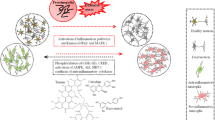
Abstract
Alzheimer’s disease (AD) is a chronic neurodegenerative disease for which there are no effective pharmaceutical drugs; their action is restricted only to symptomatic relief. Recently, it was evidenced that therapeutic interventions may delay or prevent the progression of age-related neurocognitive decline. Grape is one of the most cultivated traditional fruits in the entire world; grape-derived extracts showed several biological activities that counteract the neurodegenerative damage of AD. Grape-derived extracts are natural sources of polyphenols that could sustain a healthy brain aging through exerting anti-oxidative, anti-inflammatory, anti-acetylcholinesterase, and anti-amyloidogenic activities. In the present review, we highlight the mechanisms underlying the neuromodulating capacity of grape-derived polyphenolic extracts and compounds, especially grape seed extract, grape leaves extract, and resveratrol. However, more research work is required to estimate the most active therapeutic extracts and compounds and their brain bioavailability.
Similar content being viewed by others
Introduction
Dementia affects around 50 million people, and there are approximately 10 million new cases every year worldwide (World Health Organization (WHO) 2019). The prevalence of dementia, mostly Alzheimer’s dementia, is increasing in the whole world. Alzheimer’s disease (AD), the most common form of dementia, accounts for approximately 60 to 80% of cases; it affects almost 10% of individuals over 65 years (Alzheimer’s disease International 2016). AD nearly doubles every 5 years in individuals aged 65 to 85 years (Alzheimer’s Association 2018) and poses a great socio-economic burden for care and treatment (Olesen et al. 2012). Unfortunately, there is a lack of effective treatment strategies to combat this neurodegenerative disorder. Therefore, many researchers believe that future therapeutic approaches to slow or halt the progression of AD and preserve brain function will be most effective when administered early in the disease process (Alzheimer’s Association 2019).
AD is a proteinopathy or protein conformational disease, as it is associated with extracellular accumulation of amyloid-β (Aβ) peptide in susceptible brain regions (Hajieva 2017). AD dementia is characterized by progressive memory loss and neurocognitive decline (Scheltens et al. 2016). The key features of AD include neuroinflammation and dysfunctional cholinergic neurotransmissions (Rojo et al. 2008). AD is characterized by two histopathological hallmarks, located in the cerebral cortex and hippocampus, the extracellular accumulation of senile amyloid-β (Aβ-plaques), and the formation of neurofibrillary tangles (NFTs), composed of hyperphosphorylated tau proteins (Duyckaerts et al. 2009). The cumulative effects of both oxidative stress and neuroinflammation contribute to AD neuropathogenesis; it seems that oxidative stress precedes the deposition of Aβ-plaques (Wahlster et al. 2013). Moreover, microglial and astrocytic activation contributes to the neurodegeneration and enhances Aβ-neurotoxicity (Sadigh-Eteghad et al. 2016).
Current therapeutic strategies and searching for alternative nutraceuticals
Mild cognitive impairment (MCI) patients are more prone to develop incident AD, and most have significant brain amyloid burden (Petersen 2004). There is a deep interest to develop interventions to prevent MCI progression into AD dementia through targeting the multiple aspects of the underlying pathology of AD development (Kamphuis and Scheltens 2010); this is supposed to have the greatest therapeutic potential in prodromal AD stages. The currently available therapeutic interventions failed to completely cure AD; they only relieve symptoms and have little or no effect on slowing or reversing AD progression (Lindsley 2012). Hence, there is a pivotal need for the development of a new generation of effective, nutrition-based therapies of natural origin to enhance cognitive performance (Habtemariam 2016; Pasinetti et al. 2015). Several nutraceuticals are natural antioxidants that showed neuroprotective potentials such as polyphenols like quercetin, curcumin, and resveratrol (Kelsey et al. 2010). Consumption of Concord grape juice (Krikorian et al. 2010a), blueberry juice (Krikorian et al. 2010b), and flavanols (Desideri et al. 2012) improved verbal learning and memory formation in MCI patients.
In this review, we discuss the potential neuroprotective effect and neuromodulating activities of grape-derived polyphenols in AD, with a focus on resveratrol, grape seed extract, and grape leaves extract. Therefore, grape-derived polyphenols have been the topic of numerous studies related to neurodegeneration. Naturally occurring grape polyphenols showed prominent therapeutic potential for AD.
This review included studies from three databases “Web of Science, SCOPUS, and PubMed.” Search terms were “Alzheimer’s disease,” “polyphenols,” “Grape-derived polyphenols,” “AD-induced,” “resveratrol,” and “prevention and/or treatment of Alzheimer’s disease.” Different synonyms of the used terms such as “Alzheimer’s disease,” “AD” or “Alzheimer’s dementia,” “Grape,” “Grapevine” or “Vitis vinifera” and “Polyphenols” or “Phenolic compounds” were all included to develop a search strategy that is applied in all the searches on the databases. Studies have been first screened by title, then by abstract, and finally by reading the full text. Besides the search terms, studies were selected based on certain inclusion/eligibility and exclusion criteria. Articles were included in this review if the following inclusion criteria were met: (1) neuroprotective activity of grape polyphenols (compounds or extracts) against AD; (2) use of an interventional study design; (3) the study involved AD-induced animals; and (4) the study was published in a peer-reviewed scientific journal (editorial material, conference abstracts, retracted publications, and book chapters were excluded). The research article, to be included, should investigate the activity of grape-derived extract or compound and at least one hallmark of AD pathology such as amyloid-β. All studies were in English and published between 2008 and 2019. Moreover, the lists of references of all the included articles were screened to identify further articles meeting the inclusion criteria. The exclusion criteria included “genetic,” “AD-patients,” “dementia,” and “clinical.” This review demonstrated all the recent and relevant studies on the beneficial effects of grape-derived polyphenols in AD-induced animals as shown in Table 1.
Polyphenols
Polyphenols are naturally occurring compounds in the fruits, vegetables, tea, coffee, chocolates, legumes, cereals, and herbal beverages (Ganesan and Xu 2017). They are the most abundant class of plant secondary metabolites, with varied and complex chemical structure. Fruits like grapes, berries, apple, cherries, and pear contains up to 200–300 mg polyphenols per 100 g fresh weight (Pandey and Rizvi 2009). Polyphenols are classified based on the number of phenol rings and the chemical groups attached to the rings into “simple phenols,” which have a single aromatic ring with one or more hydroxyl groups, and the more common “polyphenolic compounds” which have multiple phenol rings (Tsao 2010). According to the number of rings and their binding affinity for different compounds, phenols can be divided into the flavonoids and the non-flavonoids (Fraga et al. 2010) (Fig. 1). Flavonoids, the largest group of polyphenols, are divided into six groups: anthocyanins, flavanones, iso-flavones, flavones, flavanols, and flavonols (D’Archivio et al. 2007). Flavonoids are composed of two aromatic rings connected through a three-carbon bridge, which form the C6-C3-C6 structural backbone (Fraga et al. 2010), such as catechins (Ciechanover and Kwon 2015). Flavonoids showed neuromodulating activities ascribed to their ability to restore the neuronal oxidant/antioxidant balance, as well as to disrupt the aggregation potential of neurotoxic proteins (Habtemariam 2016). On the other hand, non-flavonoids are composed of at least one aromatic ring that is connected to one or more hydroxyl groups, such as phenolic acids, lignans, and stilbenes (Surai 2014). Phenolic acids are present in grapes, red wine, and coffee (Han et al. 2017).
Polyphenols as possible therapeutic interventions
Polyphenols are of great therapeutic potential in neurodegenerative diseases, inflammation, dyslipidemia, and immune system diseases (Moosavi et al. 2016). Due to their multiple (pleiotropic) biological activities and potential health-promoting properties, polyphenols have been described as neuroprotective, anti-inflammatory, anti-amyloidogenic, anti-cholinesterase, anti-amnesic, hypolipidemic, and anti-aging agents (Zhang et al. 2015). Actually, the anti-oxidative and anti-aging properties of natural compounds could be of great benefit for management of neurodegenerative disorders (Borai et al. 2017; Rasool et al. 2014).
Polyphenols are dietary supplements capable of slowing down the neurocognitive decline during aging and AD (Fernández-Fernández et al. 2012). Dietary intake of polyphenols is associated with a reduced incidence of age-related dementia, improved neurocognitive decline, and delayed onset of AD dementia (Lamport et al. 2016; Pasinetti et al. 2015). Moreover, it is strongly associated with improvement of neurocognitive performance in middle-aged and aged individuals, who are either with normal aging or at risk for neurodegenerative diseases (Kesse-Guyot et al. 2012; Cimrová et al. 2011). This neurotherapeutic potential might occur through neuromodulating activities such as inhibiting neuroinflammation, inducing neurogenesis, and enhancing memory formation and consolidation (Vauzour 2012; Vauzour et al. 2008). Pharmacological research is interested in polyphenols as a nature-based treatment for brain disorders such as AD, due to their favorable safety profile and availability (Bensalem et al. 2016; Vauzour 2014; Nehlig 2013).
