Abstract
Non-stoichiometric lead free polycrystalline Sr0.8Sn0.2Bi1.75Sm0.25Nb2O9 (SSBSN) ferroelectric ceramics were synthesized through conventional solid step route method by incorporating ball milling and microwave sintering method. X-ray diffraction along with the Rietveld refinement technique confirms the single-phase orthorhombic structure with A21 crystal symmetry. Additionally, the short-range ordering was confirmed by Raman spectroscopy. Doping induced crystallite size and strain were further calculated from the Williamson-Hall plot, which comes around 150 nm and 1.48 × 10–3 respectively. A plate like morphology with an average grain size of 0.41 μm was confirmed by scanning electron microscopy (SEM). A diffuse type ferroelectric to paraelectric phase transition was recorded at 395 °C, mostly arising due to structural heterogeneity at the inter-ferroelectric phase boundary. The temperature and frequency-dependent dielectric measurement of SSBSN ceramic reveal a Maxwell–Wagner relaxation, with prominent dielectric loss in a low frequency regime perhaps due to the generation of leakage current in the SSBSN system. Frequency dependent ac conductivity indicates the polaron assisted hopping mechanism in SSBSN, which further obeys Jonscher’s formulation. The intra and intergranular contributions to impedance in SSBSN ceramics were probed by the complex impedance spectroscopy (CIS) technique. A non-Debye type relaxation mechanism in SSBSN ceramics was indicated by the Cole–Cole plot, whereas the conduction mechanism and transport properties were briefly studied using modulus spectroscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Piezoelectric and ferroelectric materials based multifunctional devices have been an indispensable element of our technological evolution due to their comprehensive applications in the electronics, sensors, underwater acoustics, transduces, photorefractive devices, tunable capacitors, pyroelectric sensors, ultrasonic medical imaging and many more. Most of these devices comprise lead-based ferroelectric materials (ex: PZT, PbTiO3) as they possess exceptional ferroelectric, piezoelectric, and pyroelectric properties. However, long-term utilization or exposure to lead can cause serious health issues for humans and the environment due to its toxicity [1, 2]. Thus, from time to time the World Health Organization (WHO) has raised serious concerns regarding the lack of proper disposal of lead and advised material scientists across the globe to search for eco-friendly alternatives [3, 4]. Among all available alternatives the Bismuth layer structured ferroelectrics (BLSF) compositions, have proved to be the most promising ferroelectric system to replace lead based devices in the future due to their exciting properties such as high Curie temperature, high dielectric constant, low polarization switching voltage, good retention behavior, minimum operating voltages, a high mechanical quality factor, and large solid solubility of dopant ions, which make them eligible candidates for numerous technological and industrial applications such as satellite communication devices, medical diagnostics, industrial automation, ultrasonic motors, transducers, etc. BLSFs were first discovered by B. Aurivillius in 1949 having a structural formula of (Bi2O2)2+ (Am−1BmO3m+1)2− where A-site is occupied by mono, dia, and trivalent ion (K+, Ca2+, Ba2+, Bi3+, etc.) or their combination allowing dodecahedral coordination, B-site is occupied by transitional elements like (Ti4+, Nb5+, Ta4+, Mo6+, etc.) suited to the octahedral coordination and m is an integer which represents the number of BO6 octahedral layer sandwiched between two perovskite slab [5,6,7,8]. Among several BLSFs, the SrBi2Nb2O9 (SBN) is the second-layered Aurivillius compound that belongs to the group of disordered uniaxial ferroelectrics with an orthorhombic crystal structure and A21am space group. SBN and its solid solution have gained substantial interest due to their less distorted NbO6 octahedra and extraordinary control of rattling space on the dielectric, ferroelectric, and piezoelectric properties. Despite having numerous useful properties, SBN also suffers from a few drawbacks, such as intense spontaneous polarization and high dielectric loss [9, 10]. Interestingly the electrical and ferroelectric properties of Bi-based SBN strongly depend on the complete or partial site substitution/modification of the parent compound [11]. Therefore, controlling these parameters became extremely important to achieve desired properties for technological applications. According to the available literature, Pritam et al. [12] have reported an enhanced dielectric constant of 120 in Sn doped SBN (Sr0.8Sn0.2Bi2Nb2O9) compared to undoped one and serves as a basis of the present work. Additionally, the literature suggests that the dielectric and ferroelectric properties of BLSFs can be strongly enhanced by rare earth substitution such as Sm3+, Ce3+, Nd3+, Pr3+, Gd3+, and Y3+ as rare earth elements are known to diminish the generation of mobile oxygen vacancies (arise due to high-temperature sintering) in the solid solution due to their peculiar inimitable electronic structure and lattice distortion [13]. Numerous studies are available on A site, B site, or Bi site modification in SBN, however very few or no reports exist on multiple site modifications in SBN, and its influence on structural, dielectric, and electrical properties in SBN is rarely available [14,15,16].
Therefore, the present work deals with the investigation of structural contribution to the electrical transport mechanism and multiple relaxation mechanism in Sr0.8Sn0.2Bi1.75Sm0.25Nb2O9 (SSBSN) ceramics synthesized through green synthesis microwave sintering (MS) method, as MS will drastically reduce the two-step sintering to single step sintering. Several dielectric-derived parameters like Z*, M*, and σ* have been thoroughly investigated to get a complete understanding of the electrical behavior of microwave sintered SSBSN ceramics.
