Abstract
An ant colony is the epitome of social organization where up to millions of individuals cooperate to survive, compete, and reproduce as a single superorganism, Female members of ant colonies typically are categorized into a reproductive queen caste and a non-reproductive worker caste. The queen(s) conveys her fertility condition and in cases, genotype status, via a suite of queen pheromones whose various functions are crucial to the superorganismal nature of ant colonies. Knowledge of these functional properties is fundamental for identifying constituent chemicals and understanding corresponding modes of actions. In this review, I summarize functional properties of ant queen pheromones learned from seven decades of behavioral experiments, and contextualize this knowledge within the broader understanding of queen pheromones in other major groups of social insects. The effects include promotion of colony integrity and coherence, maintenance of reproductive dominance of the queen, and regulation of colony social structure. Additionally, general characteristics of queen pheromones are discussed and potential avenues for future research are highlighted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The evolutionary success of social insects is attributable to their highly integrated colonial lifestyles, where numerous individual insects cooperate to survive, compete with conspecifics, and produce sexual forms (reproductive individuals) (Wilson 1971; Lin and Michener 1972). Ants (Hymenoptera: Formicidae), in particular, manifest immense diversity, abundance, and ecological impact (Wheeler 1910; Schultheiss et al. 2022). Studies of ants have illuminated the inner workings of insect societies despite the independent emergence of sociality in multiple diverse lineages and the consequent uniqueness of each major eusocial taxon (Wilson 1971).
Male ants contribute little or nothing to the maintenance and growth of the colony, confining their activities to competing for matings during their reproductive seasons. Females are generally divided into two interdependent castes that showcase a reproductive division of labor. Members of the queen caste can mate and usually monopolize egg-laying in the colony. Meanwhile the workers, normally daughters of the queen and mostly sterile, perform routine yet essential tasks such as foraging, nest building, and brood care, acting as the extended phenotype of the queen (Wheeler 1910; Hölldobler and Wilson 1990; Smith et al. 2008b; Beekman and Oldroyd 2019).
Despite a clear functional separation, the extent of morphological differentiation between the two castes nonetheless varies widely. The queen caste in many ant species is morphologically and physiologically distinct from the worker caste. Such workers are not only smaller in size, but they also lack sperm storage organs (spermathecae) and often functional ovaries, rendering them completely sterile and incapable of laying eggs (Keller et al. 2014; Trible and Kronauer 2017). In some species, workers retain reproductive potential but exhibit subtle and consistent differences from the queen caste. If the queen is missing, such workers may ascend to be the dominant reproductive females (Powell and Tschinkel 1999; Penick et al. 2021). In a minority of ant species, the two castes are not morphologically or physiologically well differentiated (Peeters 1991). Rarely, species have secondarily lost one of these castes (Peeters and Ito 2001; Monnin and Peeters 2008; Goudie and Oldroyd 2018).
Regardless of the degree of caste dimorphism, the colony must be informed of the presence of the queen or the dominant reproductive female to function optimally (Keller and Nonacs 1993). Communication in ant colonies is primarily chemical in nature, which is related to their ancestral subterranean habits (Tschinkel 2015, 2021), although visual, vibrational, and tactile signals or cues are also deployed in some instances (Hölldobler and Wilson 1990; Hölldobler 1995; Barbero et al. 2009; Golden and Hill 2016; Knaden 2019; Yilmaz and Spaethe 2022).
Chemical compounds used for intraspecific communication are termed pheromones (Karlson and Lüscher 1959). The potential durability and transmissibility of pheromones may have helped ants to evolve massive colony sizes and become the dominant invertebrates in most terrestrial ecosystems (Schultheiss et al. 2022). A variety of pheromones are deployed by ants, exemplified by alarm pheromones for threat alerts and trail pheromones for navigational guidance between various locations (Jackson and Ratnieks 2006; Grüter and Keller 2016; Vargo 2019). The studies of ant pheromones have not only deepened our knowledge of their social biology but also inspired such seemingly distinct efforts as designing network computation algorithms (Bonabeau et al. 2000).
In many eusocial insects, although the queen(s) does not actively participate in the colony maintenance and brood-rearing tasks, her presence has dramatic effects on worker brood care activities, nestmate discrimination, developmental trajectories of larvae, and colony coherence, reproductive output, and social structure (Wilson 1971; Hölldobler and Wilson 1990; Kocher and Grozinger 2011; Matsuura 2012; Ayasse and Jarau 2014; Vollet-Neto et al. 2018).
Most, if not all, of these effects are mediated through queen pheromones, a set of pheromones either exclusively produced by the queen or produced in higher quantities compared to workers (Sramkova et al. 2008; Smith et al. 2012b; Traynor et al. 2014). Thus, much like invisible wires that connect the queen to the rest of her colony and extend her influence, queen pheromones are key regulators of colony ontogeny and the emergent superorganismal features of insect societies (Wheeler 1910) (Fig. 1).
Summary of generalized functional properties of queen pheromones in ants, as exemplified in a polygyne Solenopsis invicta colony. These functions include: 1) Attracting workers to the queen; 2) Promoting colony maintenance tasks, such as brood care; 3) Inducing nestmate discrimination; 4) Inhibiting larval sexual development, through worker behaviors; 5) Inhibiting adult reproductive physiology, thereby preventing dealation, ovarian development, and egg laying in other queens, gynes, and workers; 6) Inducing worker policing (in many cases, eggs serve as agents for the dissemination of queen pheromones); 7) Inducing the execution of supernumerary queens in monogyne colonies; 8) Mediating the regulation of queen acceptance in polygyne colonies. Gray numbers indicate functional categories where a chemical basis has not yet been demonstrated
Debates remain regarding the function and mode of action of queen pheromones, which were at times considered to comprise means of queen control over reproductive activities of nestmates (Fletcher and Ross 1985) but are now widely acknowledged to be honest signals of identity and quality of the queen (Keller and Nonacs 1993; Villalta et al. 2018). Queen pheromone-induced behavioral shifts in workers thus may be explained ultimately by the alignment of their evolutionary interests with the queen’s from a kin selection perspective (Hamilton 1964; Foster et al. 2006). Workers are expected to achieve a higher inclusive fitness return associated with the ecological benefits of colony life if they help rear sisters rather than produce their own offspring in social hymenopterans, especially when the colony is headed by a single monogamous queen, the presumed ancestral condition for most eusocial insect lineages (Hughes et al. 2008; Boomsma and Gawne 2018; Kay et al. 2019). Thus, when the kinship structure changes following loss of the queen, workers prioritize their direct fitness and compete for reproduction pending establishment of a new reproductive hierarchy, rather than performing colony maintenance or other duties (Bourke 1988; Wenseleers et al. 2020b). Exceptions occur in species with obligately sterile workers, where workers are selected to maximize the rate of sexualization of the remaining brood because, by definition, they lack any route for direct fitness returns.
Pheromones can be categorized generally as having either releaser or primer effects. Releaser effects include immediate, typically behavioral responses, while primer effects are slower and progressive, often physiological responses (Conte and Hefetz 2008; Vargo 2019). A difficulty in deciphering queen pheromones stems from their multifunctional and multicomponent characteristics: at times, multiple molecules synergistically trigger a response, while in other instances, a single compound can elicit both releaser and primer effects (Grozinger et al. 2007).
Thus far, only the queen pheromones in honey bees are sufficiently well studied that a systematic understanding linking the identities of constituent chemicals, modes of action, and known functions has emerged (Keeling et al. 2003; Bortolotti and Costa 2014; Princen et al. 2019). Despite over seven decades of effort, we have only identified pheromonal compounds in a few ant species that were associated with some, but not all, known and expected functions of queen pheromones. Meanwhile, the past decade has seen considerable progress of queen pheromone identification in otherwise understudied groups of social insects such as sweat bees, wasps and termites.
This review provides a comprehensive discussion of functional properties of queen pheromones in ants, structured with a strong focus on bioassay results and the underlying natural history and sociobiology of ant colonies, which is lacking in recent reviews of similar topics (Table 1). Despite the evident limited focus on ants, the pheromonal effects discussed are largely congruent with other social insects (Table 2). I first cover the effects of queen pheromones on colony integrity and coherence. I then discuss how queen pheromones help to maintain the reproductive dominance of the queens as well as the overall reproductive division of labor in a colony. Next, I review recent studies on how queen pheromones mediate the regulation of colony social structures, specifically regarding the number of queens in the colony. Lastly, I discuss general characteristics of queen pheromones and highlight unanswered questions as a guide to future research regarding eusocial insect queen pheromones (Box 1).
Effects on colony integrity and coherence
Induction of retinue and rescue behavior
Upon examining the queen in an ant colony, one notices that she is surrounded by “retinue” workers that often lick her cuticle or otherwise groom her, a scenario commonly observed in other social insects such as honeybees and termites (Wilson 1971; Bortolotti and Costa 2014). A prominent releaser effect of the queen pheromone is attraction, enabling workers to locate the queen, so as to care for or rescue her. In a way, the queen acts as the “gravity center” that holds the colony together. This notion is evident in the rock ant Temnothorax rugatulus, a frequently emigrating species; when a colony was experimentally split into two nests under equal conditions, all members almost always reunited with the queen in one of the nests (Doering and Pratt 2016).
Early investigations pointed to the existence of chemical pheromones by showing that queens were attractive to their workers, and that this attraction capacity could be transferred in chemical extracts. Pheidole workers would adopt corpses of Lasius queens treated with Pheidole queen extracts (Stumper 1956). Queenless workers of Myrmica will generally accept queens of a closely related species (Brian 1986a, 1988a, b), which is likely due to the similarity of the chemical makeup of queen recognition signals among recently diverged taxa, a feature that ultimately may pave the way for the rise of social parasites (Lenoir et al. 2001). The chemical basis of retinue behavior pheromones was verified from experiments showing the same effect by queen corpses or queen cuticular extracts in a wide range of taxa (Watkins and Cole 1966; Jouvenaz et al. 1974; Fowler and Roberts 1982; Hölldobler and Wilson 1983; Wilson and Hölldobler 1985).
Fire ant workers (Solenopsis invicta) exhibit emphatic and swift responses to a queen exposed outside of the colony: workers will (1) quickly be attracted to her, (2) cluster around her, (3) move brood items to or around her, (4) form a pheromonal trail that the queen can follow back to the nest, and/or (5) pull the queen towards the nest, should she not move voluntarily (Glancey et al. 1983). Workers exhibit the same series of stereotyped behaviors toward a paper dummy dosed with reproductive queen hexane extract as they do toward live queens or fresh queen corpses, i.e., collectively retrieving the treated dummy into the nest and keeping it there for hours (Trible and Ross 2016; Zeng et al. 2022). Additionally, queenless workers infrequently exhibit vibrant body shaking upon retrieving a queen (Zeng, personal observation), which resembles the jerking response to a queen or king in termites (Funaro et al. 2018, 2019).
A general correlation between the intensity of attraction and the fecundity or weight of a queen was noted by many studies (Sommer and Hölldobler 1995; Hannonen et al. 2002). Such a trend is pertinent to other queen pheromone effects in the discussion that follows, and is most parsimoniously explained by a higher level of pheromone production occurring in more fertile queens (Fletcher and Blum 1983a). This collective, accurate assessment of the fertility condition of an individual is fundamental to the signaling function of queen pheromones (Keller and Nonacs 1993).