Grape polyphenols
Grape (Vitis vinifera L.) is the second most cultivated fruit in the world that is consumed fresh, dried, or processed into wine (Pari and Suresh 2008). The grape fruit is used in traditional medicine; because of its nutritional value and its high polyphenolic content, in addition to vitamins, minerals, and organic acids (Benmeziane et al. 2014). Grapes, grape seeds, grape leaves, and grape pomace are rich sources of flavonoids, including monomeric phenolic compounds such as epicatechins, procyanidins, and catechins (Surai 2014). Grape phenolics demonstrated antioxidants potential (Ismail et al. 2014; Schnee et al. 2013). For instance, polyphenols extracted from grape leaves exhibited anti-oxidant, anti-diabetic, anti-inflammatory, anti-aging effects, and anti-lipid potentials (Borai et al. 2017; Petersen and Smith 2016).
Bioactive dietary polyphenol preparation (BDPP) is a nutraceutical therapeutic combination formed of three bioactive and bioavailable grape-derived polyphenols-rich preparations (Concord grape juice, grape seed extract, and resveratrol) (Pasinetti et al. 2015). BDPP is designed to delay the conversion of MCI into AD, to multi-target the pathogenic pathways of AD progression, and to present more comprehensive coverage of AD pathogenic targets; each component exerts its unique mechanism of action (Wang et al. 2014). BDPP targets amyloid burden, synaptic plasticity, metabolic syndrome, and neurocognition, thus BDPP will demonstrate the cumulative efficiency of the three components and could be administrated to MCI individuals as a nutraceutical (Pasinetti et al. 2015).
Actually, there is a growing interest in nutritional intervention studies to confirm the neuromodulatory role of polyphenols on brain health and function, as well as to sustain the idea of healthy brain aging (Bensalem et al. 2015). At the same time, it is necessary to better define the metabolic profile, absorption profiles, and bioavailability of polyphenols to translate polyphenols-based therapeutic approach in AD animal models into a clinical therapeutic benefit (Mancuso et al., 1822; D’Archivio et al. 2010).
Grape polyphenols and Brain health
Brain-targeting grape-derived polyphenols showed promising anti-neurodegenerative properties (Wang et al. 2008). In the brain, most grape polyphenols or at least their metabolites, such as resveratrol, can cross the blood-brain barrier (BBB) in sufficient concentrations, to evoke neuroprotective effects and memory-enhancing potential through inhibiting acetylcholinesterase (AChE) activities and enhancing Aβ-clearance (Rizk et al. 2018; Borai et al. 2017). Grape polyphenols, mainly catechin, epicatechin, procyanidins, suppressed Aβ-oligomerization, and synaptic dysfunction in the brain, by interfering with specific binding sites of the Aβ-peptide (Ono et al. 2012; Ono et al. 2008). Consumption of Concord grape juice for 12 weeks resulted in amelioration of memory formation, evaluated by the Californian-learning test, in aged rats (Shukitt-Hale et al. 2006) and older humans (Krikorian et al. 2010b).
Resveratrol
Resveratrol (3,5,4′-trihydroxystilbene) belongs to stilbenes class, mainly found in the skins of mulberries, grapevines, and pomegranates (Vitrac et al. 2005). Resveratrol (RESV) is also a member of anti-microbial and low molecular weight phytoalexins that are denovo-synthesized by the plants as a defense mechanism after a pathogenic attack (Cvejic et al. 2010). The chemical structure of RESV contains two phenolic rings connected by a double bond that may undergo “cis- or trans- isomerization” by UV exposure (Song et al. 2012). RESV-derived compounds such as arachidin-1 and arachidin-3 showed health-promoting effects (Ball et al. 2015). Moreover, more than 300 RESV oligomers have been characterized (Keylor et al. 2015).
Water-soluble RESV is capable of crossing the BBB to exert its neuromodulating effects in the brain (Breuer et al. 2006), and improve neurocognitive decline (Zhao et al. 2013; Abraham and Johnson 2009). Therefore, RESV is a pleiotropic (multi-target) polyphenol that exerts neuroprotective activities through upregulation of brain-redox imbalance, interactions with signaling pathways related to neuronal function and survival, inhibition of Aβ-oligomerization, suppression of cholinesterase activity, and finally inhibiting neurodegeneration (Ahmed et al. 2016).
Grape-derived polyphenolic extracts
Grape and its different parts demonstrated an outstanding nutritional and medicinal value for thousands of years that might be ascribed to their potent anti-oxidative activities, due to the high polyphenolic content; for example, grape seed extract contains approximately 60–70% of polyphenolic compounds (Garcia-Marino et al. 2006; Nawaz et al. 2006). Therefore, regular administration of grape-derived polyphenolic extracts as nutritional intervention could halt or attenuate neurodegeneration (Moosavi et al. 2016). Administration of proanthocyanidin-rich grape seed extract demonstrated anti-inflammatory, antioxidant, antiapoptotic, anti-fibrotic, and vasodilatory effects (Xu et al. 2015; Hemmati et al. 2008). Grape seeds-derived polyphenolic compounds such as flavonoids, catechin, epicatechin, and flavan-3-ols are of great interest in pharmaceutical supplements and nutritional interventions (Garcia-Marino et al. 2006). On the other hands, grape leaves are composed of a vast range of polyphenols including anthocyanins, flavonoids, and also organic acids, like malic, oxalic, tartaric, citric, fumaric, and succinic acids (Ismail et al. 2014). These polyphenols showed an outstanding anti-oxidative potential (Schaffer et al. 2016; Lakshmi et al. 2014), and have been suggested to be used in the treatment of oxidative stress-induced neurodegenerative disorders (Schnee et al. 2013; Dani et al. 2010).
Antioxidant potential and iron-chelating activity of polyphenols
The brain is particularly susceptible to oxidative damage because it consumes a large amount of oxygen and contains high contents of polyunsaturated fatty acids (PUFAs), in addition to the presence of relatively low antioxidant defense enzymes (Wu 2005). Therefore, several in vitro and in vivo studies evidenced the neuroprotective potential of grape polyphenols that mainly attributed to their direct anti-oxidant and metal-scavenging activities (Rizk et al. 2018; Cong et al. 2016; Basli et al. 2012; Dani et al. 2010). Polyphenols are able to act as free radical scavengers and to give electrons to reactive radicals, rendering them into unreactive and more stable species (Baiomy 2016; Lakshmi et al. 2014). This anti-oxidative potential might be correlated to their complex chemical structure (Petti and Scully 2009) and phenolic content as phenolic compounds may indirectly prevent chelation of transition metal ions, thus inhibiting the generation of reactive hydroxyl radicals (HO•) (Halliwell 2008). In addition, polyphenols restrict cellular generation of reactive oxygen species (ROS) by suppressing the activity of pro-oxidant enzymes (protein kinase C, NAD(P)H oxidase, and xanthine oxidase) which participate in the intracellular electron transport chain (Procházková et al. 2011).
Clinical application of polyphenols as antioxidant-based therapy is a promising, safe, and effective approach to attenuate oxidative stress-induced neurodegenerative disorders (Bhullar and Rupasinghe 2013). For example, RESV is a potent antioxidant capable of extending the antioxidant potential by decreasing the generation of free radicals and superoxide ions (Gresele et al. 2008). RESV showed in vitro and in vivo anti-oxidative potential (Chang et al. 2012; Mikula-Pietrasik et al. 2012). Thus, RESV consumption can halt or prevent neurodegeneration, and limit or reverse the age-dependent neurocognitive decline (Bensalem et al. 2016; Vauzour 2014).
On the other side, grape-derived extracts present an important anti-oxidant activity in the neural tissues, as grape seed extract can react with free radicals and catalyzed metal ions, then terminate chain reactions by removing radical intermediates, and inhibit other oxidation reactions by being oxidized themselves (Sanchez-Moreno et al. 1999). Moreover, supplementation of grape seed extract to aged rats inhibited the accumulation of oxidative DNA damage and normalized lipid peroxidation and antioxidant activities (Balu et al. 2006). In addition, grape leaves polyphenolic extract is able to counteract the H2O2-induced enzymatic alterations (Dani et al. 2010). In our previous work, the administration of the grape leaves polyphenolic extract to AD-induced rats showed a remarkable anti-oxidative potential and supported the therapeutic efficiency of this polyphenolic extract against AD. Thus, our results demonstrated that grape leaves polyphenols attenuated AlCl3 neurotoxicity via anti-oxidative and anti-apoptotic actions (Rizk et al. 2018; Borai et al. 2017).