2 Experimental section
2.1 Materials and methods
The polycrystalline Sr0.8Sn0.2Bi1.75Sm0.25Nb2O9 (SSBSN) ceramics were synthesized using the solid-state route (SSR) method by incorporating single step microwave sintering. SSR is the simplest and most effective way to prepare inorganic bulk compositions. Usually, the SSR method is carried out by intermixing followed by high temperature sintering of a homogeneous mixture of different oxides, nitrates, and carbonates [11, 17]. During the synthesis, numerous parameters like temperature, gas flow, pressure, milling time, etc. can be easily varied to achieve the desired phase and morphology of the composition or its solid solution. Initially, the reagent grade carbonates and oxides of Bi2O3 (99.9%), SnO (99.9%), SrCO3 (99.5), Sm2O3 (99.9%), and Nb2O5 (99.95%), were used as the starting materials. The stoichiometric proportions of starting materials were extensively wet milled for 24 h. in a Retsch PM100 planetary ball mill using hard tungsten carbide balls of diameter 5 mm (the ball to. powder mass ratio was 10:1). The obtained sullery was mixed with the Polyvinyl Acetate (PVA) binder and compressed into a cylindrical pallet having a diameter of 10.8 mm and thickness of 2 mm. Further, the pallets were sintered in a closed crucible at 1000 °C for 5 h. in a microwave furnace having a frequency of 3.5 GHz.
2.2 Characterization techniques
The Phase formation of SSBSN was investigated using Bruker D8 discover equipped with Cu-Kα radiation of wavelength 1.5406 Å, operated at 40 kV and 40 mA. The data was recorded in a step size of 0.02 with a time per step of 2 s for the integrated angular range of 20°–80°. Raman spectra were recorded using a micro-Raman spectrometer equipped with a 532 nm argon-ion laser source with a magnification of 50×. The surface morphology was recorded using Carl Zeiss Merlin VP Compact FE-SEM with 5 kV acceleration voltage and 50 K magnification. The ac conductivity, impedance, and modulus spectroscopy of the prepared composition were calculated using the dielectric data, recorded on high-frequency LCR meter ZM2376 (NF corporation, Japan) with an applied oscillation level voltage of 1 V.
3 Results and discussion
3.1 Microstructural analysis
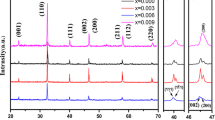
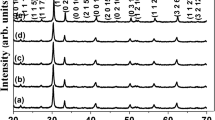
The X-ray diffraction pattern of Sn and Sm doped SBN ceramics was shown in Fig. 1a, indicating the formation of a perfect layered perovskite phase within the targeted composition. [11, 18]. The obtained diffraction peaks were indexed using orthorhombic symmetry conferring to the JCPDS card no 00-049-0607. The absence of the secondary phase signifies that the Sm and Sn ions have successfully entered their respective sites of the prepared SSBSN solid solution, thus proposing a smooth substitution. Lattice parameters of the prepared composition were found to be. 5.516 Å, 5.503 Å, and 25.038 Å respectively [16, 19]. Further, the XRD pattern of SSBSN was fitted with Rietveld refinement using Fullprof software. The refined XRD pattern is in good agreement with the experimental data having the goodness of fitting around 2.7 as shown in Fig. 1b. The crystallographic findings from the Rietveld refinement are summarized in Table 1, whereas the crystal structure of the same is demonstrated in Fig. 1c.
3.1.1 Crystallite size and the lattice strain estimation
X-ray diffraction is a powerful technique for determining numerous parameters like crystallite size, lattice strain effect, and peak broadening [17, 20,21,22]. To know the contribution of the factors mentioned above, it is essential to collect the X-ray diffraction data of standard material and estimate the instrumental broadening using Eq. 1 [23].
Here, βd is the corrected broadening, βmeasured is measured broadening, and βinstrumental is instrumental broadening. The physical broadening and instrumental broadening of the sample are estimated by FWHM (Full-width half maxima) [24]. By employing the corrected parameters, the average crystallite size can be determined by Debye Scherrer's equation depicted below.
where D is the crystallite size, λ is the wavelength of X-ray (1.5406 Å), and β is the FWHM [25]. The calculated crystallite size is around 80 nm. Based on Debye Scherer's model, only crystallite size plays a significant role in broadening of XRD peaks, however other factors like lattice strain, also known as strain-induced broadening were neglected. Numerous models, such as the Warren–Averbach method, Williamson Hall (W–H) method, etc., deal with the effect of strain and crystallite size on peak broadening discretely and can be employed to estimate the induced strain along with the crystallite size [20, 24, 26]. Among these methods, the W–H method is ideal for this purpose due to its simplified integral breath model, which noticeably differentiates between the crystallite size and strain-driven peak deformation, considering peak broadening as a function of two theta [24, 27, 28]. The total broadening of the diffraction peak is shown below.
where βhkl denotes the FWHM of a XRD peak, βsize and βstrain are the widths due to the size and strain, respectively. According to the Uniform Deformation Model (UDM), the strain is isotropic and arises in the crystalline structure due to imperfections, depicted in Eq. 4,
On rearranging the Eq. 2, 3, and 4, we get
Equation 6, known as the uniform deformation model (UDM) equation, reveals the isotropic nature of the crystals. Where D and ε are known as crystallite size and microstrain, respectively [23].