Promotion of colony maintenance
In many ant species, a newly mated queen performs all necessary tasks to initiate the growth of the colony by herself. She prepares a nest site (e.g., digs a burrow in the soil) and raises the first cohort of workers from the eggs she then lays. But when worker adults emerge, the queen stops engaging in colony maintenance tasks and transitions to acting strictly as an egg layer (Cassill et al. 2002; Majidifar et al. 2022). The continued presence of the queen not only ensures the steady production of fertilized eggs and, subsequently, additional brood and adults, but a few studies suggest that the queen also boosts the level of worker activity in brood care and acts to maintain the cohesion of the colony. In other words, the queen (or queen pheromones) may function as a “catalyst” to promote colonial development and maintenance, on top of being a “gravity center” to maintain colony cohesion, as shown in the studies below.
In broodless colonies of Cataglyphis cursor, overall worker activities were reduced after queen removal (Berton et al. 1992). Queenright workers of Myrmica sp. and Manica sp. antennated the brood more often and stayed longer with the brood than queenless workers (Vienne et al. 1998). In contrast, queenless workers tend to leave the nest, interacting more often with adult workers instead of the brood. Similarly, in Atta sexdens, workers departed more frequently from the nest, exhibited higher mortality and lowered refuse accumulation, but showed no change in foraging efficiency, when the queen was removed (Della Lucia et al. 2003; Sousa-Souto and Souza 2006). In Temnothorax curvispinosus, queenright sub-colonies outperformed queenless counterparts in various task efficiencies and were more resistant to fungal pathogens (Keiser et al. 2018). In Temnothorax crassispinus, queen presence promoted defecation within the nest which may help to suppress mold (Giehr et al. 2019).
These studies showed that the presence of the queen induced a higher level of brood care, stronger cohesion, and better overall performance of the colony. At the proximate level, these shifts in worker behavior might be manifested as secondary effects of the attractiveness of the queen causing, for instance, a more structured spatial distribution of the brood and, as a result, more efficient brood care. These results are consistent with findings in honeybees where queen mandibular pheromones are shown to stimulate a wide range of worker task performance, including foraging, defense, nest building, and brood rearing (Bortolotti and Costa 2014). However, unlike honey bees, these effects in ants remained to be associated with a chemical basis (Box 1, Q1).
Induction of nestmate discrimination
Similar to other eusocial insects, the nest of an ant colony comprises the physical structure, food storage, and brood items. It requires substantial investment to build and is valuable to looting and raiding, often from sympatric conspecific colonies (Holldobler and Michener 1980; Tschinkel 1992; Sturgis and Gordon 2012). To safeguard the nest from intruders and ensure sustainable growth, a colony must deploy effective nestmate discrimination. Each ant colony carries a set of cuticular chemical odor labels, which workers use to distinguish nestmate from non-nestmate conspecifics (Ozaki et al. 2005; Sturgis and Gordon 2012).
The presence of the queen has a significant impact on the odor label of a colony, such that queenless colonies exhibit reduced territoriality, acting as if they have lost some component of their distinct colony identity. Compared to queenless workers, workers from queenright colonies are more subject to aggression by non-nestmate conspecific workers, as well as being more aggressive themselves towards such workers.
Queenless S. invicta workers of the monogyne social form (single queen per colony) received little aggression from their original nestmates when returned to the natal queenright colony. However, if they tended a foreign queen for only 15 minutes, they were attacked by their original nestmates (Obin and Vander Meer 1989). Queenless workers also became less aggressive themselves after queen removal, becoming completely docile after about two weeks (Vander Meer and Alonso 2002). Thus, in monogyne fire ants, a colony’s unique chemical identity is attributable, at least in part, to its sole reproductive queen.
Similar phenomena were demonstrated in other species. Queenless workers of Cataglyphis niger did not show aggression towards and did not receive aggression from original nestmate workers from the queenright parent colony (Lahav et al. 1998). In Camponotus species, worker aggression towards non-nestmates largely disappeared in queenless colonies and reappeared after an unrelated queen was adopted into the colony (Carlin and Hölldobler 1983, 1986, 1987). Additionally, queens with less developed ovaries or that were incompletely inseminated had a weaker such effect than normal queens (Carlin and Hölldobler 1987).
However, as a counterexample, queen presence had little impact on nestmate discrimination in Camponotus aethiops, a discrepancy that was attributed to the presence of heritable chemical cues on workers (van Zweden et al. 2009). Another example came from Rhytidoponera confusa, where nestmate discrimination also was not affected by the presence of the queen (Crosland 1990). Queen-worker dimorphism in R. confusa is not pronounced and the colonies can reproduce without the queen (Ward 1981, 1983). Because the queen is not an indispensable component of the colony identity, it is plausible that her absence does not impact worker nestmate recognition abilities (Carlin and Hölldobler 1991).
These results point to the intricate nature of nestmate discrimination in ant colonies, which is governed by both environmentally derived cues and genetic factors (Carlin and Hölldobler 1983; Helanterä et al. 2011; Sturgis and Gordon 2012; Caliari Oliveira et al. 2022). Unraveling the specific mechanisms by which queen pheromones affect nestmate discrimination remains a challenge (Box 1, Q2). One possibility is that workers obtain queen cuticular odor cues in small colonies through common contact with the queen (Lahav et al. 1998). Another possibility is that queenless workers retain the ability to detect non-nestmates but lack the incentives to act aggressively toward them.
Maintenance of reproductive dominance
Inhibition of larval sexual development
As the hallmark of eusociality, reproductive division of labor necessitates that members of the queen caste maintain their reproductive dominance. In small colonies, the queen may manipulate larval caste fate and suppress adult reproduction through physical actions (Heinze and Smith 1990), which is frequently observed in wasp societies where caste dimorphisms are not distinct (Ross and Matthews 1991). However, in populous colonies, the effect is often achieved via an essential and well-studied class of queen primer pheromones that exert their effects onto almost all life stages of female colony members (Smith and Liebig 2017; Holman 2018).
To begin with, queen pheromones have been shown to inhibit sexualization (development as queens) of female larvae, thus biasing female development toward worker production over gyne (virgin winged queen) production.
Early studies on the subject came from Myrmica species, where the queen induces improved larval survival, earlier pupation, and lower larval and pupal weights, in apparent accord with the notion of the queen stimulating general colony function and cohesion (Brian 1957, 1986b; Brian and Carr 1960). When the queen was present, large gyne-destined larvae received less care from workers than small worker-destined larvae (Brian and Hibble 1963); moreover, the workers lethally bite gyne-destined larvae (Brian and Carr 1960; Brian 1973). The presumed pheromone was not volatile while, notably, structural and topological features of the queen played a role in the pheromonal effect (Brian 1970, 1973).
In the Pharaoh’s ant, Monomorium pharaonis, the presence of a fertile queen inhibited development of sexual brood (Petersen-Braun 1975, 1977). Unlike the case in Myrmica, this effect was disseminated specifically by the queen-laid eggs, but not queen corpses or solvent extracts (Berndt and Nitschmann 1979; Edwards 1987; Boonen and Billen 2017). Queenright workers always accept worker broods from a foreign colony but would cannibalize any introduced sexual broods (Edwards 1991). Monocyclic diterpene neocembrene, a compound produced only by egg-laying queens, was found to be a pheromonal component in M. pharaonis as it elicited weak “queen retinue” attraction as well as inhibited production of sexuals (Edwards and Chambers 1984; Oliveira et al. 2020).
Evidence from the two above examples and many other species strongly suggested that queen pheromones affect caste development through the behavior of workers, including biting the larvae to suppress growth evident by bite marks on larvae, or simply killing sexualized larvae (see Supplementary Information for more details; Table 1; Box 1, Q3). This is also the case in honey bees and stingless bees where workers control the caste fate of larvae, besides genetic and maternal factors (Bueno et al. 2023).
Adult workers probably sense queen pheromones mainly through antennal chemoreceptors, which was supported by strong electrophysiological responses of worker antennae towards queen extracts and candidate queen pheromones (D’Ettorre et al. 2004b; Holman et al. 2010; de Narbonne et al. 2016). Following perception, workers respond to queen pheromones by actively suppressing the sexual development of some larvae via the behaviors above (Box 1, Q4).
Inhibition of reproductive physiology of adult females
As the best studied effect in many groups of social insects, queen pheromones suppress physiological changes tied to the onset of reproduction in adult females in the colony, which can be measured in diverse behavioral and physiological changes in the trajectory of reproductive development, including dealation, ovarian activation, weight gain, and finally egg laying.
Dealation, or wing shedding, is the first observable indication of the onset of reproduction development in adult gynes in many ants. Take the example of S. invicta, virgin gynes in queenright colonies typically remain winged until a mating flight event, after which the newly mated gynes kick off their wings to initiate colony funding underground (Tschinkel 2013). However, these gynes can dealate as soon as 12 h after separation from fertile queens, with their alary muscles beginning to histolyze simultaneously followed by ovarian development, and oviposition starts in another 2 to 3 days (Fletcher and Blum 1981, 1983b; Vargo and Laurel 1994). By this time, the gyne begins to exhibit attractiveness in the formation of a queen retinue and to produce the inhibitory pheromones (Vargo 1999).
The fecundity of a queen is correlated to her weight and the ability to suppress reproductive development in nestmate queens. The correlation is likely due to a link between weight and the level of pheromone production (Fig. 2). Evidence comes from the fact that queenless monogyne workers consistently recognized and adopted the heavier queen of two presented as their new queen (Fletcher and Blum 1983a). Corpses of heavier queens suppressed dealation for longer than light-weight queen corpses (Fletcher and Blum 1983b; Willer and Fletcher 1986). Such an inhibitory effect acts on other egg-laying reproductive queens as well: the addition of live queens or queen corpses reduced fecundity of all nestmate queens in polygyne colonies (Vargo and Laurel 1994).
Perception of queen pheromones via the antennal sensilla leads to a downregulation of dopamine production, which in turn suppresses the production of juvenile hormones (JH) and inhibits reproductive development (Robinson and Vargo 1997; Boulay et al. 2001). JHs are critical regulators not only of reproduction, but of development and behavior, throughout the lifecycle of insects (Jindra et al. 2013). Topical treatment of alate gynes with JH or JH analogue induced dealation in S. invicta even in the presence of the queen, overriding the inhibitory effect of the queen pheromone (Vargo and Laurel 1994). Notably, JH treatment can yield opposing effects on reproductive development depending on the species and the size of the treatment doses used, suggesting a condition-dependent cost of JH and calling for more studies on the endocrinological regulation of reproduction (Robinson and Vargo 1997; Cuvillier-Hot et al. 2004; Penick et al. 2011; Holman 2012).
The inhibitory effects on worker reproduction received detailed studies in Camponotus, where eggs are again the dissemination agents of the pheromones. The addition of queen-laid eggs prohibited workers from laying eggs in queenless colonies; in addition, worker-laid eggs were less prone to destruction when applied with queen cuticular hydrocarbons (CHCs) (Endler et al. 2004). As a further support, surface chemical profiles of eggs corresponded to the cuticular chemical profiles of the respective egg-laying queen or worker (Endler et al. 2006).
In Lasius species, the compound 3-methylhentriacontane (3-MeC31) suppressed egg-laying of workers, making it the first identified queen pheromone with inhibitory effects on worker reproduction (Holman et al. 2010, 2013; Holman 2012). Further analysis indicated a slower evolution of this compound compared to other CHCs in Lasius, hinting at potential evolutionary constraints on queen signals, but did not agree with the findings in Temnothorax species (Brunner et al. 2011; Holman et al. 2013). Other studies have shown inhibitory effects on worker reproductive physiology by live queens, queen corpses, queen-laid eggs, or queen CHCs across various ant taxa (Table 1; Supplementary Information; Box 1, Q5).
