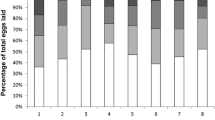
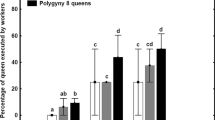
Abstract
Models based on the kin selection theory predict that in social hymenopterans, queens may favor a lower investment in the production of sexuals than workers. However, in perennial colonies, this conflict may be tuned down by colony-level selection because of the trade off between colony survival and reproductive allocation. In this study, we present a survey of sexual production in colonies of Aphaenogaster senilis, a common species of ant in the Iberian Peninsula. Similar to most species that reproduce by fission, males were found in large excess compared to gynes (172:1). Sexuals were more likely to be found in queenless than in queenright (QR) field colonies. However, we also found a few gynes and numerous males in very large QR colonies. We compared these data with those available in the literature for A. rudis, a congeneric species from North America that has independent colony founding. The sex ratio in this species was only five males for each female, and sexuals were mostly found in QR nests, irrespective of colony size. We confirmed queen inhibition of sexual production in A. senilis in laboratory experiments and provide evidence that this inhibition is mediated by a nonvolatile pheromone. To seek the potential source of such a queen pheromone, we analyzed the secretions of two conspicuous exocrine glands, the Dufour’s and postpharyngeal glands (DG and PPG, respectively) in both queens and workers. Both secretions were composed of hydrocarbons, but that of DG also contained small quantities of tetradecanal and hexadecanal. The hydrocarbon profile of the DG and PPG showed notable caste specificity suggesting a role in caste-related behavior. The PPG secretions also differed between colonies suggesting its role in colony-level recognition. We suggest that in A. senilis, there are two modes of colony fission: First, in very large colonies, gynes are produced, probably because of the dilution of the queen pheromone, and consequently one or more gynes leave the mother colony with workers and brood to found a new nest. This is beneficial at the colony level because it avoids the production of costly sexuals in small colonies. However, because the queen and workers have different optima for sexual production, we hypothesize that queens tend to overproduce the pheromone to delay their production. This in turn may drive workers to leave the mother colony during nest relocation and to produce sexuals once they are away from the queen’s influence, creating a second mode of colony fission.





Similar content being viewed by others
References
Alaux C, Jaisson P, Hefetz A (2005) Reproductive decision in semelparous social insects: a pace-maker queen in bumblebees colonies. Behav Ecol Sociobiol 59:270–277
Alaux C, Jaisson P, Hefetz A (2006) Regulation of worker reproduction in bumblebees: workers eavesdrop on a queen signal. Behav Ecol Sociobiol 60:439–446
Aron S, Keller L, Passera L (2001) Role of resource availability on sex, caste and reproductive allocation ratios in the Argentine ant Linepithema humile. J Anim Ecol 70:831–839
Backus VL (1995) Rules for allocation in a temperate forest ant—demography, natural selection, and queen–worker conflict. Am Nat 145:775–796
Bourke AFG, Chan GL (1999) Queen–worker conflict over sexual production and colony maintenance in perennial social insects. Am Nat 154:417–426
Bourke AFG, Franks NR (1995) Social evolution in ants. Princeton Univ. Press, Princeton, NJ
Bourke AFG, Ratnieks FLW (1999) Kin conflict over caste determination in social Hymenoptera. Behav Ecol Sociobiol 46:287–297
Brian MV (1973) Caste control through worker attack in the ant Myrmica. Insectes Soc 20:87–102