Figure 2 shows the W–H plot of SSBSN ceramics. The calculated value of D and ε comes around 150 nm and 1.48 × 10–3, respectively. The slope obtained from the W–H plot is found to be in a positive direction, hence generating the intrinsic strain of 1.48 × 10–3 in the system [21, 29].
3.2 Morphological analysis
Figure 3a shows the cross-sectional field-emission scanning electron microscopy (FESEM) micrograph of microwave sintered SSBSN pellet. The SEM micrographs illustrate that the different shapes and sizes of grains have been homogeneously distributed across the surface. The morphology indicates a clear grain boundary with a well-defined compact plate-like structure, which is the signature of the Bismuth layered structured ferroelectrics. Careful investigation of the high-resolution SEM image further indicates the presence of porosity in the composition, mostly arising due to evaporation of Bismuth oxide at high temperatures [30, 31]. The average grain size was calculated using Image J software at a magnification of 50 KX and a scale of 0.5 μm. Normal distribution curve fitting of the histogram was employed to calculate the mean grain size of the composition, which comes around 0.41 μm depicted in Fig. 3b.
3.3 Raman analysis
Raman spectroscopy is a chemical analysis technique, which is used to probe the structural phase transition, crystallinity, lattice symmetry, and polar nanoregions (PNR) linked with the structural disorder of ferroelectric and multiferroic materials [3]. The room temperature Raman spectra of the SSBSN ceramics in a spectral range of 100–900 cm−1 are shown in Fig. 4. A couple of intense peaks with strong Raman intensity were observed at 202.4 cm−1, 570.31 cm−1, and 838.42 cm−1; however, a bunch of weak peaks was also detected at 176.06 cm−1, 270.16 cm−1, 329.27 cm−1, 448.19 cm−1, and 686.04 cm−1 [32, 33]. A sub-intense peak was observed at 202 cm-1, which indicates the vibration of A site ions (Sr and Sn) of the pseudoperovskite blocks [34]. Peak located at 570.31 cm−1 harmonizes with rigid sublattice mode and depicts the opposite and equal displacement of positive and negative ions. Raman mode corresponds to 838.42 cm−1 attributed to the symmetric stretching of NbO6 octahedra, hence implying the supremacy of the orthorhombic phase in the SSBSN system, also confirmed from the XRD analysis of the same. Mode corresponds to 202.4 cm−1 and 686.04 cm−1 assigned to the vibrations of A site ions and asymmetric distortion of BO3 triangular group and deformation of BO4 tetrahedron respectively. The Raman mode at 270.16 cm−1 is led by a force constant due to Bi-O2 and Nb-Bi-O2 bonds. Broad peak arises due to phonon confinement and represents the nanocrystinity of the system [32, 35, 36].
3.4 Curie temperature analysis
Figure 5 illustrates the temperature-dependent dielectric constant of SSBSN ceramics measured at a variable frequency and indicates a clear ferroelectric to paraelectric phase transition at 390 °C. A smaller value of the dielectric constant was observed in the temperature range of 50–250 °C, which linearly increases with the increasing temperature, possibly due to the coupling between mobile charge carriers contributing to the polarization process [37]. A broad peak of dielectric constant was observed for SSBSN ceramics at a frequency of 100 kHz and 1 MHz, arising due to composition fluctuation in solid solution of SSBSN [34, 38,39,40]. It was reported that defects such as impurities and oxygen vacancies play a significant role in the electronic polarization of BLSFs explaining the nature of dielectric constant at higher temperatures [41].
To determine the diffuseness of a relaxor ferroelectrics, the inverse of dielectric constant and temperature obeys the Uchino and Nomura function, a modified version of Curie–Weiss law (CW) is employed in the present work, shown in Eq. 7 [42]
Here εm is the maximum value of dielectric constant at temperature Tm, C is the Curie constant and γ is a degree of diffuseness of phase transition its values range between 1 and 2. γ = 1 means normal ferroelectrics, whereas γ = 2 signifies relaxor ferroelectrics respectively [43, 44].
Figure 6 shows the plots of log (1/ε − 1/εm) as a function of log (T−Tm) of SSBSN ceramics. For both measurements, the value of γ is 1.83 and 1.98 respectively, which indicates the diffuse phase transition in SSBSN ceramics, arises due to the formation of mobile oxygen vacancies in the solid solution [2, 45].
3.5 Variation of dielectric constant and loss with frequency
Investigating the temperature and frequency dependent dielectric properties is necessary to understand the conduction mechanism, and relaxation process in solids [46]. In this regard, the frequency-dependent variation of real part of the dielectric constant of SSBSN ceramics at several temperatures is shown in Fig. 7a.
A strong dispersion behavior was observed at low frequency region, possibly due to the accumulation of space charge in SSBSN solid solution. Such resilient dispersion behavior of dielectric constant emerges to be a common feature in ferroelectrics associated with non-negligible ionic conductivity, also known as low-frequency dielectric dispersion (LFDD) [47,48,49]. A high dielectric constant was witnessed at low frequency, irrespective of temperature, possibly due to the contribution from all four types of polarization electronic, ionic, orientational, and space charge polarization to the total dielectric constant. A steady decrease in dielectric constant was observed and can be explained by Koop's theory and Maxwell–Wagner model [50]. The tanδ or dielectric loss is the energy utilized in rotating the atomic dipole in a dielectric material and it arises due to two major reasons domain wall motion under the influence of external applied field and space charge relaxation. Further, it depends on three parameters frequency, temperature, and nature of additives [51]. Figure 7b shows the frequency dependence of dielectric loss at different temperatures, a high loss was observed in low frequency regions, possibly due to the presence of space charge polarization, however, it drastically decreases at high frequency regions, thus endorsing the surge of domain wall motion in this region [12].