Another general effect of such inhibitory pheromones is a shortening of longevity. Reproduction and longevity are typically a trade-off in animals (De Loof 2011; Blacher et al. 2017). However, in social insects, reproduction and longevity are instead positively linked (Blacher et al. 2017), perhaps due to a reproductive division of labor where queens are liberated from costly daily tasks. When worker ants become reproductively active after queen removal, they also showed extended lifespans, as documented in some ant species as well as in other social insects (Tsuji et al. 1996; Kohlmeier et al. 2017; Vollet-Neto et al. 2018; Majoe et al. 2021; Negroni et al. 2021).
Harpegnathos saltator workers are fully capable of reproduction (Peeters et al. 2000). Removal of the queen (and her pheromones) prompted workers to engage in antennal duels, a ritualistic competition to re-establish hierarchy that occurs in many ponerine species (Powell and Tschinkel 1999; Peeters et al. 2000; Penick et al. 2014). Winners of these duels transition into gamergates (Sasaki et al. 2016), which had about five times the lifespan of normal workers (Yan et al. 2022). These gamergates displayed queen-like physiology, with decreased brain and optic lobe volumes, and decreased venom production. They also behaved more like queens, remaining inside the nest and hiding from intruders (Penick et al. 2021). Nevertheless, these queen-like traits can revert back to a worker-like state if a gamergate is exposed to a strong source of queen pheromones (Penick et al. 2021), such is the case in other social insects (Van Oystaeyen et al. 2014).
Induction of worker policing
Although the queen is the dominant reproductive member, workers in many ant species can potentially produce males by laying unfertilized eggs. These egg-laying workers pose a source of conflict over male parentage within the colony, as well as a cost to the colony productivity (Helanterä and Sundström 2007; Bourke and Franks 2019). The queen(s) represses reproduction of workers through pheromonal inhibition as discussed above, or through behaviors such as destruction of worker-laid eggs (Bourke 1991). Workers themselves also police reproduction of nestmate workers, a behavior documented in many social hymenopterans that might have evolved concurrently with eusociality (Ratnieks 1988; Frank 1995, 2003; Wenseleers et al. 2020a).
Typical acts of policing in social insect colonies include direct aggression toward adults or destruction of their eggs (Ratnieks and Visscher 1989; Beekman and Oldroyd 2005). Mechanistically, workers must (i) recognize queen presence through queen pheromones and (ii) correctly assess the fertility status of colony members, as well as recognize the origin of offspring through pheromones present on the egg surface or post-embryonic cuticle (Ratnieks 1995; Oi et al. 2015b). In some species, queens actively mark suspect individuals with pheromones to “command” worker policing.
Pachycondyla workers would lay viable embryonated eggs when the workers were physically separated from the queen (Dietemann and Peeters 2000). Worker-laid eggs were eaten more frequently by nestmate workers than queen-laid eggs, and such policing was more prominent when the queen was present (D’Ettorre et al. 2004a). Notably, the potential pheromonal cues by which workers distinguish egg origin were persistent and non-transferable through mutual contact between the eggs (D’Ettorre et al. 2006).
Workers in the genus Formica could distinguish nestmate eggs from non-nestmate eggs (Helanterä and Ratnieks 2009; Helanterä et al. 2014), and worker-laid eggs from queen-laid eggs, but the latter ability was displayed only when an adult queen was present (Helanterä and Sundström 2005, 2007). Corresponding to the above finding, hydrocarbon profiles of eggs displayed robust and consistent differences among species, colonies, and even among matrilines within a colony, demonstrating a link between genetic variation and potential pheromonal variation (Helanterä et al. 2014; Helanterä and d’Ettorre 2015).
In some cases, the egg-laying workers themselves, but not their eggs, were subject to policing. In Temnothorax unifasciatus, that reproductive workers were attacked, not by random nestmates, but only by a select few workers who would become dominant reproductives upon queen removal (Stroeymeyt et al. 2007). Likewise, in Novomessor cockerelli (previously Aphaenogaster cockerelli), worker-laid eggs did not differ from queen-laid eggs in their surface chemical profiles and were not policed (Smith et al. 2008a). Instead, egg-laying workers were attacked by nestmate workers (Smith et al. 2011). Reproductive status is signaled by unbranched alkanes, as the application of these compounds on non-reproductive workers induced nestmate aggression, but only in the presence of a queen (Smith et al. 2009). The queen also attacked and marked reproductive workers for aggression by discharging compounds from her Dufour’s gland onto the target worker (Smith et al. 2012a). This is similar to the finding in Dinoponera quadriceps, where high-ranking gamergates mark challengers with Dufour’s gland secretion to direct aggression by low-ranking workers (Monnin et al. 2002).
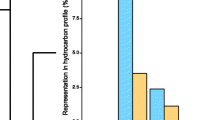
In Odontomachus brunneus, a hydrocarbon, (Z)-9-nonacosene, was identified as a fertility signal, based on three lines of evidences: (i) its higher abundance in reproductive individuals, (ii) the typical submissive gesture of nestmate workers towards workers treated with the compound (Fig. 3), and (iii) the nestmate policing (biting and pulling) of treated workers in queenright colonies (Medeiros et al. 1992; Smith et al. 2012b, 2013). The role of this compound was conserved across geographic populations, but it must function synergistically with other pheromonal chemicals (Smith et al. 2013, 2015).
Induction of execution of superfluous reproductive adults
A queenright monogyne colony is generally not expected to accept additional reproductive queens as this would decrease indirect fitness benefits to workers despite the apparent benefit of larger social groups (Hamilton 1964; Gardner et al. 2011). In species with strong caste dimorphism, superfluous queens are eliminated by workers, which may be considered as an extreme form of policing, as these queens cannot transition back to a worker-like state and contribute to colony tasks. As a requirement, workers rely on queen pheromones that signal the presence and identity of the true queen.
Monogyne Solenopsis invicta workers imprint on the pheromonal signature of their mother queen, killing any other dealate (wingless reproductive) queens presented to the colony (Fletcher and Blum 1983a; Gotzek and Ross 2007). Only when a colony is rendered queenless for a few days will it accept an unrelated queen, and the longer the colony stays queenless, the more accepting of a foreign queen it becomes (Fletcher 1986; Vander Meer and Alonso 2002). Additionally, when presented with multiple reproductive queens, such hopelessly queenless workers usually select the most physogastric one, which might be due to a higher amount of fertility signal produced by such queens (Fletcher and Blum 1983a).
In Aphaenogaster senilis, workers attack supernumerary gynes and only the oldest gyne ascends to become the sole reproductive queen (Chéron et al. 2009). In Argentine ants, Linepithema humile, queenless colonies show lower aggression towards intruder queens compared to queenright colonies, which usually kill intruder queens within 24 h (Vásquez and Silverman 2008). Here, adoption decisions were not influenced by fecundity, but by similarity of CHC profile to the nestmate queens (Vásquez and Silverman 2008; Vásquez et al. 2008). This was contrary to Camponotus floridanus, where a more fertile queen would be accepted into a queenright colony while a less fertile queen would not (Moore and Liebig 2010). Thus, there appears to be variable linkages between queen pheromones involved in nestmate recognition, fertility signaling, and regulation of reproduction in ants.
Regulation of colony social structure
An archetypical colony of social insects is composed of a single family, headed by a single queen and her offspring workers. It is less known that multiple-queen colonies occur in many ant taxa and such variation of colony social structure, being either monogyne (single-queen) or polygyne (polygyne), exist both within species and across species. Phylogenetic analysis suggested that eusociality of ants evolved under the monogyne condition, while polygyne forms subsequently evolved independently in many ant taxa (Ross and Carpenter 1991; Hughes et al. 2008). In a general sense, the evolution of social structure (from monogyny to polygyny) and the evolution of eusociality in ants, raised similar problems as to why individuals are willing to forgo personal reproductive success for the benefit of group reproductive output.
Queen pheromones are also involved in the regulation of such variation in colony social structure, an important but often overlooked class of function. Although this function of queen pheromones may be ubiquitous in diverse ant taxa (Hölldobler and Carlin 1985; Evison et al. 2012; Abril and Gómez 2019), the only such case that has received careful study to date is the regulation of colony social form in S. invicta (Box 1, Q6).
In stark contrast to the single-queen, monogyne form, the polygyne form houses multiple reproductive queens, as many as a few hundred, in a colony. The two social forms are distinct from each other in many other natural history traits, such as nest density in the wild, the average weights of alate gynes and reproductive queens, colony founding mode, and worker size distributions (Keller and Ross 1995; Gotzek and Ross 2007; Tschinkel 2013; Huang and Wang 2014). The genetic underpinning of this social form polymorphism in S. invicta and several congeners is an inversion-based selfish genetic element termed the Social b (Sb) supergene. The element spans a large portion of chromosome 16, comprising three adjacent inversions and encompassing over 500 described genes (Yan et al. 2020; Stolle et al. 2022; Helleu et al. 2022).
In monogyne colonies, all female members are homozygous for alternate, wild-type haplotype (SB), and only one SB/SB reproductive queen is tolerated. In polygyne colonies, all reproductive queens and over half of the worker population are heterozygous at the supergene locus. Polygyne workers enforce this striking genotype composition of their queens in a green-beard fashion, accepting additional Sb-carrying queens but executing SB/SB queens, including nestmate SB/SB gynes shortly after they emerge as adults (Ross and Keller 1998; Keller and Ross 1998).
The pheromonal basis of the supergene genotype signal was first demonstrated by the findings that polygyne workers rubbed against SB/SB queens were attacked by their nestmate workers (Keller and Ross 1998). Specific cuticular hydrocarbons were found to be uniquely present on the cuticle of SB/Sb queens, the abundance of which increased as the fertility of the queen increased (Eliyahu et al. 2011). Trible and Ross (2016) showed that polygyne workers showed strong preferences toward polygyne queen extracts over monogyne queen extracts, confirming the presence of a supergene pheromone. Zeng et al. (2022) then showed that a complex blend of unsaturated CHCs functioned as this signal of queen supergene status to workers (Box 1, Q7). However, beyond the recognition of supergene status, the precise mechanisms by which multiple Sb-carrying queens are permitted in polygyne colonies remain elusive. A potential general explanation may be that Sb queens are perceived as identical individuals, despite substantial variation in their fertility status and material apportionment (Ross 1988).
Discussion
Ant queen pheromones exhibit a variety of functional properties revealed by experimental analysis (summarized in Fig. 1), many of which are observed in other social insects including non-hymenopteran groups (e.g., termites), illustrating that convergent functions of queen pheromones arose across multiple independent origins of eusociality (Bortolotti and Costa 2014; Oi et al. 2015b; Funaro et al. 2018; Mitaka and Akino 2021). In essence, queen pheromones convey her presence to adult members of the colony, while signaling (1) her fertility and health condition, and at times (2) her individual identity and genotype status. The informed colony members then develop or behave accordingly to optimize the efficiency and productivity of the colony, or to adjust the colony social structure. It is reasonable to predict similar fundamental roles of queen signals in the ontogeny of other social animal colonies. Importantly, such signals may not be confined to chemical compounds but could involve multiple sensory modalities, which again necessitate a thorough understanding of their natural history to make useful predictions (Orlova and Amsalem 2021).
Several functions or properties of queen pheromones described in this review are hypothetical, because the chemical nature of the putative signals was not demonstrated experimentally. In other words, the inferred functions were associated with the presence of the queen without direct causal mechanisms or links to isolated chemicals or even crude solvent extracts from queens having been established. These studies serve as useful starting points to conceive functional frameworks for identifying specific compounds, and can provide guidance to future studies. Nonetheless, one must ask why, after decades of research focused on the problem, only very few compounds have been identified and explicitly shown to induce one or more of the discussed pheromonal effects.