Anti-inflammatory and immunomodulatory potential of polyphenols
The antioxidant capacity is strongly correlated to the immunomodulatory and anti-inflammatory role, as polyphenols can mitigate the side effects of oxidative stress generated during the immune reactions, thus promoting immunogenic function (Kamboh et al. 2015). Polyphenols exert immunomodulatory and anti-inflammatory potential through controlling enzymes and inflammatory mediators and partial regulation of the activity of transcription factors such as downregulation of the nuclear factor-kappa B (NF-κB) and affecting a number of glial and neuronal signaling pathways (Chiva-Blanch and Visioli 2012). Moreover, polyphenols demonstrated different suppressing actions on the release of pro-inflammatory cytokines, production of nitric oxide (NO) and prostaglandins (PGE2), as well as ROS generation in activated glia, and finally inhibiting microglia priming through toll-like receptors (TLR) activation (Vauzour 2014; Gonzalez-Gallego et al. 2010). This might take place through the interaction of polyphenols with molecular signaling cascades and related machinery that regulate cellular processes such as inflammation (Habauzit and Morand 2012).
There are other indirect mechanisms such as suppression of redox-sensitive transcription factors, e.g., nuclear factor-κB (NF-κB) and activator protein-1 (AP-1), upregulation of antioxidant enzymes, and inhibition of pro-oxidant enzymes (Saso and Firuzi 2014). The immunomodulatory potential of polyphenols was demonstrated by suppressing the expression of NF-κB (transcriptional regulator of inflammation and apoptosis), blocking neuroinflammation through activation of SIRT1 pathway along with modulation of inflammatory mediators (Bhullar and Rupasinghe 2013). For example, polyphenols are capable of selectively targeting NF-κB and attenuating its activation (Kuo et al. 2015; Chu 2014; Spencer et al. 2012).
RESV is a potent NF-κB inhibitor with therapeutic potential against neuroinflammation (Solberg et al. 2014). RESV administration to ovariectomized and d-galactose-induced AD rats resulted in the reduction of hippocampal NF-κB (Zhao et al. 2015). Furthermore, the oral supplementation of RESV to healthy subjects for 6 weeks showed a suppressive effect on oxidative stress and inflammation with a decrease in NF-κB binding (Ghanim et al. 2010). Quenching NF-κB by RESV and its analogues can reduce AD-associated inflammation (Lukiw 2012). RESV proves its neuroprotective potential through increasing nuclear factor erythroid 2-related factor 2 (Nrf2) expression, downregulating apoptotic enzymes like caspase-3 (Ren et al. 2011), and attenuating matrix metallopeptidase 9 (MMP-9) (Cheng et al. 2009).
Moreover, administration of grape seed procyanidins (100 or 150 mg/kg) to weaned piglets resulted in a significant increment in the concentrations of complement 4 (C4), interleukin-2 (IL-2), and immunoglobulins (IgG and IgM); ameliorated the antioxidant capacity; and reduced lipid peroxidation, as compared to controls (Hao et al. 2015). In addition, grape seeds proanthocyanidins showed immunomodulatory role in inflammatory conditions (De la Cerda-Carrasco et al. 2015). Polyphenols-rich extracts from grape seeds and grape pomace suppressed the activation of stress signals such as NF-κB and Nrf2 (Gessner et al. 2013; Zhou and Raffoul 2012).
However, the effective concentration of polyphenols to exert in vivo anti-oxidative scavenging potential is unattainable due to their very limited bioavailability and extensive metabolism in the gut and the liver. Thus, it was suggested that in lower amounts, typical of those attained in the diet, polyphenols may exert pharmacological activity (Vauzour et al. 2010).
Neuromodulating potential of polyphenols
There are several mechanisms exerted by polyphenols concerning their neuroprotective potential (Table 1), including reduction of neuroinflammation-induced neural damage, altering ROS/RNS generation, attenuation of deposition of NFTs and Aβ-plaques, and activation of signaling pathways critical in controlling synaptic plasticity (Bensalem et al. 2016). Additionally, polyphenols have a positive impact on brain health, through affecting peripheral and cerebrovascular blood flow (Jagla and Pechanova 2015; Kay et al. 2012), demonstrating their capacity to induce vascular effects capable of supporting new nerve cell growth in the hippocampus (Vauzour 2012; Spencer 2009). A higher microvascular density in association with the increase of cerebral blood flow (CBF) might induce performance by direct increase supply of glucose and oxygen in the brain (Oomen et al. 2009). Moreover, 2 g/day of grape seed extract (1 g of polyphenols) also improved endothelial function in subjects with high vascular risk (Clifton 2004).
Briefly, the molecular mechanisms underlying the neuroprotective actions of polyphenols include anti-inflammatory, anti-oxidant activities, modulation of cell signaling pathways, and anti-amyloidogenic potential through direct interaction with amyloidogenic proteins (Hügel and Jackson 2015). Additional mechanisms include direct effects on signaling pathways to promote neuronal communication, the ability to buffer against excess calcium, enhanced hippocampal neurogenesis, alterations in neuronal morphology, enhancement of stress shock proteins, alteration of inflammatory gene expression, and neuroprotection against excitotoxic stress, and reduction in inflammatory mediators such as NF-κB (Kaewkaen et al. 2012). In vitro studies showed that grape extracts are strong free radical scavengers (Bagchi et al. 2000; Fauconneau et al. 1997) and are capable of inhibiting the production of free radicals, protecting neuronal cells from oxidative stress and cellular DNA damages, and inhibiting Aβ-induced neurocytotoxicity in brain cells (Basli et al. 2012).
Grape seed polyphenolic extract is capable of attenuating neurodegeneration in transgenic AD mice, through increasing the brain bioavailability of flavan-3-ol molecules (e.g., catechin) and stimulating antioxidant mechanisms (Ho et al. 2013, Wang et al. 2012a, 2012b). In addition, this extract showed memory-enhancing potential in middle-aged and adult rats (Devi et al., 2011; Devi et al., 2006).
Therefore, a better understanding of the neuroprotective effects and the multiple molecular mechanisms of action of polyphenols on the nervous system could help design better agents/drugs for management of neurodegenerative diseases (Moosavi et al. 2016). Future work is needed to reveal the mechanistic actions of polyphenol-induced neurocognitive improvement.
The anti-amyloidogenic activity of polyphenols
The anti-amyloidogenic activity of polyphenols is primarily attributed to their ability to bind directly to Aβ-fibrils, preventing further Aβ-oligomerization, and impairing their stability through metal-chelating activity or formation of nontoxic oligomers (Rizk et al. 2018; Amit et al. 2008). This might be linked to the direct effect of polyphenols on the processing of amyloid precursor protein (APP) through the inhibition of β-secretase (amyloidogenic) and/or activation of α-secretase (non-amyloidogenic) pathways (Mori et al. 2012).
Polyphenols attenuate AD dementia by modulating Aβ-neuropathology through inhibiting Aβ-oligomerization, promoting Aβ clearance, preventing the formation of neurotoxic oligomers, and inhibiting Aβ-membrane interactions (Caruana et al. 2012; Smid et al. 2012). The ability of polyphenols to interact with proteins in the brain renders them neuroprotective (Fernandes et al. 2017). Polyphenols interact with Aβ-peptides and form polyphenol-Aβ protein interaction that blocks self-association of Aβ-42 monomers to form low molecular weight oligomers. Thus polyphenols could function as Aβ-42 inhibitors; therefore, modifying polyphenol structures will improve their pharmacokinetics and efficacy (Fig. 2). The mechanism of Aβ inhibition is driven by hydrophobic interactions that involve π-π bonding between the planar faces of the polyphenol structure and the aromatic residues of Aβ-42. Additionally, hydrogen bonding occurs between the peptide and the phenolic hydroxyl groups (Hügel and Jackson 2015).
For example, administration of the grape seed extract to transgenic AD mice, genetically modified to express APP, enhanced Aβ clearance and showed improved cognition, as compared to control mice (Liu et al. 2011). Interestingly, quercetin-3-O-glucuronide, from red wines and Concord grape juice, is capable of reaching the brain and reducing Aβ production, reducing Aβ oligomerization, and promoting neuroplasticity processes (Ho et al. 2013).