3.6 Ac conductivity analysis
To investigate the conduction process and polaron responsible for the conduction, the temperature dependent a.c conductivity as a function of frequency was studied for SSBSN ceramics, which was calculated using Eq. 8.
where f is frequency, εo and is permittivity in a vacuum, ε' is the permittivity of the medium, and tanδ is known as dielectric loss[52].
Figure 8a depicts the variation of ac conductivity as a function of frequency, the conductivity curve can be widely differentiated into three regions; the first is low-frequency regions, in this region, the ac conductivity is independent of frequency and decreases with the decrease in frequency. This decrease in conductivity corresponds to the variation of space-charge polarization at the electrode interface and generation of oxygen vacancies in the system. Here local electric field opposes the applied electric field and limits the conductivity. As we increase the frequency, a flat plateau-like region is spotted, where conductivity is independent of frequency, representing the system's dc conductivity (σdc). The third and final region, known as the high-frequency region, which usually demonstrates the dispersive behavior of conductivity, appears due to the short and long polaron hopping in the SSBSN system. The increasing trend of ac conductivity might be due to the anarchizing of cations among the neighboring sites [53]. One can notice that the nature of the curve is independent of temperature; however, the onset of the dispersion is shifted to a higher frequency as the temperature is further increased. Additionally, the behavior of a.c. conduction was examined by Jonsher’s power law shown in Eq. 9.
where A is a temperature-dependent parameter and s is a dimensionless exponent that describes the degree of interaction, whose value lies between 0 to 1, which represents the resistive and capacitive behavior of a material, respectively. The calculated value of s for SSBSN ceramics is shown in Table 2 and reveals a non-Debye type conduction mechanism.
3.7 Complex Impedance Spectroscopy
Complex impedance spectroscopy (CIS) is a powerful tool to probe the dielectric material, which tells us about the individual contribution of different electrical active regions. Usually, CIS is utilized to investigate the microscopic and macroscopic properties of the material like charge transfer resistance, diffusion process, relaxation process, transport number, etc. [54]. The complex impedance is depicted in Eq. 10.
Z* is further resolved in real (Z′) and imaginary part (Z″) of impedance and shown below.
where f is frequency, ε′ and ε″ are real and imaginary parts of the dielectric constant, and Co is known as the capacitance of vacuum[10, 55].
Figure 9a shows the variation of the real part (Z′) of impedance as a function of frequency in a temperature range of 350–550 °C. It was observed that the value of Z′ shows higher values at low-frequency regions, which drastically decreases with increasing frequency till 1 kHz. Beyond that, an independent behavior of the Z′ was observed which eventually merges at high frequency and indicates a release of space charge from the system. The value of Z′ significantly decreases with increasing temperature and indicates the negative temperature coefficients of resistance (NTCR) behavior [47, 48, 56]. Figure 9b shows the variation of the imaginary part of impedance (Z″) with frequency at different temperatures. An asymmetric broadening of the peak was observed with increasing temperature (beyond 405 °C) which indicates multiple relaxations process in the SSBSN ceramics. With the increasing frequency and temperature, the magnitude of Z″ peak was found to shift towards the high-frequency region, indicating that the relaxation mechanism spread over a wide range of frequencies. Irrespective of temperatures, the Z″ attains a constant value beyond 10 kHz, which indicates thermally driven relaxation mechanism in SSBSN ceramics [46, 47, 57].
The Cole–Cole plot of SSBSN ceramics at different temperatures is shown in Fig. 10. A linear response was observed at a lower temperature (> 400 °C) and implies the insulating behavior of the sample. As the temperature rises above 400 °C, the linear response was found to deviate into a semicircular one, indicating the rise of the conductivity in the material. Each semicircle experiences a small divergence from the origin and depicts non-Debye type relaxation process. This type of behavior in Cole–Cole plot arises due to numerous contributing factors such as grain boundary contribution, stress–strain phenomena, atomic and ionic defects distribution, etc. The emergence of two semicircles was also observed at higher temperatures, revealing that the contribution was from both grain and grain boundaries [12, 31, 53, 58]. The lack of a third semicircle rules out the interfacial contribution to total impedance of the system.