The successful identification of the honeybee queen mandibular pheromones reveals that characterization of a complete blend of queen pheromones requires a combination of reliable bioassays and sophisticated chemical approaches (Butler and Fairey 1964; Butler et al. 1997), the lack of which, perhaps, is slowing progress in ants. As reviewed in the previous sections, the effects of queen pheromones are often manifested through worker behaviors. Thus, evaluating worker response behaviors to candidate pheromonal compounds can be an effective approach to designing informative bioassays. In behavioral assays, it is valuable to practice blind experiments when possible to reduce observational bias and enhance validity of data. However, it is not always straightforward to devise an assay scoring system that is highly informative while rooted in a firm understanding of the natural history of the species and the natural context of the behavior. It cannot be overstated how important it is that investigators become intimately familiar with the social biology of the focal species in order to design and implement the most informative and meaningful bioassays.
Assigning a single numeric score to measure complex behavioral traits in a social context is difficult but can be achieved based on a sufficiently large dataset and validation of the biological meaning of the score through back-testing (Wild et al. 2021). Alternatively, it is often efficient to take measurements of distinct actions that are reliably quantifiable and use these directly, such as the number of antennation inspections by workers towards a treated glass slide (Dietemann et al. 2003). Another way to design an informative bioassay is by observing and quantifying behaviors unique to the study species. For instance, workers of Odontomachus brunneus display submissive gestures when a queen or other dominant individual is nearby (Fig. 3) (Medeiros et al. 1992), which was used as an indicator for screening pheromone compounds (Smith et al. 2012b, 2013, 2015).
A few characteristics of queen pheromones are helpful for the identification of their specific components. Candidate compounds are often uniquely present in the queen caste in species with pronounced caste dimorphism, and the amount of candidate compounds are usually correlated with fertility status (Holman et al. 2010, 2013). Consequently, a more fertile queen should exert a stronger pheromonal effect (Fletcher and Blum 1983a; Willer and Fletcher 1986; Ortius and Heinze 1999; Oi et al. 2015a). However, as exceptions are common in biology, these apparent general characteristics of queen pheromones do not always hold true. For instance, piperidine molecules, despite their abundance and a positive correlation with fertility, did not signal queen fertility in S. invicta; instead, trace polar compounds displayed the expected pheromonal effects (Eliyahu et al. 2011; Zeng et al. 2022).
Chemical ecologists often aspire to identify a single molecule with extensive, if not the complete array of, effects comprising the focal behavioral or other trait released or primed by the putative pheromone (Jacobson 2012; Ebrahim et al. 2023). That being said, such reductionist thinking often oversimplifies the complex chemical composition, multiple glandular and tissue origins, and variable functions of most insect pheromones, especially social insect queen pheromones exemplified by the well-studied honey bee queen pheromones (Keeling et al. 2003; Slessor et al. 2005; Symonds and Elgar 2008; Princen et al. 2019). Thus far, known glandular and cellular sources of pheromonal components in ants include the oenocytes, poison sac, postpharyngeal gland, metapleural gland, and Dufour’s gland (Vargo 1997; Vargo and Hulsey 2000; Yek and Mueller 2011; Kocher and Grozinger 2011). Multiple glandular sources of queen pheromones affecting a singular behavioral response have been demonstrated clearly in S. invicta (Vargo and Hulsey 2000), and we can expect the same for other ant species.
Many recent attempts to identify queen pheromones have focused on the CHCs (Van Oystaeyen et al. 2014; Oi et al. 2015b, a; Smith and Liebig 2017; Holman 2018) (Box 1, Q8), which is grounded in their essential signaling roles in the colony social life and the relative ease of their quantification and identification (Martin and Drijfhout 2009; Kroiss et al. 2011). While studies on CHCs remain important, more studies are starting to highlight other molecule classes playing roles of queen pheromones (Smith et al. 2016; Villalta et al. 2018; Steitz and Ayasse 2020). Once we obtain a holistic blend of identified pheromonal compounds of the proper ratio that encapsulates the full range of functional properties in even a few model ant species, with advances in the neurophysiology of odor reception and genetic basis of reproductive division of labor, we will grasp more fully how queen pheromones work to regulate colony social life of ants (Yan and Liebig 2021) (Box 1, Q9).
Data Availability
There is no data in the sense of any generated dataset. All the info is present in the text and supplementary information.
References
Abril S, Gómez C (2019) Factors triggering queen executions in the Argentine ant. Sci Rep 9:10427. https://doi.org/10.1038/s41598-019-46972-5
Alaux C, Jaisson P, Hefetz A (2004) Queen influence on worker reproduction in bumblebees (Bombus terrestris) colonies. Insectes Soc 51:287–293. https://doi.org/10.1007/s00040-004-0741-5
Ayasse M, Jarau S (2014) Chemical Ecology of bumble bees. Annu Rev Entomol 59:299–319. https://doi.org/10.1146/annurev-ento-011613-161949
Barbero F, Thomas JA, Bonelli S et al (2009) Queen ants make distinctive sounds that are mimicked by a butterfly social parasite. Science 323:782–785. https://doi.org/10.1126/science.1163583
Beekman M, Oldroyd BP (2019) Conflict and major transitions — why we need true queens. Curr Opin Insect Sci 34:73–79. https://doi.org/10.1016/j.cois.2019.03.009
Beekman M, Oldroyd BP (2005) Honeybee workers use cues other than egg viability for policing. Biol Let 1:129–132. https://doi.org/10.1098/rsbl.2005.0294
Berndt KP, Nitschmann J (1979) The physiology of reproduction in the pharaoh’s ant (Monomorium pharaonis L.) 2. Unmated Queens Insect Soc 26:137–145
Berton F, Lenoir A, Le Roux G, Le Roux A. M (1992) Effect of orphaning on the effectiveness of queen attraction and on worker behavioral repertoire in Cataglyphis cursor (Hymenoptera: Formicidae). Sociobiol
Blacher P, Huggins TJ, Bourke AF (2017) Evolution of ageing, costs of reproduction and the fecundity–longevity trade-off in eusocial insects. Proc R Soc B 284:20170380
Bonabeau E, Dorigo M, Theraulaz G (2000) Inspiration for optimization from social insect behaviour. Nature 406:39–42. https://doi.org/10.1038/35017500
Boomsma JJ, Gawne R (2018) Superorganismality and caste differentiation as points of no return: how the major evolutionary transitions were lost in translation: Superorganisms, eusociality and major transitions. Biol Rev 93:28–54. https://doi.org/10.1111/brv.12330
Boonen S, Billen J (2017) Caste regulation in the ant Monomorium pharaonis (L.) with emphasis on the role of queens. Insectes Soc 64:113–121
Bortolotti L, Costa C (2014) Chemical Communication in the Honey Bee Society. In: Neurobiology of Chemical Communication. CRC Press/Taylor & Francis, p 57
Boulay R, Cerdá X, Fertin A et al (2009) Brood development into sexual females depends on the presence of a queen but not on temperature in an ant dispersing by colony fission, Aphaenogaster senilis. Ecological Entomol 34:595–602. https://doi.org/10.1111/j.1365-2311.2009.01108.x
Boulay R, Hefetz A, Cerdá X et al (2007) Production of sexuals in a fission-performing ant: dual effects of queen pheromones and colony size. Behav Ecol Sociobiol 61:1531–1541. https://doi.org/10.1007/s00265-007-0385-3
Boulay R, Hooper-Bui LM, Woodring J (2001) Oviposition and oogenesis in virgin fire ant females Solenopsis invicta are associated with a high level of dopamine in the brain: Oviposition and oogenesis in virgin fire ant females. Physiol Entomol 26:294–299. https://doi.org/10.1046/j.0307-6962.2001.00250.x
Bourke AF, Franks NR (2019) Social evolution in ants. In: Social Evolution in Ants. Princeton University Press
Bourke AFG (1988) Worker reproduction in the higher eusocial hymenoptera. Q Rev Biol 63:291–311
Bourke AFG (1991) Queen behaviour, reproduction and egg cannibalism in multiple-queen colonies of the ant Leptothorax acervorum. Anim Behav 42:295–310. https://doi.org/10.1016/S0003-3472(05)80561-5
Bourke AFG (1993) Lack of experimental evidence for pheromonal inhibition of reproduction among queens in the ant Leptothorax acervorum. Anim Behav 45:501–509. https://doi.org/10.1006/anbe.1993.1061
Breed MD, Stiller TM, Blum MS, Page RE (1992) Honeybee nestmate recognition: effects of queen fecal pheromones. J Chem Ecol 18:1633–1640. https://doi.org/10.1007/BF00993235
Brian M (1970) Communication between queens and larvae in the ant Myrmica. Anim Behav 18:467–472
Brian MV (1986a) Bonding between workers and queens in the ant genus Myrmica. Anim Behav 34:1135–1145. https://doi.org/10.1016/S0003-3472(86)80173-7
Brian MV (1988a) The control of Myrmica rubra workers by queens of their own and other Myrmica species and the interaction between queenless groups of workers. Physiol Entomol 13:1–7. https://doi.org/10.1111/j.1365-3032.1988.tb00902.x
Brian MV (1988b) Queen selection by worker groups of the ant Myrmica rubra L. Anim Behav 36:914–925. https://doi.org/10.1016/S0003-3472(88)80174-X
Brian MV (1957) The growth and development of colonies of the ant Myrmica. Insectes Soc 4:177–190
Brian MV (1986b) The importance of daylength and queens for larval care by young workers of the ant Myrmica rubra L. Physiol Entomol 11:239–249. https://doi.org/10.1111/j.1365-3032.1986.tb00411.x
Brian MV (1973) Queen recognition by brood-rearing workers of the ant Myrmica rubra L. Anim Behav 21:691–698. https://doi.org/10.1016/S0003-3472(73)80093-4
Brian MV, Carr CAH (1960) The influence of the queen on brood rearing in ants of the genus Myrmica. J Insect Physiol 5:81–94. https://doi.org/10.1016/0022-1910(60)90034-2
Brian MV, Hibble J (1963) Larval size and the influence of the queen on growth in Myrmica. Ins Soc 10:71–81. https://doi.org/10.1007/BF02223523
Brian MV, Rigby C (1978) The trophic eggs of Myrmica rubra L. Ins Soc 25:89–110. https://doi.org/10.1007/BF02224488