Activation of AMP-activated protein kinase (AMPK) could be regarded as an anti-amyloidogenic strategy (Vingtdeux et al. 2010; Wang et al. 2010a, 2010b; Vingtdeux et al. 2008). Orally administered RESV can cross the BBB to activate autophagy and brain AMPK signaling, to decrease microglial activation, to reduce Aβ production and deposition in the brain, and to promote Aβ clearance in AD murine model (Solberg et al. 2014; Capiralla et al. 2012; Vingtdeux et al. 2010). Furthermore, treatment of APP-transgenic mice with RESV reduced Aβ-42 deposition in the brain and protected from Aβ-induced neurotoxicity (Huang et al. 2011; Karuppagounder et al. 2009). This anti-amyloidogenic potential of RESV might take place through the ability of RESV to prevent Aβ-oligomerization, as well as its ability to disrupt existing Aβ deposits (Ghobeh et al. 2014). Moreover, RESV enhances the binding of transthyretin, a transporter protein, to Aβ-oligomers and therefore preventing Aβ-plaque aggregation (Ribeiro et al. 2012). Resveratrol and its derivatives strongly inhibited the fibrillization of Aβ-peptides (Richard et al. 2011). In addition to AMPK activation, RESV exerts its neuroprotective potential through activation of protein kinase C, which stimulates α-secretase enzyme and consequently the non-amyloidogenic pathway, resulting in a reduction in the Aβ production (Bastianetto et al. 2015). Quercetin also displayed anti-amyloidogenic potential and reversed Aβ-induced neurotoxicity in a cell system over-expressing APP Swedish mutation (APPswe), which is associated with early-onset familial AD (Jimenez-Aliaga et al. 2011). In addition, quercetin-3-O-glucuronide, quercetin metabolite, is capable of destabilizing the brain-targeted formation of neurotoxic Aβ-oligomers and improving neuroplasticity processes, through the activation of the c-Jun N-terminal kinases and the mitogen-activated protein kinase signaling pathways (Ho et al. 2013).
Recently, the grape leaves polyphenolic extract was found to reduce Aβ-induced oxidative stress, thus suggesting that this natural extract is a promising neuroprotective agent against Aβ-induced neuroinflammation in AlCl3-induced AD-rats (Rizk et al. 2018). In addition, the grape seed extract inhibited the oligomerization of Aβ-peptides and improved neurocognitive function in AD models (Bhullar and Rupasinghe 2013; Liu et al. 2011). Therefore, preventing the accumulation of neurotoxic Aβ-deposits in the brain might provide a useful therapeutic intervention against AD (Guerrero-Munoz et al. 2014). The ability of polyphenols to improve synaptic transmission by elevating cAMP, targeting multiple signaling pathways, and reducing Aβ-neurotoxicity suggest their therapeutic potential against age-related disorders like AD and dementia (Bhullar and Rupasinghe 2013).
On the other hand, polyphenols inhibit abnormal tau phosphorylation and tau aggregation (Ho and Pasinetti 2010; Wang et al. 2008). Polyphenolic grape-derived extracts such as proanthocyanidins-enriched grape seed extracts are capable of interfering with tau-induced neurotoxicity by inhibiting abnormal aggregation of hyperphosphorylated tau (Santa-Maria et al. 2012; Wang et al. 2010a, 2010b), possibly through non-covalent interactions of polyphenols with tau proteins (Ho et al. 2009).
Polyphenols and anti-acetylcholinesterase activity
Acetylcholine (ACh) deficiency is one of the main pathological hallmarks of AD, which renders acetylcholinesterase (AChE) inhibitors as important AD drugs (Khan et al. 2009). Polyphenols could act as AChE inhibitors by accessing the brain and exerting a memory-enhancing effect, for example, Vitis-derived polyphenolic extracts inhibit brain/serum AChE in AD-rats (Bhullar and Rupasinghe 2013; Fernández-Fernández et al. 2012). In our previous study, AD rats administrated the grape leaves polyphenolic extract showed cognitive recovery, assessed by T-maze, through increasing ACh and IL-6, inhibiting AChE, increasing BDNF (the classic neurotrophic factor) level, and finally enhancing neuronal survival and plasticity (Borai et al. 2017). Similarly, administration of pure flavanols to senile rats increased BDNF levels and enhanced memory formation (Rendeiro et al. 2013). Moreover, quercetin improved neurocognitive ability and exhibited neuroprotection against trimethyltin-induced neurotoxicity by inhibiting AChE (Choi et al. 2012). In addition, quercetin inhibited AChE activity and improved neurocognitive function in streptozotocin-induced mice (Tota et al. 2010) (Fig. 2).
Brain localization and bioavailability of polyphenols
The chemical structure of polyphenols determines the rate and the extent of absorption and the nature of the blood-circulating metabolites, and dietary intake of polyphenols increased the antioxidant capacity, indicating their absorption through the gut barrier (Rodrigo et al. 2011); only approximately 5–10% of polyphenols are absorbed and metabolized in biochemical pathways (Chiva-Blanch and Visioli 2012). Moreover, polyphenols, or at least key metabolites, can cross BBB, accumulate in the brain in sufficient and pharmacologically relevant concentrations, and exert neuroprotective actions through modulating brain health and function (Bensalem et al. 2016; Ho et al. 2013). Both antioxidant and neuromodulating potential of polyphenols or their metabolites depend on their bioavailability and their BBB-crossing capacity (Pandareesh et al. 2015; Del Rio et al. 2013). Their brain permeability is totally controlled by the degree of lipophilicity and polarity of each polyphenolic compound (Youdim et al. 2003). In addition, stereoactive interaction of polyphenols with specific efflux transporters expressed on endothelial cells of the BBB is another factor (Faria et al. 2011). For example, RESV is selectively localized in different brain regions; this might be attributed to specific metabolism of RESV in the brain or its diffusion rate (Pasinetti et al. 2015). Brain-penetrating polyphenol metabolites, such as quercetin-3-O-glucoside and 3′-O-methyl-epicatechin-5-O-β-glucuronide, are capable of modulating Aβ-neuropathogenic mechanisms and promoting synaptic plasticity by enhancing cAMP response element-binding protein (CREB) signal transduction, which is involved in memory formation and consolidation (Ho et al. 2013, Wang et al. 2012a, 2012b).
However, further work is needed to better understand polyphenol absorption, metabolism, tissue distribution, intracellular accumulation and excretion, and brain bioavailability. Furthermore, the structural changes and chemical biotransformation of polyphenols during metabolism and interaction with the BBB within the target brain need more clarification to determine their cerebral bioavailability (Bensalem et al. 2015). Actually, resolving the bioavailability of natural polyphenols is more challenging than with synthetic compounds (Rubio et al. 2014), because resident gut microbiota generates secondary metabolites (Van Duynhoven et al. 2011). This involves de-glycosylation, followed by the breakdown of phenolic rings into phenolic acids and aldehydes. After absorption, metabolites can be glucuronidated, sulfated, and/or methylated and are detected in the bloodstream, urine, and fecal samples (Chen et al. 2014). Only picomolar or nanomolar concentrations of intact polyphenols can reach the brain after oral administration (Rojo et al. 2016). Therefore, repeated ingestion of polyphenols over time is necessary to keep a high concentration of polyphenols in plasma (Baba et al. 2001; De Boer et al. 2005).
Despite poor bioavailability and increased metabolism (Juan et al. 2010), RESV showed neuroprotective potential in AD animal models (Walle 2011); moreover, it has been suggested that some of RESV metabolites such as RESV-3-sulfate and RESV-3-O-glucuronide and di- and tri-sulfated derivatives may be responsible for its neuroprotective activity (Wenzel et al. 2005).
Future studies should focus on administrating bioactive and BBB-permeable polyphenols in either monomeric forms or herbal formulae (Hügel and Jackson 2015). Moreover, further pre-clinical work is needed to determine the most neuroactive nutraceutical formulations, whether through the diet or supplement, to design and perform informative clinical trials (Bensalem et al. 2015). Therefore, it is necessary to define the “true bioactive molecules” and the role of metabolism in the pharmacology of ingested polyphenols (Rojo et al. 2016).
Administration of high dosage of polyphenols can lead to hepatotoxicity and nephrotoxicity due to increased hepatic biotransformations of polyphenols; therefore, the optimal dosage of single compounds or polyphenol-enriched extracts may increase their therapeutic efficacy (Albarracin et al. 2012). Based on chemical drug design, it is possible to ameliorate polyphenol bioavailability through designing and developing novel synthetic polyphenolic compounds with similar structures that favor BBB accessibility (Hajieva 2017; Ohlow et al. 2012).
Delivery system of polyphenols
To ensure better polyphenol delivery into the brain, new delivery systems are developed to overcome limitations related to the bioavailability of polyphenols and to improve their pharmacokinetics. This might include encapsulation of bioactive polyphenols in phospholipid nanoparticles (entrapment of polyphenols in lipid vesicles), their incorporation with biodegradable polymers, or their modifications by using adjuvants as absorption enhancers (Hügel and Jackson 2015; Mignet et al. 2013). However, administration of nanoparticle preparations for prolonged periods may give rise to the toxicity of the carriers that encapsulate active compounds (Mancuso et al. 2012).
Several resveratrol carriers were developed, for instance, cyclodextrin-RESV inclusion complexes (Soo et al. 2016), chitosan-coated lipid microparticles (Scalia et al. 2015), N-trimethylchitosan-g-palmitic acid surface-modified solid lipid nanoparticles (Ramalingam and Ko 2016), protein nanoparticles (Joye et al. 2015), and nano-carriers with gold and silver nanoparticles (Park et al. 2016). In AD rat model, intraperitoneal administration of nano-encapsulated RESV increased its bioavailability and its concentration in the brain and finally resulted in better neuroprotective potential than free RESV (Frozza et al. 2013; Frozza et al. 2010). This proves that the high bioavailability of RESV in lipid core nanocapsules is contributing to the therapeutic potential against AD (Frozza et al. 2013).