3.8 Complex modulus spectroscopy (CMS)
Unlike the resistive part, the capacitive element also significantly contributes to the impedance spectra of the material. Thus, it might be possible that the researcher can observe a single semicircle in a CIS but can observe multiple peaks in the modulus plot. Thus, to precisely know the contribution of resistive or capacitive components, we must need to investigate our data for the complex modulus spectroscopy (CMS). CMS is a convenient technique to examine the transport properties of dynamical systems[59]. Mathematically CMS is depicted in Eq. 13[55, 60]
M* can be further resolved into real (M′) and imaginary component (M″)
Figure 11a illustrates the frequency dependent variation of the real component of modulus at some selected temperatures. It was observed that M′ achieves a constant value above 1 kHz, but approaches zero at the lower frequency region, thus confirming the existence of interfacial or atomic polarization in the SSBSN solid solution. A strong dispersion behavior is observed at the high-frequency region irrespective of temperature, which contributes to the conduction phenomena that arise due to the movement of the charge carrier under the action of an applied electric field in the studied temperature region [55, 61]. A shift in the dispersion region is also observed at high temperatures and supports the long-range mobility of the charge carrier. Figure 11b shows the variation of the imaginary part of the modulus of SSBSN ceramics at several temperatures, a substantial shift in the peak of M″ observed with increasing temperature, indicating a thermally driven relaxation process in solid solution [62]. A nonsymmetrical broad peak is perceived for all measurements, which demonstrates multiple relaxation processes in SSBSN ceramics with different time constants. Finally, a decrease in the magnitude of M″ peak is detected with the increasing temperature, which undoubtedly specifies the surge in the dielectric constant of the SSBSN ceramics [19, 31, 63].
4 Conclusion
In summary, we have successfully prepared novel Sr0.8Sn0.2Bi1.75Sm0.25Nb2O9 (SSBSN) ferroelectric ceramics through single step solid state route method by incorporating microwave sintering technique, which drastically reduced the processing parameters (temperature and time) of SSBSN ceramics. XRD and Raman spectra validate the defect free orthorhombic crystal structure with A21am space group. Additionally, the Rietveld refinement and Williamson Hall method were used to calculate lattice parameters, atomic position, and strain in SSBSN matrix. A plate like morphology with an average grain size of 0.41 μm was confirmed by scanning electron microscopy (SEM). Temperature dependent dielectric measurement (Tc) indicates diffuse type phase transition, which arises due to the presence of an interacting ferroelectric cluster on the SSBSN. Further, the degree of diffusiveness (γ) was checked by Uchino and Nomura function, which comes around 1.8. Impedance analysis depicts the non-Debye type conduction mechanism with the NTCR behavior, whereas grain, and grain boundary contribution was revealed by Cole Plot. Modulus spectroscopy indicates that the relaxation process in SSBSN was governed by hopping of mobile oxygen vacancies. Ffrequency dependent AC conductivity demonstrates the dispersive behavior of conductivity, arising due to the polaron hopping in the SSBSN matrix. The obtained results support that the SSBSN can be used as a potential candidate for high frequency power transformer applications.
Data and code availability
The datasets studied and investigated during the current study are accessible from the corresponding author upon reasonable request, as the experimental data of current study is confidential and still unpublished.
References
V.K. Sharma, R. Nathawat, S.S. Rathore, Dielectric properties correlation with microstructure in ABi4Ti4O15 (A = Sr, Ba) bismuth layered ferroelectrics. Mater. Adv. 3, 4890–4898 (2022). https://doi.org/10.1039/d2ma00333c
Z. Zhou, X. Dong, S. Huang, H. Yan, Dielectric relaxation of La3+-modified Bi3TiNbO 9 aurivillius phase ceramics. J. Am. Ceram. Soc. 89, 2939–2942 (2006). https://doi.org/10.1111/j.1551-2916.2006.01166.x
S. Sahoo, D.K. Pradhan, S. Kumari et al., Structural heterogeneity induced enhancement of physical properties in Sm-modified K0.5Na0.5NbO3. J. Am. Ceram. Soc. (2023). https://doi.org/10.1111/jace.19612
A.F. Lima, Optical properties, energy band gap and the charge carriers’ effective masses of the R3c BiFeO3 magnetoelectric compound. J. Phys. Chem. Solids (2020). https://doi.org/10.1016/j.jpcs.2020.109484
P. Fang, H. Fan, Z. Xi et al., Structure and electrical properties of bismuth and sodium modified SrBi2Nb2O9 ferroelectric ceramics. J. Alloys Compd. 550, 335–338 (2013). https://doi.org/10.1016/j.jallcom.2012.10.147