Brunner E, Kroiss J, Trindl A, Heinze J (2011) Queen pheromones in Temnothorax ants: control or honest signal? BMC Evol Biol 11:55. https://doi.org/10.1186/1471-2148-11-55
Bueno FGB, dos Santos CF, Otesbelgue A et al (2023) The queens of the stingless bees: from egg to adult. Insectes Soc 70:43–57. https://doi.org/10.1007/s00040-022-00894-0
Butler CG, Callow RK, Johnston NC (1997) The isolation and synthesis of queen substance, 9-oxodec-trans-2-enoic acid, a honeybee pheromone. Proc R Soc B 155:417–432. https://doi.org/10.1098/rspb.1962.0009
Butler CG, Fairey EM (1964) Pheromones of the honeybee: biological studies of the mandibular gland secretion of the queen. J Apic Res 3:65–76. https://doi.org/10.1080/00218839.1964.11100085
Caliari Oliveira R, Van Zweden J, Wenseleers T (2022) similarities in recognition cues lead to the infiltration of non-nestmates in an ant species. J Chem Ecol 48:16–26. https://doi.org/10.1007/s10886-021-01325-3
Carlin NF, Hölldobler B (1983) Nestmate and kin recognition in interspecific mixed colonies of ants. Science 222:1027–1029
Carlin NF, Hölldobler B (1986) The kin recognition system of carpenter ants (Camponotus spp.): I. Hierarchical Cues in Small Colonies. Behav Ecol Sociobiol 19:123–134
Carlin NF, Hölldobler B (1987) The kin recognition system of carpenter ants (Camponotus spp.): II. Larger Colonies Behav Ecol Sociobiol 20:209–217
Carlin NF, Hölldobler B (1991) The role of the queen in ant nestmate recognition: reply to Crosland. Anim Behav 41:525–527. https://doi.org/10.1016/S0003-3472(05)80855-3
Cassill D, Tschinkel WR, Vinson SB (2002) Nest complexity, group size and brood rearing in the fire ant, Solenopsis invicta. Insectes Soc 49:158–163. https://doi.org/10.1007/s00040-002-8296-9
Chéron B, Doums C, Fédérici P, Monnin T (2009) Queen replacement in the monogynous ant Aphaenogaster senilis: supernumerary queens as life insurance. Anim Behav 78:1317–1325. https://doi.org/10.1016/j.anbehav.2009.08.016
Conte YL, Hefetz A (2008) Primer Pheromones in social hymenoptera. Annu Rev Entomol 53:523–542. https://doi.org/10.1146/annurev.ento.52.110405.091434
Crosland MWJ (1990) The influence of the queen, colony size and worker ovarian development on nestmate recognition in the ant Rhytidoponera confusa. Anim Behav 39:413–425. https://doi.org/10.1016/S0003-3472(05)80404-X
Cuvillier-Hot V, Lenoir A, Crewe R et al (2004) Fertility signalling and reproductive skew in queenless ants. Anim Behav 68:1209–1219. https://doi.org/10.1016/j.anbehav.2003.11.026
De Loof A (2011) Longevity and aging in insects: is reproduction costly; cheap; beneficial or irrelevant? A critical evaluation of the “trade-off” concept. J Insect Physiol 57:1–11. https://doi.org/10.1016/j.jinsphys.2010.08.018
de Narbonne MM, van Zweden JS, Bello JE, et al (2016) Biological activity of the enantiomers of 3-methylhentriacontane, a queen pheromone of the ant Lasius niger. J Exp Biol. https://doi.org/10.1242/jeb.136069
Dejean A, Passera L (1974) Ponte des ouvrières et inhibition royale chez la Fourmi Temnothorax recedens (Nyl.) (Formicidae, Myrmicinae). Insectes Soc 21:343–355. https://doi.org/10.1007/BF02331564
Della Lucia TMC, Peternelli EFO, Lacerda FG et al (2003) Colony behavior of Atta sexdens rubropilosa (Hymenoptera: Formicidae) in the absence of the queen under laboratory conditions. Behav Proc 64:49–55. https://doi.org/10.1016/S0376-6357(03)00078-0
D’Ettorre P, Heinze J, Ratnieks FLW (2004a) Worker policing by egg eating in the ponerine ant Pachycondyla inversa. Proc R Soc B 271:1427–1434. https://doi.org/10.1098/rspb.2004.2742
D’Ettorre P, Heinze J, Schulz C et al (2004b) Does she smell like a queen? Chemoreception of a cuticular hydrocarbon signal in the ant Pachycondyla inversa. J Exp Biol 207:1085–1091. https://doi.org/10.1242/jeb.00865
D’Ettorre P, Tofilski A, Heinze J, Ratnieks FLW (2006) Non-transferable signals on ant queen eggs. Naturwissenschaften 93:136–140. https://doi.org/10.1007/s00114-005-0075-9
Dietemann V, Peeters C (2000) Queen Influence on the Shift from Trophic to Reproductive Eggs Laid by Workers of the Ponerine Ant Pachycondyla Apicalis. Insectes Soc 47:223–228. https://doi.org/10.1007/PL00001707
Dietemann V, Peeters C, Liebig J et al (2003) Cuticular hydrocarbons mediate discrimination of reproductives and nonreproductives in the ant Myrmecia gulosa. Proc Natl Acad Sci USA 100:10341–10346. https://doi.org/10.1073/pnas.1834281100
Doering GN, Pratt SC (2016) Queen location and nest site preference influence colony reunification by the ant Temnothorax rugatulus. Insectes Soc 63:585–591. https://doi.org/10.1007/s00040-016-0503-1
Ebrahim SA, Dweck HK, Weiss BL, Carlson JR (2023) A volatile sex attractant of tsetse flies. Science 379:eade1877
Edwards JP (1987) Caste regulation in the pharaoh’s ant Monomorium pharaonis: the influence of queens on the production of new sexual forms. Physiol Entomol 12:31–39. https://doi.org/10.1111/j.1365-3032.1987.tb00721.x
Edwards JP (1991) Caste regulation in the pharaoh’s ant Monomorium pharaonis: recognition and cannibalism of sexual brood by workers. Physiol Entomol 16:263–271. https://doi.org/10.1111/j.1365-3032.1991.tb00565.x
Edwards JP, Chambers J (1984) Identification and source of a queen-specific chemical in the pharaoh’s ant, Monomorium pharaonis (L.). J Chem Ecol 10:1731–1747
Eliyahu D, Ross KG, Haight KL et al (2011) Venom alkaloid and cuticular hydrocarbon profiles are associated with social organization, queen fertility status, and queen genotype in the fire ant Solenopsis invicta. J Chem Ecol 37:1242–1254. https://doi.org/10.1007/s10886-011-0037-y
Endler A, Liebig J, Hölldobler B (2006) Queen fertility, egg marking and colony size in the ant Camponotus floridanus. Behav Ecol Sociobiol 59:490–499. https://doi.org/10.1007/s00265-005-0073-0
Endler A, Liebig J, Schmitt T et al (2004) Surface hydrocarbons of queen eggs regulate worker reproduction in a social insect. Proc Natl Acad Sci USA 101:2945–2950. https://doi.org/10.1073/pnas.0308447101
Evison SEF, Ferreira RS, D’Ettorre P et al (2012) Chemical Signature and Reproductive Status in the Facultatively Polygynous ant Pachycondyla Verenae. J Chem Ecol 38:1441–1449. https://doi.org/10.1007/s10886-012-0195-6
Fletcher DC (1986) Triple action of queen pheromones in the regulation of reproduction in fire ant (Solenopsis invicta) colonies. In: International Society of Invertebrate Reproduction. Int Symp 4:305–316
Fletcher DJC, Blum MS (1983a) Regulation of queen number by workers in colonies of social insects. Science 219:312–314. https://doi.org/10.1126/science.219.4582.312
Fletcher DJC, Blum MS (1981) Pheromonal Control of dealation and oogenesis in virgin queen fire ants. Science 212:73–75. https://doi.org/10.1126/science.212.4490.73
Fletcher DJC, Blum MS (1983b) The inhibitory pheromone of queen fire ants: effects of disinhibition on dealation and oviposition by virgin queens. J Comp Physiol 153:467–475. https://doi.org/10.1007/BF00612601
Fletcher DJC, Ross KG (1985) Regulation of Reproduction in Eusocial Hymenoptera. Annu Rev Entomol 30:319–343. https://doi.org/10.1146/annurev.en.30.010185.001535
Foster K, Wenseleers T, Ratnieks F (2006) Kin selection is the key to altruism. Trends Ecol Evol 21:57–60. https://doi.org/10.1016/j.tree.2005.11.020
Fowler HG, Roberts RB (1982) Entourage pheromone in carpenter ant (Camponotus pennsylvanicus) (Hymenoptera: Formicidae) Queens. J Kansas Entomol Soc 55:568–570
Frank SA (1995) Mutual policing and repression of competition in the evolution of cooperative groups. Nature 377:520–522. https://doi.org/10.1038/377520a0
Frank SA (2003) Repression of Competition and the Evolution of Cooperation. Evolution 57:693–705. https://doi.org/10.1111/j.0014-3820.2003.tb00283.x
Funaro CF, Böröczky K, Vargo EL, Schal C (2018) Identification of a queen and king recognition pheromone in the subterranean termite Reticulitermes flavipes. Proc Natl Acad Sci USA 115:3888–3893. https://doi.org/10.1073/pnas.1721419115
Funaro CF, Schal C, Vargo EL (2019) Queen and king recognition in the subterranean termite, Reticulitermes flavipes: Evidence for royal recognition pheromones. PLOS ONE 14:e0209810. https://doi.org/10.1371/journal.pone.0209810
Gamboa GJ, Reeve HK, Pfennig DW (1986) The Evolution and Ontogeny of Nestmate Recognition in Social Wasps. Annu Rev Entomol 31:431–454. https://doi.org/10.1146/annurev.en.31.010186.002243
Gardner A, West SA, Wild G (2011) The genetical theory of kin selection. J Evol Biol 24:1020–1043. https://doi.org/10.1111/j.1420-9101.2011.02236.x
Giehr J, Czaczkes TJ, Heinze J (2019) Sanitary behavior in queenright and queenless ant colonies. Behav Proc 164:86–90. https://doi.org/10.1016/j.beproc.2019.04.017
Gilley DC (2001) The Behavior of Honey Bees (Apis mellifera ligustica) during Queen Duels. Ethology 107:601–622. https://doi.org/10.1046/j.1439-0310.2001.00692.x
Glancey BM, Lofgren CS, Rocca JR, Tumlinson JH (1983) Behavior of disrupted colonies of Solenopsis invicta towards queens and pheromone-treated surrogate queens placed outside the nest. Sociobiology 7:
Gobin B, Billen J, Peeters C (1999) Policing behaviour towards virgin egg layers in a polygynous ponerine ant. Anim Behav 58:1117–1122. https://doi.org/10.1006/anbe.1999.1245
Golden TMJ, Hill PSM (2016) The evolution of stridulatory communication in ants, revisited. Insectes Soc 63:309–319. https://doi.org/10.1007/s00040-016-0470-6
Gotzek D, Ross KG (2007) Genetic regulation of colony social organization in fire ants: an integrative overview. Q Rev Biol 82:201–226. https://doi.org/10.1086/519965
Goudie F, Oldroyd BP (2018) The distribution of thelytoky, arrhenotoky and androgenesis among castes in the eusocial Hymenoptera. Insectes Soc 65:5–16. https://doi.org/10.1007/s00040-017-0597-0
Grozinger CM, Fischer P, Hampton JE (2007) Uncoupling primer and releaser responses to pheromone in honey bees. Naturwissenschaften 94:375–379. https://doi.org/10.1007/s00114-006-0197-8
Grüter C, Keller L (2016) Inter-caste communication in social insects. Curr Opin Neurobiol 38:6–11. https://doi.org/10.1016/j.conb.2016.01.002
Hamilton WD (1964) The genetical evolution of social behaviour. J Theoretical Biol 7:1–16. https://doi.org/10.1016/0022-5193(64)90038-4
Hannonen M, Sledge MF, Turillazzi S, Sundström L (2002) Queen reproduction, chemical signalling and worker behaviour in polygyne colonies of the ant Formica fusca. Anim Behav 64:477–485. https://doi.org/10.1006/anbe.2002.4001