Brian MV (1979) Habitat differences in sexual production by two co-existent ants Tetramorium caespitum, Lasius alienus, in southern England. J Anim Ecol 48:943–953
Bulmer MG (1983) Sex ratio theory in social insects with swarming. J Theor Biol 100:329–340
Butler CG (1959) The source of the substance produced by a queen honeybee (Apis mellifera) which inhibits development of the ovaries of the workers of its colony. Proc R Entomol Soc Lond 34:137–138
Butler L (1961) The scent of queen honey bees (Apis mellifera) that causes partial inhibition of queen rearing. J Insect Physiol 7:258–264
Cerdá X, Dahbi A, Retana J (2002) Spatial patterns, temporal variability, and the role of multi-nest colonies in a monogynous Spanish desert ant. Ecol Entomol 27:7–15
Craig R (1980) Sex investment ratios in social Hymenoptera. Am Nat 116:311–323
Cuvillier-Hot V, Cobb M, Malosse C, Peeters C (2001) Sex, age and ovarian activity affect cuticular hydrocarbons in Diacamma ceylonense, a queenless ant. J Insect Physiol 47:485–493
Cuvillier-Hot V, Lenoir A, Crewe R, Malosse C, Peeters C (2004) Fertility signaling and reproductive skew in queenless ants. Anim Behav 68:1209–1219
de Menten L, Fournier D, Brent C, Passera L, Vargo EL, Aron S (2005) Dual mechanism of queen influence over sex ratio in the ant Pheidole pallidula. Behav Ecol Sociobiol 58:527–533
Dietemann V, Peeters C, Liebig J, Thivet V, Hölldobler B (2003) Cuticular hydrocarbons mediate discrimination of reproductives and non reproductives in the ant Myrmecia gulosa. Proc Natl Acad Sci USA 100:10341–10346
Dor R, Katzav-Gozansky T, Hefetz A (2005) Dufour’s gland pheromone as a reliable fertility signal among honeybee (Apis mellifera) workers. Behav Ecol Sociobiol 58:270–276
Elmes GW, Wardlaw JC (1982) A population study of the ants Myrmica sabuleti and Myrmica scabrinodis living at two sites in the south of England. II. Effect of above-nest vegetation. J Anim Ecol 51:665–680
Endler A, Liebig J, Schmitt T, Parker JE, Jones GR, Schreier P, Hölldobler B (2004) Surface hydrocarbons of queen eggs regulate worker reproduction in a social insect. Proc Natl Acad Sci USA 101:2945–2950
Gotwald WH Jr (1995) Army ants: the biology of social predation. Cornell Univ. Press, Ithaca, NY
Hammond RL, Keller L (2004) Conflict over male parentage in social insects. PLoS Biol 2:1472–1482
Headley AE (1949) A population study of the ant Aphaenogaster fulva ssp. aquia Buckley (Hymenoptera, Formicidae). Ann Entomol Soc Am, 42:265–272
Heinze J, Stengl B, Sledge MF (2002) Worker rank, reproductive status and cuticular hydrocarbon signature in the ant, Pachycondyla cf. inversa. Behav Ecol Sociobiol 52:59–65
Herbers JM, Banschbach VS (1998) Food supply and reproductive allocation in forest ants: repeated experiments give different results. Oikos 83:145–151
Herbers JM, Banschbach VS (1999) Plasticity of social organization in a forest ant species. Behav Ecol Sociobiol 45:451–465
Herbers JM, DeHeer CJ, Foitzik S (2001) Conflict over sex allocation drives conflict over reproduction in perennial social insects. Am Nat 158:178–192
Hölldobler B, Carlin NF (1989) Colony founding, queen control and worker reproduction in the ant Aphaenogaster (=Novomessor) cockerelli (Hymenoptera: Formicidae). Psyche 96:131–151
Hoover SER, Keeling CI, Winston ML, Slessor KN (2003) The effect of queen pheromones on worker honey bee ovary development. Naturwissenschaften 90:477–480
Iwanishi S, Ohkawara K (2005) The mechanism of the queen signal in regulation of worker reproduction in the myrmicine ant Aphaenogaster smythiesi japonica. Ethol Ecol Evol 17:27–39
Iwanishi S, Hasegawa E, Ohkawara K (2003) Worker oviposition and policing behaviour in the myrmicine ant Aphaenogaster smythiesi japonica Forel. Anim Behav 66:513–519