Conclusion and future directions
This review highlighted the potential therapeutic neuroprotective and/or neurorestorative potential of grape-derived polyphenols, with a special focus on grape-derived polyphenols. This takes place through the modulation of several mechanisms involved in AD such as oxidative stress, neuroinflammation, and Aβ-plaque formation. Grape-derived polyphenols showed anti-oxidative, anti-inflammatory, anti-amyloidogenic, and neuromodulating activities. Therefore, grape-derived polyphenols could be regarded as ideal candidates for counteracting the multifactorial nature of AD. Further research is required to develop novel formulations or to design chemical analogs to polyphenols with neuroprotective potential. In addition, there is an urgent need to translate the positive outcome of therapeutic intervention of polyphenols in pre-clinical studies, using AD animal models, into clinical application in AD-patients, to allow the incorporation of polyphenols in clinical treatment or dietary interventions to suppress AD progression and to decide the pharmacological relevance of polyphenols to humans.
Abbreviations
- ACh:
-
Acetylcholine
- AChE:
-
Acetylcholinesterase
- AD:
-
Alzheimer’s disease
- AMPK:
-
AMP-activated protein kinase
- AP-1:
-
Activator protein-1
- Aβ-plaques:
-
Amyloid-β plaques
- BBB:
-
Blood-brain barrier
- BDPP:
-
Bioactive dietary polyphenol preparation
- CBF:
-
Cerebral blood flow
- CNS:
-
Central nervous system
- MCI:
-
Mild cognitive impairment
- NFTs:
-
Neurofibrillary tangles
- NF-κB:
-
Nuclear factor kappa light chain enhancer of activated B cells
- NF-κB:
-
Nuclear factor-kappa B
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- PUFAs:
-
Polyunsaturated fatty acids
- RESV:
-
Resveratrol
- ROS:
-
Reactive oxygen species
- TLR:
-
Toll-like receptors
- WHO:
-
World Health Organization
References
Abraham J, Johnson RW (2009) Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res 12(6):445–453
Ahmed T, Javed S, Javed S, Tariq A, Šamec D, Tejada S, Nabavi SF, Braidy N, Nabavi SM (2016) Resveratrol and Alzheimer’s disease: mechanistic insights. Mol Neurobiol 54(4):2622–2635
Albarracin SL, Stab B, Casas Z, Sutachan JJ, Samudio I, Gonzalez J et al (2012) Effects of natural antioxidants in neurodegenerative disease. Nutr Neurosci 15:1–9
Alzheimer’s Association (2018) Alzheimer’s disease facts and figures. Alzheimers Dement 14(3):367–429
Alzheimer’s Association (2019) Alzheimer’s disease facts and figures. Alzhimers Demen 15(3):321–387
Alzheimer’s Disease International. The global voice on dementia. 2016. Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2016.pdf, Accessed 22 Jul 2016
Amit T, Avramovich-Tirosh Y, Youdim MBH, Mandel S (2008) Targeting multiple Alzheimer’s disease etiologies with multimodal neuroprotective and neurorestorative iron chelators. FASEB J 22(5):1296–1305
Baba S, Osakabe N, Natsume M, Muto Y, Takizawa T, Terao J (2001) In vivo comparison of the bioavailability of (+)-catechin, (−)-epicatechin and their mixture in orally administered rats. J Nutr 131:2885–2891
Bagchi D, Bagchi M, Stohs SJ, Das DK, Ray SD, Kuszynski CA, Joshi SS, Pruess HG (2000) Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicol 148:187–197
Baiomy AA (2016) Protective role of grape seeds extract against cadmium toxicity in the lung of male Wistar rats. J Cyto Histo S5:004
Ball JM, Medina-Bolivar F, Defrates K, Hambleton E, Hurlburt ME, Fang L, Yang T, Nopo-Olazabal L et al (2015) Investigation of stilbenoids as potential therapeutic agents for rotavirus gastroenteritis. Adv Virol 2015:293524
Balu M, Sangeetha P, Murali G, Panneerselvam C (2006) Modulatory role of grape seed extract on age-related oxidative DNA damage in central nervous system of rats. Brain Res Bul 68:469–473
Basli A, Soulet S, Chaher N, Mérillon JM, Chibane M, Monti JP, Richard T (2012) Wine polyphenols: potential agents in neuroprotection. Oxidative Med Cell Longev 2012:805762
Bastianetto S, Ménard C, Quirion R (2015) Neuroprotective action of resveratrol. Biochim Biophys Acta 1852:1195–1201
Benmeziane F, Djamai R, Cadot Y, Seridi R (2014) Optimization of extraction parameters of phenolic compounds from Algerian fresh table grapes, (Vitis Vinifera). Int Food Res J 21(3):1061–1065
Bensalem J, Dal-Pan A, Gillard E, Calon F, V’eronique P (2015) Protective effects of berry polyphenols against age-related cognitive impairment. Nut Aging 3:89–106
Bensalem J, Servant L, Alfos S, Gaudout D, Layé S, Pallet V, Lafenetre P (2016) Dietary polyphenol supplementation prevents alterations of spatial navigation in middle-aged mice. Front Behav Neurosci 10:9
Bhullar KS, Rupasinghe H (2013) Polyphenols: multipotent therapeutic agents in neurodegenerative diseases. Oxidative Med Cell Longev 2013:891748
Borai IH, Ezz MK, Rizk MZ, Aly HF, El-Sherbiny M, Matloub AA, Fouad GI (2017) Therapeutic impact of grape leaves polyphenols on certain biochemical and neurological markers in AlCl3-induced Alzheimer's disease. Biomed Pharmacother 93:837–851
Breuer C, Wolf G, Andrabi SA, Lorenz P, Horn TF (2006) Blood-brain barrier permeability to the neuroprotectant oxyresveratrol. Neurosci Lett 393(2):113–118
Capiralla H, Vingtdeux V, Zhao H, Sankowski R, Al-Abed Y, Davies P et al (2012) Resveratrol mitigates lipopolysaccharide- and Abeta-mediated microglial inflammation by inhibiting the TLR4/NF-kappaB/STAT sig-naling cascade. J Neurochem 120:461–472
Caruana M, Neuner J, Hogen T, Schmidt F, Kamp F, Scerri C et al (2012) Polyphenolic compounds are novel protective agents against lipid membrane damage by alpha-synuclein aggregates in vitro. Biochim Biophys Acta 1818:2502–2510
Chang CC, Chang CY, Huang JP, Hung LM (2012) Effect of resveratrol on oxidative and inflammatory stress in liver and spleen of streptozotocin-induced type 1diabetic rats. Chin J Physiol 55(3):192–201
Chen Z, Zheng S, Li L, Jiang H (2014) Metabolism of flavonoids in human: a comprehensive review. Curr Drug Metab 15:48–61
Cheng G, Zhang X, Gao D, Jiang X, Dong W (2009) Resveratrol inhibits MMP-9 expression by up-regulating PPAR α expression in an oxygen glucose deprivation-exposed neuron model. Neurosci Lett 451(2):105–108
Chiva-Blanch G, Visioli F (2012) Polyphenols and health: moving beyond antioxidants. J Berry Res 2:63–71
Choi GN, Kim JH, Kwak JH et al (2012) Effect of quercetin on learning and memory performance in ICR mice under neurotoxic trimethyltin exposure. Food Chem 132(2):1019–1024
Chu AJ (2014) Antagonism by bioactive polyphenols against inflammation: a systematic view. Inflamm Allergy Drug Targets 13:34–64
Ciechanover A, Kwon YT (2015) Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Exp Mol Med 47:e147
Cimrová B, Budáč S, Melicherová U, Jergelová M, Jagla F (2011) Electrophysiological evidence of the effect of natural polyphenols upon the human higher brain functions. Neuro Endocrinol Lett 32(4):464–468
Clifton PM (2004) Effect of grape seed extract and quercetin on cardiovascular and endothelial parameters in high-risk subjects. J Biomed Biotechnol 2004:272–278
Cvejic JM, Djekic SV, Petrovic AV, Atanackovic MT, Jovic SM, Brceski ID, Gojkovic-Bukarica LC (2010) Determination of trans- and cis-resveratrol in Serbian commercial wines. J Chromatogr Sci 48:229–234
D’Archivio M, Filesi C, DiBenedetto R, Gargiulo R, Giovannini C, Masella R (2007) Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanita 43:348–361
Dani C, Oliboni LS, Agostini F, Funchal C, Serafini L, Henriques JA, Salvador M (2010) Phenolic content of grapevine leaves (Vitis labrusca var. Bordo) and its neuroprotective effect against peroxide damage. Toxicol in Vitro 24:148–153
D’Archivio M, Filesi C, Varì R, Scazzocchio B, Masella R (2010) Bioavailability of the polyphenols: status and controversies. Int J Mol Sci 11(4):1321–1342