Heinze J, Smith TA (1990) Dominance and fertility in a functionally monogynous ant. Behav Ecol Sociobiol 27:1–10. https://doi.org/10.1007/BF00183306
Helanterä H, d’Ettorre P (2015) A comparative study of egg recognition signature mixtures in Formica ants. Evolution 69:520–529. https://doi.org/10.1111/evo.12590
Helanterä H, Lee YR, Drijfhout FP, Martin SJ (2011) Genetic diversity, colony chemical phenotype, and nest mate recognition in the ant Formica fusca. Behav Ecol 22:710–716. https://doi.org/10.1093/beheco/arr037
Helanterä H, Martin SJ, Ratnieks FLW (2014) Recognition of nestmate eggs in the ant Formica fusca is based on queen derived cues. Curr Zool 60:131–136. https://doi.org/10.1093/czoolo/60.1.131
Helanterä H, Ratnieks FLW (2009) Two independent mechanisms of egg recognition in worker Formica fusca ants. Behav Ecol Sociobiol 63:573–580. https://doi.org/10.1007/s00265-008-0692-3
Helanterä H, Sundström L (2005) Worker reproduction in the ant Formica fusca. J Evolution Biol 18:162–171. https://doi.org/10.1111/j.1420-9101.2004.00777.x
Helanterä H, Sundström L (2007) Worker policing and nest mate recognition in the ant Formica fusca. Behav Ecol Sociobiol 61:1143–1149. https://doi.org/10.1007/s00265-006-0327-5
Helleu Q, Roux C, Ross KG, Keller L (2022) Radiation and hybridization underpin the spread of the fire ant social supergene. Proc Natl Acad Sci USA 119:e2201040119. https://doi.org/10.1073/pnas.2201040119
Hölldobler B (1995) The chemistry of social regulation: multicomponent signals in ant societies. Proc Natl Acad Sci USA 92:19–22. https://doi.org/10.1073/pnas.92.1.19
Hölldobler B, Carlin NF (1985) Colony Founding, queen dominance and oligogyny in the Australian Meat Ant Iridomyrmex purpureus. Behav Ecol Sociobiol 18:45–58
Hölldobler B, Carlin NF (1989) Colony founding, queen control and worker reproduction in the ant Aphaenogaster (= Novomessor) cockerelli (Hymenoptera: Formicidae). Psyche 96:131–151
Holldobler B, Michener CD (1980) Mechanisms of identification and discrimination in social Hymenoptera. Mechanisms of identification and discrimination in social Hymenoptera 35–58
Hölldobler B, Wilson EO (1990) The ants. Belknap Press of Harvard University Press, Cambridge, Mass
Hölldobler B, Wilson EO (1983) Queen control in colonies of weaver ants (Hymenoptera: Formicidae). Ann Entomol Soc Am 76:235–238. https://doi.org/10.1093/aesa/76.2.235
Holman L (2018) Queen pheromones and reproductive division of labor: a meta-analysis. Behav Ecol. https://doi.org/10.1093/beheco/ary023
Holman L (2012) Costs and constraints conspire to produce honest signaling: insights from an ant queen pheromone. Evolution 66:2094–2105. https://doi.org/10.1111/j.1558-5646.2012.01603.x
Holman L, Jørgensen CG, Nielsen J, d’Ettorre P (2010) Identification of an ant queen pheromone regulating worker sterility. Proc R Soc B 277:3793–3800. https://doi.org/10.1098/rspb.2010.0984
Holman L, Lanfear R, d’Ettorre P (2013) The evolution of queen pheromones in the ant genus Lasius. J Evol Biol 26:1549–1558. https://doi.org/10.1111/jeb.12162
Holman L, van Zweden JS, Oliveira RC et al (2017) Conserved queen pheromones in bumblebees a reply to Amsalem et al. PeerJ 5:e3332. https://doi.org/10.7717/peerj.3332
Hoover SER, Keeling CI, Winston ML, Slessor KN (2003) The effect of queen pheromones on worker honey bee ovary development. Naturwissenschaften 90:477–480. https://doi.org/10.1007/s00114-003-0462-z
Huang YC, Wang J (2014) Did the fire ant supergene evolve selfishly or socially? BioEssays 36:200–208. https://doi.org/10.1002/bies.201300103
Hughes WOH, Oldroyd BP, Beekman M, Ratnieks FLW (2008) Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science 320:1213–1216. https://doi.org/10.1126/science.1156108
Ichinose K, Boulay R, Cerdá X, Lenoir A (2009) Influence of queen and diet on nestmate recognition and cuticular hydrocarbon differentiation in a fission-dispersing ant. Aphaenogaster Senilis Jzoo 26:681–685. https://doi.org/10.2108/zsj.26.681
Ikan R, Gottlieb R, Bergmann ED, Ishay J (1969) The pheromone of the queen of the Oriental hornet, Vespa orientalis. J Insect Physiol 15:1709–1712. https://doi.org/10.1016/0022-1910(69)90003-1
Imperatriz-Fonseca VL, Zucchi R (1995) Virgin queens in stingless bee (Apidae, Meliponinae) colonies: a review. Apidologie 26:231–244. https://doi.org/10.1051/apido:19950305
Ishay I, Ikan R, Bergmann ED (1965) The presence of pheromones in the Oriental hornet, Vespa orientalis F. J Insect Physiol 11:1307–1309. https://doi.org/10.1016/0022-1910(65)90123-X
Jackson DE, Ratnieks FLW (2006) Communication in ants. Curr Biol 16:R570–R574. https://doi.org/10.1016/j.cub.2006.07.015
Jacobson M (2012) Insect sex pheromones. Elsevier
Jarau S, van Veen JW, Aguilar I, Ayasse M (2010) A scientific note on virgin queen acceptance in stingless bees: evidence for the importance of queen aggression. Apidologie 41:38–39. https://doi.org/10.1051/apido/2009045
Jindra M, Palli SR, Riddiford LM (2013) The Juvenile hormone signaling pathway in insect development. Annu Rev Entomol 58:181–204. https://doi.org/10.1146/annurev-ento-120811-153700
Jouvenaz DP, Banks WA, Lofgren CS (1974) Fire ants: attraction of workers to queen secretions. Ann Entomol Soc Am 67:442–444
Kaminski L-A, Slessor KN, Winston ML et al (1990) Honeybee response to queen mandibular pheromone in laboratory bioassays. J Chem Ecol 16:841–850. https://doi.org/10.1007/BF01016494
Karlson P, Lüscher M (1959) ‘Pheromones’: a new term for a class of biologically active substances. Nature 183:55–56. https://doi.org/10.1038/183055a0
Katzav-Gozansky T, Boulay R, Soroker V, Hefetz A (2006) Queen pheromones affecting the production of queen-like secretion in workers. J Comp Physiol A 192:737–742. https://doi.org/10.1007/s00359-006-0110-0
Kay T, Lehmann L, Keller L (2019) Kin selection and altruism. Curr Biol 29:R438–R442. https://doi.org/10.1016/j.cub.2019.01.067
Keeling CI, Slessor KN, Higo HA, Winston ML (2003) New components of the honey bee ( Apis mellifera L.) queen retinue pheromone. Proc Natl Acad Sci USA 100:4486–4491. https://doi.org/10.1073/pnas.0836984100
Keiser CN, Vojvodic S, Butler IO et al (2018) Queen presence mediates the relationship between collective behaviour and disease susceptibility in ant colonies. J Anim Ecol 87:379–387. https://doi.org/10.1111/1365-2656.12696
Keller L, Nonacs P (1993) The role of queen pheromones in social insects: queen control or queen signal? Anim Behav 45:787–794. https://doi.org/10.1006/anbe.1993.1092
Keller L, Ross KG (1995) Gene by environment interaction: effects of a single gene and social environment on reproductive phenotypes of fire ant queens. Funct Ecol 9:667. https://doi.org/10.2307/2390159
Keller L, Ross KG (1998) Selfish genes: a green beard in the red fire ant. Nature 394:573–575. https://doi.org/10.1038/29064
Keller RA, Peeters C, Beldade P (2014) Evolution of thorax architecture in ant castes highlights trade-off between flight and ground behaviors. eLife 3:e01539. https://doi.org/10.7554/eLife.01539
Klobuchar E, Deslippe R (2002) A queen pheromone induces workers to kill sexual larvae in colonies of the red imported fire ant (Solenopsis invicta). Naturwissenschaften 89:302–304. https://doi.org/10.1007/s00114-002-0331-1
Knaden M (2019) Learning and processing of navigational cues in the desert ant. Curr Opin Neurobiol 54:140–145. https://doi.org/10.1016/j.conb.2018.10.005
Kocher SD, Grozinger CM (2011) Cooperation, conflict, and the evolution of queen pheromones. J Chem Ecol 37:1263–1275. https://doi.org/10.1007/s10886-011-0036-z
Kohlmeier P, Negroni MA, Kever M et al (2017) Intrinsic worker mortality depends on behavioral caste and the queens’ presence in a social insect. Sci Nat 104:1–7
Konishi T, Matsuura K (2021) Royal presence promotes worker and soldier aggression against non-nestmates in termites. Insect Soc 68(1):15–21
Kroiss J, Svatoš A, Kaltenpoth M (2011) Rapid identification of insect cuticular hydrocarbons using gas chromatography—ion-trap mass spectrometry. J Chem Ecol 37:420–427. https://doi.org/10.1007/s10886-011-9933-4
Lahav S, Soroker V, Vander Meer RK, Hefetz A (1998) Nestmate recognition in the ant Cataglyphis niger: do queens matter? Behav Ecol Sociobiol 43:203–212. https://doi.org/10.1007/s002650050482
Lenoir A, D’Ettorre P, Errard C, Hefetz A (2001) Chemical ecology and social parasitism in ants. Annu Rev Entomol 46:573–599. https://doi.org/10.1146/annurev.ento.46.1.573
Lin N, Michener CD (1972) Evolution of sociality in insects. Q Rev Biol 47:131–159. https://doi.org/10.1086/407216
Lopez-Vaamonde C, Brown RM, Lucas ER et al (2007) Effect of the queen on worker reproduction and new queen production in the bumble bee Bombus terrestris. Apidologie 38:171–180. https://doi.org/10.1051/apido:2006070
Lubertazzi D (2012) The biology and natural history of Aphaenogaster rudis. Psyche A: J Entomol 2012:e752815. https://doi.org/10.1155/2012/752815
Majidifar V, Psalti MN, Coulm M, et al (2022) Ontogeny of superorganisms: social control of queen specialization in ants. bioRxiv
Majoe M, Libbrecht R, Foitzik S, Nehring V (2021) Queen loss increases worker survival in leaf-cutting ants under paraquat-induced oxidative stress. Phil Trans R Soc B 376:20190735. https://doi.org/10.1098/rstb.2019.0735
Martin S, Drijfhout F (2009) A review of ant cuticular hydrocarbons. J Chem Ecol 35:1151–1161. https://doi.org/10.1007/s10886-009-9695-4
Matsuura K (2012) Multifunctional queen pheromone and maintenance of reproductive harmony in termite colonies. J Chem Ecol 38:746–754. https://doi.org/10.1007/s10886-012-0137-3
Matsuura K, Himuro C, Yokoi T et al (2010) Identification of a pheromone regulating caste differentiation in termites. Proc Natl Acad Sci USA 107:12963–12968. https://doi.org/10.1073/pnas.1004675107
Medeiros FNS, Lopes LE, Moutinho PRS et al (1992) Functional polygyny, agonistic interactions and reproductive dominance in the neotropical ant Odontomachus chelifer (Hymenoptera, Formicidae, Ponerinae). Ethology 91:134–146. https://doi.org/10.1111/j.1439-0310.1992.tb00857.x
Melathopoulos AP, Winston ML, Pettis JS, Pankiw T (1996) Effect OF QUEEN MANDIBULAR PHEROMONE ON INITIATION AND MAINTENANCE OF QUEEN CELLS IN THE HONEY BEE (Apis mellifera L.). Can Entomol 128:263–272. https://doi.org/10.4039/Ent128263-2