S. Inai, J. Sato, R. Aoyagi et al., Piezoelectric properties of V and Ba substituted SrBi2 Nb 2O9 ceramics. Ferroelectrics 358, 148–152 (2007). https://doi.org/10.1080/00150190701537109
Z. Tang, J. Chen, B. Yang, S. Zhao, Energy storage performances regulated by layer selection engineering for doping in multi-layered perovskite relaxor ferroelectric films. Appl. Phys. Lett. (2019). https://doi.org/10.1063/1.5087045
S. Supriya, Tailoring layered structure of bismuth-based aurivillius perovskites: recent advances and future aspects. Coord. Chem. Rev. 479, 215010 (2023)
S. Supriya, Recent trends and morphology mechanisms of rare-earth based BiFeO3 nano perovskites with excellent photocatalytic performances. J. Rare Earths 41, 331–341 (2023)
A. Pritam, S. Kumar, V. Shrivastava et al., Probing the effect of LaFeO3 on the structural, electrical, and dielectric properties of Sr0.8Sn0.2Bi2Nb2O9 processed through solid state route method. Mater. Chem. Phys. 316, 129089 (2024). https://doi.org/10.1016/j.matchemphys.2024.129089
S. Supriya, Highly tunable multifunctional rare earth based Bi0.5-xCexNa0.5TiO3 perovskites via site selective doping engineering. Mater. Chem. Phys. (2022). https://doi.org/10.1016/j.matchemphys.2022.126233
A. Pritam, V. Shrivastava, Hole/electron transport layers in tin-doped SBLN nano materials for hybrid solar cell applications. J. Mater. Sci. Mater. Electron. 30, 11054–11062 (2019). https://doi.org/10.1007/s10854-019-01447-1
M. Afqir, A. Tachafine, D. Fasquelle et al., Dielectric properties of gadolinium-doped SrBi2Nb2O9 ceramics. J. Mater. Sci. Mater. Electron. 29, 1289–1297 (2018). https://doi.org/10.1007/s10854-017-8034-8
D. Nelis, J.M. Calderon-Moreno, M. Popa et al., Formation and micro-Raman spectroscopic study of Aurivilius and fluorite-type SrBi2Nb2O9 nanocrystallites obtained using an “amorphous citrate” route. J. Eur. Ceram. Soc. 26, 409–415 (2006). https://doi.org/10.1016/j.jeurceramsoc.2005.07.049
S.N. Kumar, P. Kumar, D.K. Agrawal, Structural, dielectric and ferroelectric properties of SBN ceramics synthesized by microwave reactive sintering technique. Ceram. Int. 38, 5243–5250 (2012). https://doi.org/10.1016/j.ceramint.2012.03.034
J. Zhang, G. Wang, F. Gao et al., Influence of Sr/Ba ratio on the dielectric, ferroelectric and pyroelectric properties of strontium barium niobate ceramics. Ceram. Int. 39, 1971–1976 (2013). https://doi.org/10.1016/j.ceramint.2012.08.048
S. Supriya, Effect of sintering temperature and micro structural analysis on sol-gel derived silver bismuth titanate ceramics. Mater. Res. Bull. 96, 290–295 (2017). https://doi.org/10.1016/j.materresbull.2017.01.018
Y. Wu, M.J. Forbess, S. Seraji et al., Doping effect in layer structured SrBi2Nb2O9ferroelectrics. J. Appl. Phys. 90, 5296–5302 (2001). https://doi.org/10.1063/1.1413236
I. Coondoo, N. Panwar, A. Tomar et al., Impedance spectroscopy and conductivity studies in SrBi 2(Ta 1-xW x) 2O9 ferroelectric ceramics. Phys. B Condens. Matter 407, 4712–4720 (2012). https://doi.org/10.1016/j.physb.2012.09.024
K.A. Aly, N.M. Khalil, Y. Algamal, Q.M.A. Saleem, Estimation of lattice strain for zirconia nano-particles based on Williamson–Hall analysis. Mater. Chem. Phys. 193, 182–188 (2017). https://doi.org/10.1016/j.matchemphys.2017.01.059
R. Sivakami, S. Dhanuskodi, R. Karvembu, Estimation of lattice strain in nanocrystalline RuO2 by Williamson–Hall and size-strain plot methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 152, 43–50 (2016). https://doi.org/10.1016/j.saa.2015.07.008
C.L. Hobday, S. Krause, S.M.J. Rogge et al., Perspectives on the influence of crystal size and morphology on the properties of porous framework materials. Front. Chem. (2021). https://doi.org/10.3389/fchem.2021.772059
P. Shunmuga Sundaram, T. Sangeetha, S. Rajakarthihan et al., XRD structural studies on cobalt doped zinc oxide nanoparticles synthesized by coprecipitation method: Williamson–Hall and size-strain plot approaches. Phys. B Condens. Matter 595, 412342 (2020). https://doi.org/10.1016/j.physb.2020.412342
D. Nath, F. Singh, R. Das, X-ray diffraction analysis by Williamson–Hall, Halder–Wagner and size-strain plot methods of CdSe nanoparticles—a comparative study. Mater. Chem. Phys. 239, 122021 (2020). https://doi.org/10.1016/j.matchemphys.2019.122021
T.S. Velayutham, N.I.F. Salim, W.C. Gan, W.H. Abd. Majid, Effect of cerium addition on the microstructure, electrical and relaxor behavior of Sr0.5Ba0.5Nb2O6 ceramics. J. Alloys Compd. 666, 334–340 (2016). https://doi.org/10.1016/j.jallcom.2015.12.248