Julian GE, Fewell JH, Gadau J, Johnson RA, Larrabee D (2002) Genetic determination of the queen caste in an ant hybrid zone. Proc Natl Acad Sci USA 99:8157–8160
Katzav-Gozansky T, Boulay R, Soroker V, Hefetz A (2004) Queen-signal modulation of worker pheromonal composition in honeybees. Proc R Soc Lond B 271:2065–2069
Katzav-Gozansky T, Boulay R, Soroker V, Hefetz A (2006) Queen pheromones affecting the production of queen-like secretion in workers. J Comp Physiol A 192:737–742
Keller L, Nonacs P (1993) The role of queen pheromones in social insects: queen control or queen signal? Anim Behav 45:787–794
Lahav S, Soroker V, Hefetz A, Vander Meer RK (1999) Direct behavioral evidence for hydrocarbons as ant recognition discriminators. Naturwissenschaften 86:246–249
Ledoux A (1971) Un nouveau mode de bouturage de société chez la fourmi Aphaenogaster senilis Mayr. C R Acad Sci Ser D Sci Nat 273:83–85
Ledoux A (1976a) Bouturage expérimental de colonie chez la fourmi Aphaenogaster senilis Mayr. C R Acad Sci Ser D Sci Nat 283:1061–1063
Ledoux A (1976b) Inhibition exercée sur l’apparition de nouvelles femelles ailées, par le femelle reine pondeuse chez Aphaenogaster senilis (Hyméoptère Formicoidea). C R Acad Sci Ser D Sci Nat 283:1197–200
Ledoux A (1984) Sur la présence d’ouvrières à parthénogenèse thélytoque observée chez Aphaenogaster senilis (Mayr.) (Hyménoptère Formicoidea). C R Acad Sci Ser III Sci Vie 299:859–861
Lenoir A, Fresneau D, Errard C, Hefetz A (1999) Individuality and colonial identity in ants. In: Detrain C, Deneubourg JL, Pasteels J (eds) Information processing in social insects. Birkhauser, Basel, pp 219–237
Lenoir A, Hefetz A, Simon T, Soroker V (2001) Comparative dynamics of gestalt odour formation in two ant species Camponotus fellah and Aphaenogaster senilis (Hymenoptera: Formicidae). Physiol Entomol 26:275–283
Liebig J, Peeters C, Oldham NJ, Markstadter C, Hölldobler B (2000) Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? Proc Natl Acad Sci USA 97:4124–4131
Lim SP, Lee CY (2005) Effects of queen body parts on the production of new sexuals in the Pharaoh’s ant, Monomorium pharaonis (Hymenoptera: Formicidae). Sociobiology 46:677–688
Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996) SAS system for mixed models. SAS Institute, Cary
Mehdiabadi N, Reeve HK, Mueller UG (2003) Queens versus workers: sex-ratio conflicts in eusocial insects. Trends Ecol Evol 18:88–93
Monnin T, Ratnieks FL, Jones GR, Beard R (2002) Pretender punishment induced by chemical signaling in a queenless ant. Nature 419:61–65
Oster G, Wilson EO (1978) Caste and ecology in the social insects. Princeton Univ. Press, Princeton, NJ
Pamilo P (1991) Evolution of colony characteristics in social insects. I. Sex allocation. Am Nat 137:83–107
Pearcy M, Aron S (2006) Local resource competition and sex ratio in the ant Cataglyphis cursor. Behav Ecol:569–574
Peeters C, Monnin T, Malosse C (1999) Cuticular hydrocarbons correlated with reproductive status in a queenless ant. Proc R Soc Lond B 266:1323–1327
Pettis JS, Winston ML, Collins AM (1995) Suppression of queen rearing in European and Africanized honey bees Apis mellifera L. by synthetic queen mandibular pheromone. Insectes Soc 42:113–121
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge Univ. Press, Cambridge
Ratnieks FLW (1988) Reproductive harmony via mutual policing by workers in eusocial Hymenoptera. Am Nat 132:217–236
Seeley TD (1995) The wisdom of the hive: the social physiology of honey bee colonies. Harvard Univ. Press, London
Sundström L (1995) Sex allocation and colony maintenance in monogyne and polygyne colonies of Formica truncorum (Hymenoptera: Formicidae): the impact of kinship and mating structure. Am Nat 146:182–201