Mitaka Y, Akino T (2021) A review of termite pheromones: multifaceted, context-dependent, and rational chemical communications. Frontiers in Ecology and Evolution 8:
Monnin T, Peeters C (2008) How many gamergates is an ant queen worth? Naturwissenschaften 95:109–116. https://doi.org/10.1007/s00114-007-0297-0
Monnin T, Ratnieks FLW, Jones GR, Beard R (2002) Pretender punishment induced by chemical signalling in a queenless ant. Nature 419:61–65. https://doi.org/10.1038/nature00932
Moore D, Liebig J (2010) Mixed messages: fertility signaling interferes with nestmate recognition in the monogynous ant Camponotus floridanus. Behav Ecol Sociobiol 64:1011–1018. https://doi.org/10.1007/s00265-010-0916-1
Mori K, Otsuka T (1985) Synthesis of the enantiomers of 5-hexadecanolide, the pheromone of the queen of the oriental hornet, Vespa orientalis, employing enzymic resolution of (±)-2-aminotridecanoic acid as the key-step. Tetrahedron 41(3):547–551
Negroni MA, Macit MN, Stoldt M et al (2021) Molecular regulation of lifespan extension in fertile ant workers. Phil Trans R Soc B 376:20190736. https://doi.org/10.1098/rstb.2019.0736
Nunes TM, Mateus S, Favaris AP et al (2014) Queen signals in a stingless bee: suppression of worker ovary activation and spatial distribution of active compounds. Sci Rep 4:7449. https://doi.org/10.1038/srep07449
Obin MS, Vander Meer RK (1989) Nestmate recognition in fire ants (Solenopsis invicta Buren). Do Queens Label Workers? Ethology 80:255–264. https://doi.org/10.1111/j.1439-0310.1989.tb00744.x
Oi CA, Millar JG, van Zweden JS, Wenseleers T (2016) Conservation of queen pheromones across two species of vespine wasps. J Chem Ecol 42:1175–1180. https://doi.org/10.1007/s10886-016-0777-9
Oi CA, van Zweden JS, Oliveira RC et al (2015a) The origin and evolution of social insect queen pheromones: novel hypotheses and outstanding problems. BioEssays 37:808–821. https://doi.org/10.1002/bies.201400180
Oi CA, Van Oystaeyen A, Caliari Oliveira R et al (2015b) Dual Effect of Wasp Queen Pheromone in Regulating Insect Sociality. Curr Biol 25:1638–1640. https://doi.org/10.1016/j.cub.2015.04.040
Oliveira RC, Warson J, Sillam-Dussès D et al (2020) Identification of a queen pheromone mediating the rearing of adult sexuals in the pharaoh ant Monomorium pharaonis. Biol Lett 16:20200348. https://doi.org/10.1098/rsbl.2020.0348
Orlova M, Amsalem E (2021) Bumble bee queen pheromones are context-dependent. Sci Rep 11:16931. https://doi.org/10.1038/s41598-021-96411-7
Ortius D, Heinze J (1999) Fertility signaling in queens of a North American ant. Behav Ecol Sociobiol 45:151–159. https://doi.org/10.1007/s002650050548
Ozaki M, Wada-Katsumata A, Fujikawa K et al (2005) Ant Nestmate and non-nestmate discrimination by a chemosensory sensillum. Science 309:311–314. https://doi.org/10.1126/science.1105244
Passera L (1980) The inhibitory function of queens in the ant Plagiolepis pygmaea Latr.: the act of pheromones. Insectes Soc
Passera L, Aron S, Bach D (1995) Elimination of sexual brood in the Argentine ant Linepithema humile: queen effect and brood recognition. Entomol Exp Appl 75:203–212. https://doi.org/10.1111/j.1570-7458.1995.tb01928.x
Peeters C (1991) The occurrence of sexual reproduction among ant workers. Biol J Lin Soc 44:141–152. https://doi.org/10.1111/j.1095-8312.1991.tb00612.x
Peeters C, Ito F (2001) Colony dispersal and the evolution of queen morphology in social hymenoptera. Ann Rev Entomol 46:601–630. https://doi.org/10.1146/annurev.ento.46.1.601
Peeters C, Liebig J, Hölldobler B (2000) Sexual Reproduction by Both Queens and Workers in the Ponerine Ant Harpegnathos Saltator. Insect Soc 47:325–332. https://doi.org/10.1007/PL00001724
Penick CA, Brent CS, Dolezal K, Liebig J (2014) Neurohormonal changes associated with ritualized combat and the formation of a reproductive hierarchy in the ant Harpegnathos saltator. J Exp Biol 217:1496–1503
Penick CA, Ghaninia M, Haight KL et al (2021) Reversible plasticity in brain size, behaviour and physiology characterizes caste transitions in a socially flexible ant (Harpegnathos saltator). Proc R Soc B 288:1948. https://doi.org/10.1098/rspb.2021.0141
Penick CA, Liebig J, Brent CS (2011) Reproduction, dominance, and caste: endocrine profiles of queens and workers of the ant Harpegnathos saltator. J Comp Physiol A 197:1063–1071
Petersen-Braun M (1975) Untersuchungen zur Sozialen Organisation der Pharaoameise Monomorium pharaonis L. (Hymenoptera, Formicidae) I. Der Brutzyklus und seine Steuerung durch Population seigene Faktoren. Insectes Soc 22:269–291
Petersen-Braun M (1977) Untersuchungen zur sozialen Organisation der Pharaoameise Monomorium pharaonis L. (Hymenoptera, Formicidae) II. Die Kastendeterminierung Insectes Soc 24:303–318
Pettis JS, Winston ML, Collins AM (1995) Suppression of queen rearing in European and Africanized honey beesApis mellifera L. by synthetic queen mandibular gland pheromone. Ins Soc 42:113–121. https://doi.org/10.1007/BF01242447
Powell S, Tschinkel WR (1999) Ritualized conflict in Odontomachus brunneus and the generation of interaction-based task allocation: a new organizational mechanism in ants. Anim Behav 58:965–972. https://doi.org/10.1006/anbe.1999.1238
Princen SA, Oliveira RC, Ernst UR et al (2019) Honeybees possess a structurally diverse and functionally redundant set of queen pheromones. Proc R Soc B 286:20190517. https://doi.org/10.1098/rspb.2019.0517
Ratnieks FLW (1988) Reproductive Harmony via Mutual Policing by Workers in Eusocial Hymenoptera. Am Nat 132:217–236
Ratnieks FLW (1995) Evidence for a queen-produced egg-marking pheromone and its use in worker policing in the honey bee. J Apic Res 34:31–37. https://doi.org/10.1080/00218839.1995.11100883
Ratnieks FLW, Visscher PK (1989) Worker policing in the honeybee. Nature 342:796–797. https://doi.org/10.1038/342796a0
Robinson GE, Vargo EL (1997) Juvenile hormone in adult eusocial hymenoptera: gonadotropin and behavioral pacemaker. Arch Insect Biochem Physiol 35:559–583. https://doi.org/10.1002/(SICI)1520-6327(1997)35:4%3c559::AID-ARCH13%3e3.0.CO;2-9
Ross KG (1988) Differential reproduction in multiple-queen colonies of the fire ant Solenopsis invicta (Hymenoptera: Formicidae). Behav Ecol Sociobiol 23:341–355
Ross KG, Carpenter JM (1991) Phylogenetic analysis and the evolution of queen number in eusocial Hymenoptera. J Evol Biol 4:117–130. https://doi.org/10.1046/j.1420-9101.1991.4010117.x
Ross KG, Keller L (1998) Genetic control of social organization in an ant. Proc Natl Acad Sci USA 95:14232–14237
Ross KG, Matthews RW (1991) The social biology of wasps. Cornell University Press
Sasaki T, Penick CA, Shaffer Z et al (2016) A simple behavioral model predicts the emergence of complex animal hierarchies. Am Nat 187:765–775
Schultheiss P, Nooten SS, Wang R et al (2022) The abundance, biomass, and distribution of ants on Earth. Proc Natl Acad Sci USA 119:e2201550119. https://doi.org/10.1073/pnas.2201550119
Slessor KN, Winston ML, Le Conte Y (2005) Pheromone communication in the honeybee (Apis mellifera L.). J Chem Ecol 31:2731–2745. https://doi.org/10.1007/s10886-005-7623-9
Smith AA, Hölldober B, Liebig J (2009) Cuticular Hydrocarbons reliably identify cheaters and allow enforcement of altruism in a social insect. Curr Biol 19:78–81. https://doi.org/10.1016/j.cub.2008.11.059
Smith AA, Hölldobler B, Liebig J (2008a) Hydrocarbon Signals explain the pattern of worker and egg policing in the ant Aphaenogaster cockerelli. J Chem Ecol 34:1275–1282. https://doi.org/10.1007/s10886-008-9529-9
Smith AA, Hölldobler B, Liebig J (2011) Reclaiming the crown: queen to worker conflict over reproduction in Aphaenogaster cockerelli. Naturwissenschaften 98:237–240. https://doi.org/10.1007/s00114-011-0761-8
Smith AA, Hölldobler B, Liebig J (2012a) Queen-specific signals and worker punishment in the ant Aphaenogaster cockerelli: the role of the Dufour’s gland. Anim Behav 83:587–593. https://doi.org/10.1016/j.anbehav.2011.12.024
Smith AA, Liebig J (2017) The evolution of cuticular fertility signals in eusocial insects. Curr Opin Insect Sci 22:79–84. https://doi.org/10.1016/j.cois.2017.05.017
Smith AA, Millar JG, Hanks LM, Suarez AV (2012b) Experimental evidence that workers recognize reproductives through cuticular hydrocarbons in the ant Odontomachus brunneus. Behav Ecol Sociobiol 66:1267–1276. https://doi.org/10.1007/s00265-012-1380-x
Smith AA, Millar JG, Hanks LM, Suarez AV (2013) A conserved fertility signal despite population variation in the cuticular chemical phenotype of the trap-jaw ant Odontomachus brunneus. J Exp Bio. https://doi.org/10.1242/jeb.089482
Smith AA, Millar JG, Suarez AV (2015) A social insect fertility signal is dependent on chemical context. Biol Lett 11:20140947. https://doi.org/10.1098/rsbl.2014.0947
Smith AA, Millar JG, Suarez AV (2016) Comparative analysis of fertility signals and sex-specific cuticular chemical profiles of Odontomachus trap-jaw ants. J Exp Biol 219:419–430. https://doi.org/10.1242/jeb.128850
Smith CR, Toth AL, Suarez AV, Robinson GE (2008b) Genetic and genomic analyses of the division of labour in insect societies. Nat Rev Genet 9:735–748. https://doi.org/10.1038/nrg2429
Sommer K, Hölldobler B (1995) Colony founding by queen association and determinants of reduction in queen number in the antLasius niger. Anim Behav 50:287–294. https://doi.org/10.1006/anbe.1995.0244
Sousa-Souto L, Souza DJ (2006) Queen influence on workers behavior of the leaf-cutting ant Atta sexdens rubropilosa (Forel, 1908). Braz J Biol 66:503–508. https://doi.org/10.1590/S1519-69842006000300016
Sramkova A, Schulz C, Twele R et al (2008) Fertility signals in the bumblebee Bombus terrestris (Hymenoptera: Apidae). Naturwissenschaften 95:515–522. https://doi.org/10.1007/s00114-008-0353-4
Steitz I, Ayasse M (2020) Macrocyclic lactones act as a queen pheromone in a primitively eusocial sweat bee. Curr Biol 30:1136-1141.e3. https://doi.org/10.1016/j.cub.2020.01.026
Steitz I, Brandt K, Biefel F et al (2019) Queen recognition signals in two primitively eusocial halictid bees: evolutionary conservation and caste-specific perception. Insects 10:416. https://doi.org/10.3390/insects10120416
Stolle E, Pracana R, López-Osorio F et al (2022) Recurring adaptive introgression of a supergene variant that determines social organization. Nat Commun 13:1180. https://doi.org/10.1038/s41467-022-28806-7
