbiol Biotechnol 80:389–397
Minard RD, Liu SY, Bollag J-M (1981) Oligomers and quinones from 2,4-dichlorophenol. J Agric Food Chem 29:250–253
Morozova OV, Shumakovich GP, Gorbacheva MA, Shleev SV, Yaropolov AI (2007) “Blue” laccases. Biochemistry (Mosc) 72:1136–1150
Nagai M, Sato T, Watanabe H, Saito K, Kawata M, Enei H (2002) Purification and characterization of an extracellular laccase from the edible mushroom Lentinula edodes, and decolorization of chemically different dyes. Appl Microbiol Biotechnol 60:327–335
Ncanana S, Baratto L, Roncaglia L, Riva S, Burton SG (2007) Laccase-mediated oxidation of totarol. Adv Synth Catal 349:1507–1513
Niedermeyer THJ, Mikolasch A, Lalk M (2005) Nuclear amination catalyzed by fungal laccases: reaction products of p-hydroquinones and primary aromatic amines. J Org Chem 70:2002–2008
Piontek K, Antorini M, Choinowski T (2002) Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-Å resolution containing a full complement of coppers. J Biol Chem 277:37663–37669
Rodgers CJ, Blanford CF, Giddens SR, Skamnioti P, Armstrong FA, Gurr SJ (2010) Designer laccases: a vogue for high-potential fungal enzymes? Trends Biotechnol 28:63–72
Schröder M, Pereira L, Rodríguez Couto S, Erlacher A, Schöning K-U, Cavaco-Paulo A, Gübitz GM (2007) Enzymatic synthesis of Tinuvin. Enzym Microbiol Technol 40:1748–1752
Solomon EI, Chen P, Metz M, Lee SK, Palmer AE (2001) Oxygen binding, activation, and reduction to water by copper proteins. Angew Chem Int Ed 40:4570–4590
Solomon EI, Augustine AJ, Yoon J (2008) O2 reduction to H2O by the multicopper oxidases. Dalton Trans 30:3921–3932
Tadesse MA, D’Annibale A, Galli C, Gentili P, Sergi F (2008) An assessment of the relative contributions of redox and steric issues to laccase specificity towards putative substrates. Org Biomol Chem 6:868–878
Tauber MM, Gübitz GM, Rehorek A (2008) Degradation of azo dyes by oxidative processes—laccase and ultrasound treatment. Bioresour Technol 99:4213–4220
Torres E, Bustos-Jaimes I, Le Borgne S (2003) Potential use of oxidative enzymes for the detoxification of organic pollutants. Appl Catal B Environ 46:1–15
Wesenberg D, Kyriakides I, Agathos SN (2003) White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol Adv 22:161–187
Xu F (1996) Oxidation of phenols, anilines, and benzenethiols by fungal laccases: correlation between activity and redox potentials as well as halide inhibition. Biochemistry 35:7608–7614
Xu F (1997) Effects of redox potential and hydroxide inhibition on the pH activity profile of fungal laccases. J Biol Chem 272:924–928
Xu F (1999) Laccase. In: Fleckinger MC, Drew SW (eds) Encyclopedia of bioprocess technolog.: fermentation, biocatalysis, and bioseparation. Wiley, New York, pp 1545–1553
Xu F, Shin W, Brown SH, Wahleithner JA, Sundaram UM, Solomon EI (1996) A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim Biophys Acta 1292:303–311
Xu F, Berka RM, Wahleithner JA, Nelson BA, Shuster JR, Brown SH, Palmer AE, Solomon EI (1998) Site-directed mutations in fungal laccase: effect on redox potential, activity and pH profile. Biochem J 334:63–70
Xu F, Kulys JJ, Duke K, Li KC, Krikstopaitis K, Deussen HJW, Abbate E, Galinyte V, Schneider P (2000) Redox chemistry in laccase-catalyzed oxidation of N-hydroxy compounds. Appl Environ Microbiol 66:2052–2056
Yaver DS, Xu F, Golightly EJ, Brown KM, Brown SH, Rey MW, Schneider P, Halkier T, Mondorf K, Dalbøge H (1996) Purification, characterization, molecular cloning, and expression of two laccase genes from the white rot basidiomycete Trametes villosa. Appl Environ Microbiol 62:834–841
Acknowledgments
We thank M. Lalk (Institute of Pharmacy, University of Greifswald) for providing NMR data. R. Jack is gratefully acknowledged for help in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hahn, V., Mikolasch, A. & Schauer, F. Cleavage and synthesis function of high and low redox potential laccases towards 4-morpholinoaniline and aminated as well as chlorinated phenols. Appl Microbiol Biotechnol 98, 1609–1620 (2014). https://doi.org/10.1007/s00253-013-4984-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4984-9