De Boer VC, Dihal AA, Van Der Woude H, Arts IC, Wolffram S, Alink GM et al (2005) Tissue distribution of quercetin in rats and pigs. J Nutr 135:1718–1725
De la Cerda-Carrasco A, López-Solís R, Núñez-Kalasic H, Peña-Neira A, Obreque-Slier E (2015) Phenolic composition and antioxidant capacity of pomaces from four grape varieties (Vitis vinífera L.). J Sci Food Agric 95:1521–15275
Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A (2013) Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 18:1818–1892
Desideri G, Kwik-Uribe C, Grassi D et al (2012) Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the Cocoa, Cognition, and Aging (CoCoA) study. Hyperten. 60:794–801
Devi A, Jolitha AB, Ishii N (2006) Grape seed proanthocyanidin extract (GSPE) and antioxidant defense in the brain of adult rats. Med Sci Monit 12(4):BR124–BR129
Devi AS, Chandrasekar BK, Manjula KR, Ishii N (2011) Grape seed proanthocyanidin lowers brain oxidative stress in adult and middle-aged rats. Exp Gerontol 46(11):958–964
Duyckaerts C, Delatour B, Potier MC (2009) Classification and basic pathology of Alzheimer disease. Acta Neuropathol 118:5–36
Faria A, Pestana D, Teixeira D, Couraud PO, Romero I, Weksler B et al (2011) Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food Funct 2:39–44
Fauconneau B, Waffo-Teguo P, Huguet F, Barrier L, Decendit A, Merillon JM (1997) Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro tests. Life Sci 61:2103–2110
Fernandes I, Pérez-Gregorio R, Soares S, Mateus N, de Freitas V (2017) Wine flavonoids in health and disease prevention. Mol. 22:292
Fernández-Fernández L, Comes G, Bolea I, Valente T, Ruiz J et al (2012) LMN diet, rich in polyphenols and polyunsaturated fatty acids, improves mouse cognitive decline associated with aging and AD. Behav Brain Res 228(2):261–271
Fraga CG, Galleano M, Verstraeten SV, Oteiza PI (2010) Basic biochemical mechanisms behind the health benefits of polyphenols. Mol Asp Med 31:435–445
Frozza RL, Bernardi A, Hoppe JB et al (2013) Neuroprotective effects of resveratrol against Abeta administration in rats are improved by lipid-core nanocapsules. Mol Neurobiol 47:1066–1080
Frozza RL, Bernardi A, Paese K, Hoppe JB et al (2010) Characterization of trans-resveratrol-loaded lipid-core nanocapsules and tissue distribution studies in rats. J Biomed Nanotechnol 6:694–703
Ganesan K, Xu B (2017) A critical review on polyphenols and health benefits of black soybeans. Nutrients. 9(5):455
Garcia-Marino M, Rivas-Gonzalo JC, Ibanez E, Garcia-Moreno C (2006) Recovery of catechins and proanthocyanidins from winery by-products using subcritical water extraction. Anal Chim Acta 563:44–50
Gessner DK, Fiesel A, Most E, Dinge SJ, Wen G, Ringseis R, Eder K (2013) Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress-responsive transcription factors NF-κB and Nrf2 in the duodenal mucosa of pigs. Acta Vet Scand 55:18–28
Ghanim H, Sia CL, Abuaysheh S, Korzeniewski K, Patnaik P, Marumganti A et al (2010) An antiinflammatory and reactive oxygen species suppressive effects of an extract of Polygonum cuspidatum containing resveratrol. J Clin Endocrinol Metab 95:E1–E8
Ghobeh M, Ahmadian S, Meratan AA, Ebrahim-Habibi A, Ghasemi A, Shafizadeh M, Nemat-Gorgani M (2014) Interaction of Abeta (25-35) fibrillation products with mitochondria: effect of small-molecule natural products. Biopolym. 102(6):473–486
Gonzalez-Gallego J, Garcia-Mediavilla MV, Sanchez-Campos S, Tunon MJ (2010) Fruit polyphenols, immunity, and inflammation. Br J Nutr 104(3):S15–S27
Gresele P, Pignatelli P, Guglielmini G et al (2008) Resveratrol, at concentrations attainable with moderate wine consumption, stimulates human platelet nitric oxide production. J Nutr 138(9):1602–1608
Guerrero-Munoz MJ, Castillo-Carranza DL, Kayed R (2014) Therapeutic approaches against common structural features of toxic oligomers shared by multiple amyloidogenic proteins. Biochem Pharmacol 88:468–478
Habauzit V, Morand C (2012) Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: an update for clinicians. Ther Adv Chronic Dis 3(2):87–106
Habtemariam S (2016) The therapeutic potential of Rosemary (Rosmarinus officinalis) Diterpenes for Alzheimer’s disease. Evid Based Complement Alternat Med 2680409:14
Hajieva P (2017) The effect of polyphenols on protein degradation pathways: implications for neuroprotection. Mol 22(1):159
Halliwell B (2008) Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys 476:107–112
Han X, Shen T, Lou H (2017) Dietary polyphenols and their biological significance. Int J Mol Sci 8:950–988
Hao R, Li Q, Zhao J, Li H, Wang W, Gao J (2015) Effects of grape seed procyanidins on growth performance, immune function and antioxidant capacity in weaned piglets. Livest Sci 178:237–242
Hemmati AA, Nazari Z, Samei M (2008) A comparative study of grape seed extract and vitamin E effects on silica-induced pulmonary fibrosis in rats. Pulm Pharmacol Ther 21:668–674
Ho L, Ferruzzi MG, Janle EM, Wang J, Gong B, Chen TY et al (2013) Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J 27:769–781
Ho L, Pasinetti GM (2010) Polyphenolic compounds for treating neurodegenerative disorders involving protein misfolding. Expert Rev Proteom 7(4):579–589
Ho L, Yemul S, Wang J, Pasinetti GM (2009) Grape seed polyphenolic extract as a potential novel therapeutic agent in tauopathies. JAD. 16:433–439
Huang TC, Lu KT, Wo YY, Wu YJ, Yang YL (2011) Resveratrol protects rats from A훽 induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS ONE 6(12):e29102
Hügel HM, Jackson N (2015) Polyphenols for the prevention and treatment of dementia diseases. Neural Regen Res 10(11):1756–1758
Ismail FH, Khalil MH, Al Seif FA, El-magdoub F, Abdel Rahman AN (2014) Biosynthesis of gold nanoparticles using extract of grape (Vitis Vinifera) leaves and seeds. PNN. 3:1–12
Jagla F, Pechanova O (2015) Age-related cognitive impairment as a sign of geriatric neurocardiovascular interactions: may polyphenols play a protective role? Oxidative Med Cell Longev 2015:721514
Jimenez-Aliaga K, Bermejo-Bescos P, Benedi J, Martin-Aragon S (2011) Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci 89:939–945
Joye IJ, Davidov-Pardo G, Ludescher RD, McClements DJ (2015) Fluorescence quenching study of resveratrol binding to zein and gliadin: towards a more rational approach to resveratrol encapsulation using water-insoluble proteins. Food Chem 185:261–267
Juan ME, Maijo M, Planas JM (2010) Quantification of trans-resveratrol and its metabolites in rat plasma and tissues by HPLC. J Pharm Biomed Anal 51:391–398
Kaewkaen P, Tong-Un T, Wattanathorn J, Muchimapura S, Kaewrueng W, Wongcharoenwanakit S (2012) Mulberry fruit extract protects against memory impairment and hippocampal damage in animal model of vascular dementia. Evid Based Complement Alternat Med 2012:263520
Kamboh AA, Arain MA, Mughal MJ, Zaman A, Arain ZM, Soomro AH (2015) Flavonoids: health promoting phytochemicals for animal production—a review. J Anim Health Prod 3:6–13
Kamphuis PJ, Scheltens P (2010) Can nutrients prevent or delay onset of Alzheimer's disease? J Alzheimers Dis 20:765–775
Karuppagounder SS et al (2009) Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer's disease. Neurochem Int 54(2):111–118
Kay CD, Hooper L, Kroon PA, Rimm EB, Cassidy A (2012) Relative impact of flavonoid composition, dose and structure on vascular function: a systematic review of randomised controlled trials of flavonoid-rich food products. Mol Nutr Food Res 56:1605–1616
Kelsey NA, Wilkins HM, Linseman DA (2010) Nutraceutical antioxidants as novel neuroprotective agents. Mol. 15(11):7792–7814
Kesse-Guyot E, Fezeu L, Andreeva VA et al (2012) Total and specific polyphenol intakes in midlife are associated with cognitive function measured 13 years later. J Nutr 142(1):76–83
Keylor MH, Matsuura BS, Stephenson CR (2015) Chemistry and biology of resveratrol-derived natural products. Chem Rev 115(17):8976–9027