Stroeymeyt N, Brunner E, Heinze J (2007) “Selfish worker policing” controls reproduction in a Temnothorax ant. Behav Ecol Sociobiol 61:1449–1457. https://doi.org/10.1007/s00265-007-0377-3
Stumper R (1956) Sur les sécrétions attractives des fourmis femelles. Comptes Rendus Hebdomadaires Des Seances De L Academie Des Sci 242:2487–2489
Sturgis SJ, Gordon DM (2012) Nestmate recognition in ants (Hymenoptera: Formicidae): a review. Myrmecol News 16:101–110
Sun Q, Hampton JD, Merchant A et al (2020) Cooperative policing behaviour regulates reproductive division of labour in a termite. Proc R Soc B 287:20200780. https://doi.org/10.1098/rspb.2020.0780
Symonds MRE, Elgar MA (2008) The evolution of pheromone diversity. Trends Ecol Evol 23:220–228. https://doi.org/10.1016/j.tree.2007.11.009
Tarpy DR, Fletcher DJC (2003) “Spraying” behavior during queen competition in honey bees. J Insect Behavior 16:425–437. https://doi.org/10.1023/A:1024884211098
Traynor KS, Le Conte Y, Page RE (2014) Queen and young larval pheromones impact nursing and reproductive physiology of honey bee (Apis mellifera) workers. Behav Ecol Sociobiol 68:2059–2073. https://doi.org/10.1007/s00265-014-1811-y
Trible W, Kronauer DJC (2017) Caste development and evolution in ants: it’s all about size. J Exp Biol 220:53–62. https://doi.org/10.1242/jeb.145292
Trible W, Ross KG (2016) Chemical communication of queen supergene status in an ant. J Evol Biol 29:502–513. https://doi.org/10.1111/jeb.12799
Tschinkel WR (2015) The architecture of subterranean ant nests: beauty and mystery underfoot. J Bioecon 17:271–291. https://doi.org/10.1007/s10818-015-9203-6
Tschinkel WR (2021) Ant architecture: the wonder, beauty, and science of underground nests. Princeton University Press, Princeton, New Jersey
Tschinkel WR (1992) Brood raiding and the population dynamics of founding and incipient colonies of the fire ant, Solenopsis invicta. Ecological Entomol 17:179–188. https://doi.org/10.1111/j.1365-2311.1992.tb01176.x
Tschinkel WR (2013) The fire ants. Belknap Press
Tsuji K, Egashira K, Hölldobler B (1999) Regulation of worker reproduction by direct physical contact in the ant Diacamma sp. from Japan. Anim Behav 58:337–343
Tsuji K, Nakata K, Heinze J (1996) Lifespan and Reproduction in a Queenless Ant. Naturwissenschaften 83:577–578. https://doi.org/10.1007/BF01141985
Van Doorn A (1986) Investigations into the regulation of dominance behaviour and of the division of labour in bumblebee colonies (Bombus terrestris). Netherlands J Zool 37:255–276
van Doorn A, Chrambach A (1989) Retinue Behaviour in Bumblebee Workers (Bombus terrestris L.). J Apic Res 28:66–70. https://doi.org/10.1080/00218839.1989.11100823
Van Oystaeyen A, Oliveira RC, Holman L et al (2014) Conserved class of queen pheromones stops social insect workers from reproducing. Science 343:287–290. https://doi.org/10.1126/science.1244899
van Zweden JS, Dreier S, d’Ettorre P (2009) Disentangling environmental and heritable nestmate recognition cues in a carpenter ant. J Insect Physiol 55:159–164. https://doi.org/10.1016/j.jinsphys.2008.11.001
Vander Meer R, Alonso LE (2002) Queen primer pheromone affects conspecific fire ant ( Solenopsis invicta ) aggression. Behav Ecol Sociobiol 51:122–130. https://doi.org/10.1007/s002650100417
Vargo E (1988) A bioassay for a primer pheromone of queen fire ants (Solenopsis invicta) which inhibits the production of sexuals. Insectes Soc 35:382–392. https://doi.org/10.1007/BF02225813
Vargo EL (2019) Primer Pheromones in ants. In: Vander Meer RK, Breed MD, Espelie KE, Winston ML (eds) Pheromone Communication in Social Insects, 1st edn. CRC Press, pp 293–313
Vargo EL (1999) Reproductive development and ontogeny of queen pheromone production in the fire ant Solenopsis invicta. Physiol Entomol 24:370–376. https://doi.org/10.1046/j.1365-3032.1999.00153.x
Vargo EL (1997) Poison Gland of Queen Fire Ants (Solenopsis invicta) is the Source of a Primer Pheromone. Naturwissenschaften 84:507–510. https://doi.org/10.1007/s001140050435
Vargo EL, Fletcher DJC (1986a) Evidence of pheromonal queen control over the production of male and female sexuals in the fire ant, Solenopsis invicta. J Comp Physiol 159:741–749. https://doi.org/10.1007/BF00603727
Vargo EL, Fletcher DJC (1986b) Queen number and the production of sexuals in the fire ant, Solenopsis invicta (Hymenoptera: Formicidae). Behav Ecol Sociobiol 19:41–47. https://doi.org/10.1007/BF00303841
Vargo EL, Hulsey CD (2000) Multiple glandular origins of queen pheromones in the fire ant Solenopsis invicta. J Insect Physiol 46:1151–1159. https://doi.org/10.1016/S0022-1910(99)00226-7
Vargo EL, Laurel M (1994) Studies on the mode of action of a queen primer pheromone of the fire ant Solenopsis invicta. J Insect Physiol 40:601–610. https://doi.org/10.1016/0022-1910(94)90147-3
Vargo EL, Passera L (1991) Pheromonal and behavioral queen control over the production of gynes in the argentine ant Iridomyrmex humilis (Mayr). Behav Ecol Sociobiol 28:161–169
Vásquez GM, Schal C, Silverman J (2008) Cuticular hydrocarbons as queen adoption cues in the invasive Argentine ant. J Exp Biol 211:1249–1256. https://doi.org/10.1242/jeb.017301
Vásquez GM, Silverman J (2008) Queen acceptance and the complexity of nestmate discrimination in the Argentine ant. Behav Ecol Sociobiol 62:537–548. https://doi.org/10.1007/s00265-007-0478-z
Vienne C, Errard C, Lenoir A (1998) Influence of the queen on worker behaviour and queen recognition behaviour in ants. Ethology 104:431–446. https://doi.org/10.1111/j.1439-0310.1998.tb00081.x
Villalta I, Abril S, Cerdá X, Boulay R (2018) Queen Control or queen signal in ants: what remains of the controversy 25 years after Keller and Nonacs’ seminal paper? J Chem Ecol 44:805–817. https://doi.org/10.1007/s10886-018-0974-9
Vollet-Neto A, Koffler S, dos Santos CF et al (2018) Recent advances in reproductive biology of stingless bees. Insectes Soc 65:201–212. https://doi.org/10.1007/s00040-018-0607-x
Ward PS (1983) Genetic Relatedness and colony organization in a species complex of ponerine ants: I. phenotypic and genotypic composition of colonies. Behav Ecol Sociobiol 12:285–299
Ward PS (1981) Ecology and Life History of theRhytidoponera Impressa Group (Hymenoptera: Formicidae)II. Colony origin, seasonal cycles, and reproduction. Psyche: J Entomol 88:109–126. https://doi.org/10.1155/1981/39593
Watkins JF, Cole TW (1966) Attraction of army ant workers to secretions of their queens. Texas J Sci 18:254
Wenseleers T, Oi CA, Caliari Oliveira R (2020a) Worker Policing. In: Starr CK (ed) Encyclopedia of Social Insects. Springer International Publishing, Cham, pp 1–8
Wenseleers T, Princen S, Caliari Oliveira R, Oi CA (2020b) Conflicts of interest within colonies. In: Starr CK (ed) Encyclopedia of Social Insects. Springer International Publishing, Cham, pp 1–15
Wheeler WM (1910) Ants: their structure, development and behavior. Columbia University Press, New York
Wild B, Dormagen DM, Zachariae A et al (2021) Social networks predict the life and death of honey bees. Nat Commun 12:1110. https://doi.org/10.1038/s41467-021-21212-5
Willer DE, Fletcher DJC (1986) Differences in inhibitory capability among queens of the ant Solenopsis invicta. Physiol Entomol 11:475–482. https://doi.org/10.1111/j.1365-3032.1986.tb00441.x
Wilson EO (1971) The insect societies. Massachusetts, USA, Harvard University Press, Cambridge
Wilson EO, Hölldobler B (1985) Caste-specific techniques of defense in the polymorphic ant Pheidole embolopyx (Hymenoptera: Formicidae). Insectes Soc 32:3–22
Winston ML, Higo HA, Colley SJ et al (1991) The role of queen mandibular pheromone and colony congestion in honey bee (Apis mellifera L.) reproductive swarming (Hymenoptera: Apidae). J Insect Behav 4:649–660. https://doi.org/10.1007/BF01048076
Winston ML, Slessor KN, Willis LG et al (1989) The influence of queen mandibular pheromones on worker attraction to swarm clusters and inhibition of queen rearing in the honey bee (Apis mellifera L.). Insectes Soc 36:15–27
Yamamoto Y, Matsuura K (2011) Queen pheromone regulates egg production in a termite. Biol Let 7:727–729. https://doi.org/10.1098/rsbl.2011.0353
Yan H, Liebig J (2021) Genetic basis of chemical communication in eusocial insects. Genes Dev 35:470–482. https://doi.org/10.1101/gad.346965.120
Yan H, Opachaloemphan C, Carmona-Aldana F et al (2022) Insulin signaling in the long-lived reproductive caste of ants. Science 377:1092–1099. https://doi.org/10.1126/science.abm8767
Yan Z, Martin SH, Gotzek D et al (2020) Evolution of a supergene that regulates a trans-species social polymorphism. Nat Ecol Evol 4:240–249. https://doi.org/10.1038/s41559-019-1081-1
Yek SH, Mueller UG (2011) The metapleural gland of ants. Biol Rev 86:774–791. https://doi.org/10.1111/j.1469-185X.2010.00170.x
Yilmaz A, Spaethe J (2022) Colour vision in ants (Formicidae, Hymenoptera). Phil Trans R Soc B 377:20210291. https://doi.org/10.1098/rstb.2021.0291
Zanette LRS, Miller SDL, Faria CMA et al (2012) Reproductive conflict in bumblebees and the evolution of worker policing. Evolution 66:3765–3777. https://doi.org/10.1111/j.1558-5646.2012.01709.x
Zeng H, Millar JG, Chen L et al (2022) Characterization of queen supergene pheromone in the red imported fire ant using worker discrimination assays. J Chem Ecol 48:109–120. https://doi.org/10.1007/s10886-021-01336-0
Acknowledgements
I extend my heartfelt thanks to my Ph.D. advisor, Kenneth Ross, as well as Brendan Hunt, Buck Trible, Taka Sasaki, and colleagues in the Ross and Hunt labs for their insightful comments and support. I am also thankful to editor James Traniello, Jürgen Heinze and other anonymous reviewers for their valuable feedback and constructive criticism on the initial submission. Furthermore, I am obliged to Adrian Smith for graciously providing the photos of Odontomachus brunneus that served as the basis for the drawings in Figure 3.
Funding
HZ is supported by the following U.S. NSF grants: #1354479 to Kenneth G. Ross, #1755130 to Brendan G. Hunt and Kenneth G. Ross, and #1754476 to Sarah Kocher and Brendan G. Hunt.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Communicated by J. Heinze
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zeng, H. Functional properties of ant queen pheromones as revealed by behavioral experiments. Behav Ecol Sociobiol 77, 113 (2023). https://doi.org/10.1007/s00265-023-03378-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03378-8