B. Rajesh Kumar, B. Hymavathi, X-ray peak profile analysis of solid-state sintered alumina doped zinc oxide ceramics by Williamson–Hall and size-strain plot methods. J. Asian Ceram. Soc. 5, 94–103 (2017). https://doi.org/10.1016/j.jascer.2017.02.001
S. Harjo, Y. Tomota, S. Torii, T. Kamiyama, Residual thermal phase stresses in α-γ Fe-Cr-Ni alloys measured by a neutron diffraction time-of-flight method. Mater. Trans. 43, 1696–1702 (2002). https://doi.org/10.2320/matertrans.43.1696
H. Irfan, K.M. Racik, S. Anand, Microstructural evaluation of CoAl2O4 nanoparticles by Williamson–Hall and size–strain plot methods. J. Asian Ceram. Soc. 6, 54–62 (2018). https://doi.org/10.1080/21870764.2018.1439606
S. Devesa, A.P. Rooney, M.P. Graça et al., Williamson-hall analysis in estimation of crystallite size and lattice strain in Bi1.34Fe0.66Nb1.34O6.35 prepared by the sol-gel method. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 263, 114830 (2021). https://doi.org/10.1016/j.mseb.2020.114830
Z. Yao, H. Li, M. Ma et al., Preparation and electrical properties of (1–x)SrBi2Nb2O9-xBiFeO3 lead-free piezoelectric ceramics. Ceram. Int. 42, 5391–5396 (2016). https://doi.org/10.1016/j.ceramint.2015.12.075
A. Pritam, V. Shrivastava, Microwave derived porosity in tin-doped Sr1-xSnxBi1.95La0.05Nb2O9 nano materials for non-Debye conduction and photocatalytic activity. Phys. B Condens. Matter 593, 412320 (2020). https://doi.org/10.1016/j.physb.2020.412320
P. Mojumdar, R. Shaily, R. Bokolia, Structural properties of strontium bismuth niobate (SrBi2Nb2O9) ferroelectric ceramics. Mater. Today Proc. 47, 4661–4665 (2021)
B. Harihara Venkataraman, T. Fujiwara, T. Komatsu, Synthesis and characterization of rare-earth doped SrBi2Nb2O9 phase in lithium borate based nanocrystallized glasses. J. Solid State Chem. 182, 1538–1544 (2009). https://doi.org/10.1016/j.jssc.2009.04.004
R. Singh, V. Luthra, R.S. Rawat, R.P. Tandon, Structural, dielectric and piezoelectric properties of SrBi2Nb2O9 and Sr0.8Bi2.2Nb2O9 ceramics. Ceram. Int. 41, 4468–4478 (2015). https://doi.org/10.1016/j.ceramint.2014.11.139
R. Singh, M. Gautam, V. Luthra, S. Subramanian, Exploration of the structural, dielectric, ferroelectric, and piezoelectric properties in non-stoichiometric Sr 1−X Bi 2+2X/3 Nb 2 O 9 ceramics. ECS J. Solid State Sci. Technol. 12, 103005 (2023). https://doi.org/10.1149/2162-8777/acff82
G.Z. Liu, C. Wang, H.S. Gu, Lu.H. Bin, Raman scattering study of La-doped SrBi2Nb2O 9 ceramics. J. Phys. D Appl. Phys. 40, 7817–7820 (2007). https://doi.org/10.1088/0022-3727/40/24/034
Z.Y. Zou, X.K. Lan, W.Z. Lu et al., Novel high Curie temperature Ba2ZnSi2O7 ferroelectrics with low-permittivity microwave dielectric properties. Ceram. Int. 42, 16387–16391 (2016). https://doi.org/10.1016/j.ceramint.2016.07.040
Z. Yang, R. Gu, L. Wei, H. Ren, Phase formation, microstructure and dielectric properties of Sr 0.53Ba0.47Nb2-xTaxO6 ceramics. J. Alloys Compd. 504, 211–216 (2010). https://doi.org/10.1016/j.jallcom.2010.05.093
H. Du, Y. Li, H. Li et al., Relaxor behavior of bismuth layer-structured ferroelectric ceramic with m = 2. Solid State Commun. 148, 357–360 (2008). https://doi.org/10.1016/j.ssc.2008.05.017
S.B. Krupanidhi, A. Sharma, A.K. Singh, V. Tuli, Recent advances in functional materials and devices (Springer Nature Singapore, Singapore, 2024)
A. Tanwar, M. Verma, V. Gupta, K. Sreenivas, A-site substitution effect of strontium on bismuth layered CaBi 4Ti4O15 ceramics on electrical and piezoelectric properties. Mater. Chem. Phys. 130, 95–103 (2011). https://doi.org/10.1016/j.matchemphys.2011.05.081
P. Fang, H. Fan, J. Li et al., The dielectric properties and the relaxation phase transition of copper substituted SrBi2 Nb2 O9 ferroelectric ceramics. Solid State Commun. 149, 2074–2077 (2009). https://doi.org/10.1016/j.ssc.2009.08.016
D. Kajewski, Z. Ujma, K. Szot, M. Pawełczyk, Dielectric properties and phase transition in SrBi2Nb2O9-SrBi2Ta2O9 solid solution. Ceram. Int. 35, 2351–2355 (2009). https://doi.org/10.1016/j.ceramint.2009.01.013
J. Zhang, M. Chao, E. Liang, M. Li, Synthesis and dielectric properties of textured SrBi 2Nb 2O 9 ceramics via laser rapid solidification. J. Alloys Compd. 521, 150–154 (2012). https://doi.org/10.1016/j.jallcom.2012.01.099
F. Rubio-Marcos, J.J. Romero, M.G. Navarro-Rojero, J.F. Fernandez, Effect of ZnO on the structure, microstructure and electrical properties of KNN-modified piezoceramics. J. Eur. Ceram. Soc. 29, 3045–3052 (2009). https://doi.org/10.1016/j.jeurceramsoc.2009.04.026
T.V. Kumar, Dielectric relaxation, ionic conduction and complex impedance studies on NaNo3 fast ion conductor. Int. J. Mater. Sci. Appl. 2, 173 (2013). https://doi.org/10.11648/j.ijmsa.20130206.12
K. Sambasiva Rao, D. Madhava Prasad, P. Murali Krishna, T.S. Latha, Structure, dielectric and impedance properties of barium strontium samarium bismuth niobate ceramic. Ceram. Silik. 52, 190–200 (2008)