Khan MH, Orhan I et al (2009) Cholinesterase inhibitory activities of some flavonoid derivatives and chosen xanthone and their modecular docking studies. Chem Biol Interact 181(3):383–389
Kong D, Yan Y, He XY et al (2019) Effects of resveratrol on the mechanisms of antioxidants and estrogen in Alzheimer’s disease. BioMed Res Inter 2019:8983752
Krikorian R, Nash TA, Shidler MD, Shukitt-Hale B, Joseph JA (2010a) Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br J Nutr 103:730–734
Krikorian R, Shidler MD, Nash TA et al (2010b) Blueberry supplementation improves memory in older adults. J Agric Food Chem 58:3996–4000
Kuo PH, Lin CI, Chen YH, Chiu WC, Lin SH (2015) A high cholesterol diet enriched with polyphenols from Oriental plums (Prunus salicina) improves cognitive function and lowers brain cholesterol levels and neurodegenerative-related protein expression in mice. Br J Nutr 113:1550–1557
Lakshmi BVS, Sudhakar M, Anisha M (2014) Neuroprotective role of hydroalcoholic extract of Vitis Vinifera against aluminium-induced oxidative stress in rat brain. Neurotox. 41:73–79
Lamport DJ, Pal D, Macready AL et al (2016) The effects of flavanone-rich citrus juice on cognitive function and cerebral blood flow: an acute, randomised, placebo-controlled cross-over trial in healthy, young adults. Br J Nutr 116:2160–2168
Lindsley CW (2012) Alzheimer’s disease: development of disease-modifying treatments is the challenge for our generation. ACS Chem Neurosci 3:804–805
Liu P, Kemper LJ, Wang J, Zahs KR, Ashe KH, Pasinetti GM (2011) Grape seed polyphenolic extract specifically decreases aβ*56 in the brains of Tg2576 mice. JAD. 26(4):657–666
Lukiw WJ (2012) NF-kappaB-regulated, proinflammatory miRNAs in Alzheimer’s disease. Alzheimers Res Ther 4(6):47
Mancuso C, Siciliano R, Barone E, Preziosi P (2012) Natural substances and Alzheimer’s disease: from preclinical studies to evidence based medicine. Biochim Biophys Acta 1822:616–624
Mignet N, Seguin J, Chabot GG (2013) Bioavailability of polyphenol liposomes: a challenge ahead. Pharmaceu. 5:457–471
Mikula-Pietrasik J, Kuczmarska A, Rubis B et al (2012) Resveratrol delays replicative senescence of human mesothelial cells via mobilization of antioxidative and DNA repair mechanisms. Free Radic Biol Med 52(11–12):2234–2245
Moosavi F, Hosseini R, Saso L, Firuzi O (2016) Modulation of neurotrophic signaling pathways by polyphenols. Drug Des Devel Ther 10:23–42
Mori T, Rezai-Zadeh K, Koyama N et al (2012) Tannic acid is a natural beta-secretase inhibitor that prevents cognitive impairment and mitigates Alzheimer-like pathology in transgenic mice. J Biol Chem 287(9):6912–6927
Nawaz H, Shi J, Mittal GS, Kakuda Y (2006) Extraction of polyphenols from grape seeds and concentration by ultrafiltration. Sep Purif Technol 48:176–181
Nehlig A (2013) The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Br J Clin Pharmacol 75(3):716–727
Ohlow MJ, Granold M, Schreckenberger M, Moosmann B (2012) Is the chromanol head group of vitamin E nature’s final truth on chain-breaking antioxidants? FEBS Lett 586:711–716
Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jonsson B, Group CS et al (2012) The economic cost of brain disorders in Europe. Eur J Neurol 19:155–162
Ono K, Condron MM, Ho L et al (2008) Effects of grape seed derived polyphenols on amyloid β-protein self-assembly and cytotoxicity. J Biol Chem 283(47):32176–32187
Ono K, Li L, Takamura Y, Yoshiike Y, Zhu L, Han F, Mao X, Ikeda T, Takasaki J et al (2012) Phenolic compounds prevent amyloid β-protein oligomerization and synaptic dysfunction by site-specific binding. J Biol Chem 287:14631–14643
Oomen CA, Farkas E, Roman V, Van Der Beek EM, Luiten PG, Meerlo P, Meerlo P (2009) Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci 1(4):2009
Pandareesh MD, Mythri RB, Srinivas Bharath MM (2015) Bioavailability of dietary polyphenols: factors contributing to their clinical application in CNS diseases. Neurochem Int 89:198–208
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med Cell Longev 2(5):270–278
Pari L, Suresh A (2008) Effect of grape (Vitis Vinifera L.) leaf extract on alcohol induced oxidative stress in rats. Food Chem Toxicol 46(5):1627–1623
Park S, Cha SH, Cho I, Park S, Park Y et al (2016) Antibacterial nanocarriers of resveratrol with gold and silver nanoparticles. Mat Sci Eng C 58:1160–1169
Pasinetti G, Jun W, Lap H, Wei Z, Lauren D (2015) Roles of resveratrol and other grape-derived polyphenols in Alzheimer's disease prevention and treatment. Biochim et Biophys Acta 1852(6):1202–1208
Petersen KS, Smith C (2016) Ageing-associated oxidative stress and inflammation are alleviated by products from grapes. Oxid Med Cell Longev 201:6236309 12 pages. Hindawi Publishing Corporation
Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256(3):183–194
Petti S, Scully C (2009) Polyphenols, oral health and disease: a review. J Dent 37:413–423
Procházková D, Boušová I, Wilhelmová N (2011) Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 82:513–523
Qi Y, Shang L, Liao Z et al (2019) Intracerebroventricular injection of resveratrol ameliorated Aβ-induced learning and cognitive decline in mice. Metab Brain Dis 34:257
Ramalingam P, Ko YT (2016) Improved oral delivery of resveratrol from N-trimethyl chitosan-g-palmitic acid surface-modified solid lipid nanoparticles. Colloid Surf B 139:52–61
Rasool M, Malik A, Qureshi MS et al (2014) Recent updates in the treatment of neurodegenerative disorders using natural compounds. Evid Based Complement Alternat Med 2014:979730
Ren J, Fan C, Chen N, Huang J, Yang Q (2011) Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf-2 and HO-1 in rats. Neurochem Res 36(12):2352–2362
Rendeiro C, Vauzour D, Rattray M, Waffo-Teguo P, Merillon JM, Butler LT et al (2013) Dietary levels of pure flavonoids improve spatial memory performance and increase hippocampal brain-derived neurotrophic factor. PLoS One 8(5):e63535
Ribeiro CA, Saraiva MJ, Cardoso I (2012) Stability of the transthyretin molecule as a key factor in the interaction with abeta peptide–relevance in Alzheimer’s disease. PLoS One 7(9):e45368
Richard T, Poupard P, Nassra M, Papastamoulis Y, Iglesias ML, Krisa S et al (2011) Protective effect of epsilon-viniferin on beta-amyloid peptide aggregation investigated by electrospray ionization mass spectrometry. Bioorg Med Chem 19:3152–3155
Rizk MZ, Borai IH, Ezz MK, El-Sherbiny M, Aly HF, Matloub A, Fouad GI (2018) Possible therapeutic role of grape (Vitis vinifera) leaves polyphenolic extract in the regression of aluminium-induced Alzheimer's disease in rats. J Mater Environ Sci 9(7):2098–2108
Rodrigo R, Miranda A, Vergara L (2011) Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin Chim Acta 412(5–6):410–424
Rojo LE, Fernandez JA, Maccioni AA, Jimenez JM, Maccioni RB (2008) Neuroinflammation: implications for the pathogenesis and molecular diagnosis of Alzheimer's disease. Arch Med Res 39:1–16
Rojo LE, Villaguala C, Avello M, Pastene E (2016) Polyphenols in neurodegeneration and neuroendocrine alterations: a metabolomic approach. Rev Farmacol Chile 9(1):29
Rubio L, Macia A, Motilva MJ (2014) Impact of various factors on pharmacokinet-ics of bioactive polyphenols: an overview. Curr Drug Metab 15:62–76
Sadigh-Eteghad S, Majdi A, Mahmoudi J, Golzari SE, Talebi M (2016) Astrocytic and microglial nicotinic acetylcholine receptors: an overlooked issue in Alzheimer’s disease. J Neural Transm 123(12):1359–1367
Sanchez-Moreno C, Larrauri JA, Saura-Calixto F (1999) Free radical scavenging capacity and inhibition of lipid oxidation of lipid oxidation of wine, grape juices and related polyphenolic constituents. Food Res Int 32:407–412
Santa-Maria I, Diaz-Ruiza C, Ksiezak-Reding H, Chen A, Ho L et al (2012) GSPE interferes with tau aggregation in vivo: implication for treating tauopathy. Neurobiol Aging 33:2072–2081