Talbot M (1951) Populations and hibernating conditions of the ant Aphaenogaster (Attomyrma) rudis Emery (Hymenoptera: Formicidae). Ann Entomol Soc Am 44:302–307
Thomas ML, Parry LJ, Allan RA, Elgar MA (1999) Geographic affinity, cuticular hydrocarbons and colony recognition in the Australian meat ant Iridomyrmex purpureus. Naturwissenschaften 86:87–92
Vander Meer RK, Glancey BM, Lofgren CS, Glover A, Tumlinson JH, Rocca J (1980) The poison sac of red imported fire ant queens: source of a pheromone attractant. Ann Entomol Soc Am 73:609–612
Vander Meer RK, Morel L (1998) Nestmate recognition in ants. In: Vander Meer RK, Breed M, Winston M, Espelie KE (eds) Pheromone communication in social insects. Westview, Boulder, CO, pp 79–103
Vargo EL (1988) A bioassay for a primer pheromone of queen fire ants (Solenopsis invicta) which inhibits the production of sexuals. Insectes Soc 35:382–392
Vargo EL (1997) Poison gland of queen fire ants (Solenopsis invicta) is the source of a primer pheromone. Naturwissenschaften 84:507–510
Vargo EL, Hulsey CD (2000) Multiple glandular origins of queen pheromones in the fire ant Solenopsis invicta. J Insect Physiol 46:1151–1159
Vargo EL, Passera L (1991) Pheromonal and behavioral queen control over the production of gynes in the Argentine ant Iridomyrmex humilis (Mayr). Behav Ecol Sociobiol 28:161–169
Vargo EL, Passera L (1992) Gyne development in the Argentine ant Iridomyrmex humilis role of overwintering and queen control. Physiol Entomol 17:193–201
Volny VP, Gordon DM (2002) Genetic basis for queen–worker dimorphism in a social insect. Proc Natl Acad Sci USA 99:6108–6111
Wagner D, Tissot M, Cuevas W, Gordon DM (2000) Harvester ants utilize cuticular hydrocarbons in nestmate recognition. J Chem Ecol 26:2245–2257
Walin L, Seppä P (2001) Resource allocation in the red ant Myrmica ruginodis—an interplay of genetics and ecology. J Evol Biol 14:694–707
Wheeler DE (1994) Nourishment in ants: patterns in individuals and societies. In: Hunt JH, Nalepa CA (eds) Nourishment and evolution in insect societies. Westview, Boulder, CO, pp 245–278
Wilson EO (1971) The insect societies. Harvard Univ. Press, Cambridge.
Winston M, Higo HA, Slessor KN (1990) Effects of various dosages of queens mandibular gland pheromone on the inhibition of queen rearing in the honey bee (Hymenoptera: Apidae). Ann Entomol Soc Am 83:234–238
Woyciechowski M, Łomnicki A (1987) Multiple mating of queens and the sterility of workers among eusocial Hymenoptera. J Theor Biol 128:317–327
Zar JH (1984) Biostatistical analyses. Princeton Hall, London
Acknowledgments
This work was supported by “Ecodoca” (European Community—Access to Research Infrastructure action of the Improving Human Potential Program at Doñana Biological Station) to AL and AH and “Picasso” no. 09137XM to AL and XC. RB and XC were supported by grant CGL2006-04968/BOS. Jean-Philippe Christides helped in the chemical analyses. Colony collection and demographic data were obtained with the help of Fernando Amor, Angel Barroso, Ana Carvajal, Raymond Jegat, Isabel Luque, Thibaut Monnin, and Javier Retana. We thank Ray Heithaus and David Lubertazzi for valuable information on A. rudis and Saturo Iwanishi for interesting comments on the manuscript. We thank Naomi Paz for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Heinze
Rights and permissions
About this article
Cite this article
Boulay, R., Hefetz, A., Cerdá, X. et al. Production of sexuals in a fission-performing ant: dual effects of queen pheromones and colony size. Behav Ecol Sociobiol 61, 1531–1541 (2007). https://doi.org/10.1007/s00265-007-0385-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-007-0385-3