B. Harihara Venkataraman, K.B.R. Varma, Frequency-dependent dielectric characteristics of ferroelectric SrBi 2Nb2O9 ceramics. Solid State Ion. 167, 197–202 (2004). https://doi.org/10.1016/j.ssi.2003.12.020
P. Goel, K.L. Yadav, Effect of V+5 doping on structural and dielectric properties of SrBi2Nb2O9 synthesized at low temperature. Phys. B Condens. Matter 382, 245–251 (2006). https://doi.org/10.1016/j.physb.2006.02.033
J. Li, Y. Pu, X. Wang et al., Effect of yttrium doping on the structure, dielectric multiferroic and magnetodielectric properties of Bi5Ti3FeO15 ceramics. J. Mater. Sci. Mater. Electron. 31, 4345–4353 (2020). https://doi.org/10.1007/s10854-020-02992-w
V. Shrivastava, Lattice strain driven dielectric insulation response of Sr1-xCaxBi2Nb2O9 ceramics. J. Adv. Dielectr. 6, 1650021-1-1650021–6 (2016). https://doi.org/10.1142/S2010135X16500211
V. Shrivastava, A. Pritam, A. Joshi, Integrating third phase transition and CO/CO2 contamination in microwave tailored Bi2Mo1−xWxO6 nano materials. J. Mater. Sci. Mater. Electron. 29, 17388–17396 (2018). https://doi.org/10.1007/s10854-018-9836-z
M.P. Dasari, K. Sambasiva Rao, P. Murali Krishna, G. Gopala Krishna, Barium strontium bismuth niobate layered perovskites: dielectric, impedance and electrical modulus characteristics. Acta Phys Pol A 119, 387–394 (2011). https://doi.org/10.12693/APhysPolA.119.387
S. Khasa, P. Singh, S. Sanghi et al., Science and technology structural analysis and dielectric characterization of aurivillius type CaSrBi2Nb2O9 ceramics. J. Integr. Sci. Technol. 2, 13–21 (2014)
K. Sambasiva Rao, D. Madhava Prasad, P. Murali Krishna et al., Impedance and modulus spectroscopy studies on Ba0.1Sr0.81La0.06Bi2Nb2O9 ceramic. Mater. Sci. Eng. B 133, 141–150 (2006). https://doi.org/10.1016/j.mseb.2006.06.030
A. Laha, S. Saha, S.B. Krupanidhi, Analysis of alternating current conduction and impedance spectroscopy study of BaBi2Nb2O9 thin films. Mater. Res. Soc. Symp. Proc. 688, 241–246 (2002). https://doi.org/10.1557/proc-688-c7.21.1
K. Sambasiva Rao, D. Madhava Prasad, P. Murali Krishna et al., Frequency and temperature dependence of electrical properties of barium and gadolinium substituted SrBi2Nb2O9 ceramics. J. Mater. Sci. 42, 7363–7374 (2007). https://doi.org/10.1007/s10853-007-1555-4
B.H. Venkataraman, K.B.R. Varma, Microstructural, dielectric, impedance and electric modulus studies on vanadium—doped and pure strontium bismuth niobate (SrBi2 Nb2O9) ceramics. J. Mater. Sci. Mater. Electron. 16, 335–344 (2005). https://doi.org/10.1007/s10854-005-1144-8
A. Pritam, V. Shrivastava, Deviations from Vegard’s law and intense Raman scattering in low-doped Bi2Mo1−xWxO6 (x = 0–10%) ceramics. Phys. Status Solidi B Basic Res. (2020). https://doi.org/10.1002/pssb.201900450
P.P.S. Bhadauria, J. Kolte, Impedance and AC conductivity analysis of La-substituted 0.67BiFeO3–0.33BaTiO3 solid solution. Appl. Phys. A Mater. Sci. Process. (2022). https://doi.org/10.1007/s00339-022-05600-2
S.K. Patri, P.L. Deepti, R.N.P. Choudhary, B. Behera, Dielectric, impedance and modulus spectroscopy of BaBi2Nb2O9. J. Electroceram. 40, 338–346 (2018). https://doi.org/10.1007/s10832-018-0135-0
D. Dhak, P. Dhak, P. Pramanik, Influence of substitution on dielectric and impedance spectroscopy of Sr 1–x Bi 2 + y Nb 2 O 9 ferroelectric ceramics synthesized by chemical route. Appl. Surf. Sci. 254, 3078–3092 (2008). https://doi.org/10.1016/j.apsusc.2007.10.097
P. Khatri, B. Behera, V. Srinivas, R.N.P. Choudhary, Complex impedance spectroscopic properties of Ba3V 2O8 ceramics. Adv. Mater. Sci. Eng. 2008, 1–6 (2008). https://doi.org/10.1155/2008/746256
Acknowledgements
Anurag Pritam and Saurav Sain express gratitude towards the Shiv Nadar Foundation for providing a research fellowship.
Funding
Open Access funding provided by the IReL Consortium
Author information
Authors and Affiliations
Contributions
Anurag Pritam: conceptualization, analysis, investigation, and writing original draft, Sourav Sain: investigation, Gourav Bhattacharya: investigation, Susanta Sinha Roy: supervision and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest or any personal relationship that could have influenced the work reported in this paper.
Ethical approval
This work does not deal with any experiment regarding embryo and human tissue.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pritam, A., Bhattacharya, G., Sain, S. et al. Multiple relaxation mechanisms in SrBi2Nb2O9 ceramic tweaked by tin and samarium incorporation in assistance with single-step microwave sintering. Appl. Phys. A 130, 342 (2024). https://doi.org/10.1007/s00339-024-07482-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-024-07482-y