Saso L, Firuzi O (2014) Pharmacological applications of antioxidants: lights and shadows. Curr Drug Targets 15:1177–1199
Scalia S, Trotta V, Iannuccelli V, Bianchi A (2015) Enhancement of in vivo human skin penetration of resveratrol by chitosan-coated lipid microparticles. Colloid Surf B. 135:42–49
Schaffer TK, Wohlenberg MF, Medeiros N, Martins JB, Agostini F, Funchal C, Dani C (2016) Evaluation of antioxidant activity of grapevine leaves extracts (Vitis Labrusca) in liver of Wistar rats. An Acad Bras Cienc 88(1):187–196
Scheltens P, Blennow K, Breteler MM, De Strooper B, Frisoni GB, Salloway S et al (2016) Alzheimer’s disease. Lancet. 388:505–517
Schnee S, Queiroz EF, Voinesco F, Marcourt L, Dubuis PH, Wolfender JL, Gindro K (2013) Vitis Vinifera canes, a new source of antifungal compounds against Plasmopara Viticola, Erysiphe Necator, and Botrytis Cinerea. J Agric Food Chem 61(23):5459–5467
Shukitt-Hale B, Carey A, Simon L, Mark DA, Joseph JA (2006) Effects of Concord grape juice on cognitive and motor deficits in aging. Nut. 22:295–302
Smid SD, Maag JL, Musgrave IF (2012) Dietary polyphenol-derived protection against neurotoxic β-amyloid protein: from molecular to clinical. Food Funct 3:1242–1250
Solberg NO, Chamberlin R, Vigil JR, Deck LM, Heidrich JE, Brown DC (2014) Optical and SPION-enhanced MR imaging shows that trans-stilbene inhibitors of NF-kappa B concomitantly lower Alzheimer’s disease plaque formation and microglial activation in AbetaPP/PS-1 transgenic mouse brain. JAD 40(1):191–212
Song YM, Ha YM, Kim JA, Chung KW, Uehara Y, Lee KJ, Chun P, Byun Y et al (2012) Synthesis of novel azo-resveratrol, azooxyresveratrol and their derivatives as potent tyrosinase inhibitors. Bioorg Med Chem Lett 22(24):7451–7455
Soo E, Thakur S, Qu Z, Jambhrunkar S, Parekh HS, Popat A (2016) Enhancing delivery and cytotoxicity of resveratrol through a dual nanoencapsulation approach. J Colloid Interface Sci 462:368–374
Spencer JP (2009) The impact of flavonoids on memory: physiological and molecular considerations. Chem Soc Rev 38:1152–1161
Spencer JP, Vafeiadou K, Williams RJ, Vauzour D (2012) Neuroinflammation: modulation by flavonoids and mechanisms of action. Mol Asp Med 33:83–97
Surai PF (2014) Polyphenol compounds in the chicken/animal diet: from the past to the future. J Anim Physiol An N 98:19–31
Tota S, Awasthi H, Kamat PK, Nath C, Hanif K (2010) Protective effect of quercetin against intracerebral streptozotocin induced reduction in cerebral blood flow and impairment of memory in mice. Behav Brain Res 209(1):73–79
Tsao R (2010) Chemistry and biochemistry of dietary polyphenols. Nutr. 2:1231–1246
Van Duynhoven J, Vaughan EE, Jacobs DM, Kemperman RA, Van Velzen EJ, Gross G et al (2011) Metabolic fate of polyphenols in the human superorgan-ism. Proc Natl Acad Sci 108(1):4531–4538
Vauzour D (2012) Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxidative Med Cell Longev 2012:914273
Vauzour D (2014) Effect of flavonoids on learning, memory and neurocognitive performance: relevance and potential implications for Alzheimer’s disease pathophysiology. J Sci Food Agric 94(6):1042–1056
Vauzour D, Rodriguez-Mateos A, Corona G, Oruna-Concha MJ, Spencer JP (2010) Polyphenols and human health: prevention of disease and mechanisms of action. Nutr. 2:1106–1131
Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JP (2008) The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr 3:115–126
Vingtdeux V et al (2008) Therapeutic potential of resveratrol in Alzheimer's disease. BMC Neurosci 9(2):S6
Vingtdeux V et al (2010) AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem 285(12):9100–9113
Vitrac X, Bornet A, Vanderlinde R, Valls J, Richard T et al (2005) Determination of stilbenes (δ- viniferin, trans-astringin, trans-piceid, cis-and trans-resveratrol, ε- viniferin) in Brazilian wines. J Agric Food Chem 53(14):5664–5669
Wahlster L, Arimon M, Nasser-Ghodsi N, Post KL, Serrano-Pozo A, Uemura K, Berezovska O (2013) Presenilin-1 adopts pathogenic conformation in normal aging and in sporadic Alzheimer’s disease. Acta Neuropathol 125:187–199
Walle T (2011) Bioavailability of resveratrol. Ann N Y Acad Sci 1215:9–15
Wang J, Bi W, Cheng A, Freire D, Vempati P et al (2014) Targeting multiple pathogenic mechanisms with polyphenols for the treatment of Alzheimer’s disease-experimental approach and therapeutic implications. Front Aging Neurosci 6:42
Wang J, Ferruzzi MG, Ho L, Blount J, Janle EM, Gong B et al (2012a) Brain-targeted proanthocyanidin metabolites for Alzheimer's disease treatment. J Neurosci 32(15):5144–5150
Wang J, Ferruzzi MG, Ho L et al (2012b) Brain-targeted proanthocyandinin metabolites for Alzheimer’s disease treatment. J Neurosci 32(15):5144–5150
Wang J, Ho L, Zhao W et al (2008) Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer's disease. J Neurosci 28(25):6388–6392
Wang J, Santa-Maria I, Ho L, Ksiezak-Reding H, Ono K, Teplow DB, Pasinetti GM (2010b) Grape derived polyphenols attenuate tau neuropathology in a mouse model of Alzheimer's disease. JAD. 22:653–661
Wang J et al (2010a) The role of Sirt1: at the cross road between promotion of longevity and protection against Alzheimer's disease neuropathology. Biochim Biophys Acta 1804(8):1690–1694
Wang X, Ma S, Yang B, Huang T, Meng N, Xu L, Li Q (2018) Resveratrol promotes hUC-MSCs engraftment and neural repair in a mouse model of Alzheimer’s disease. Behav Brain Res 339:297–304
Wenzel E, Soldo T, Erbersdobler H, Somoza V (2005) Bioactivity, and metabolism of trans-resveratrol orally administered to Wistar rats. Mol Nutr Food Res 49(5):482–494
World Health Organization (WHO). Dementia [online]. 2019. Available at: https://www.who.int/en/news-room/fact-sheets/detail/dementia. Accessed 14 Jul 2019
Wu WM. An Investigation into the neuroprotective effects of estrogen and progesterone in a model of homocysteine-induced neurodegeration. PhD Thesis. Rhodes University. 2005
Xu ZC, Yin J, Zhou B, Liu YT, Yu Y, Li GQ (2015) Grape seed proanthocyanidin protects liver against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress. World J Gastroenterol 21(24):7468–7477
Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C (2003) Interaction between flavonoids and the blood-brain barrier: in vitro studies. J Neurochem 85(1):180–192
Zhang YJ, Gan RY, Li S, Zhou Y, Li AN, Xu DP, Li HB (2015) Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Mol. 20:21138–21156
Zhao HF, Li N, Wang Q, Cheng XJ, Li XM, Liu TT (2015) Resveratrol decreases the insoluble Abeta1-42 level in hippocampus and protects the integrity of the blood-brain barrier in AD rats. Neurosc. 310:641–649
Zhao YN, Li WF, Li F, Zhang Z, Dai YD, Xu AL, Qi C, Gao JM et al (2013) Resveratrol improves learning and memory in normally aged mice through micro RNA-CREB pathway. Biochem Biophys Res Commun 435(4):597–602
Zhou K, Raffoul JJ (2012) Potential anticancer properties of grape antioxidants. J Oncol 2012:803294
Acknowledgements
I would like to acknowledge the National Research center (NRC) for supporting and facilitating this work. The authors are grateful to all the researchers whom we cited in this review for their significant and valuable research.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
One author (GF) independently screened the titles and abstracts of the publications. A second (MR) revised the collected articles. The corresponding author wrote and approved the final manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ibrahim Fouad, G., Zaki Rizk, M. Possible neuromodulating role of different grape (Vitis vinifera L.) derived polyphenols against Alzheimer’s dementia: treatment and mechanisms. Bull Natl Res Cent 43, 108 (2019). https://doi.org/10.1186/s42269-019-0149-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-019-0149-z