Abstract
Several epidemiological studies indicate that moderate consumption of red wine is associated with a lower incidence of dementia and Alzheimer's disease. Red wine is enriched in antioxidant polyphenols with potential neuroprotective activities. Despite scepticism concerning the bioavailability of these polyphenols, in vivo data have clearly demonstrated the neuroprotective properties of the naturally occurring polyphenol resveratrol in rodent models for stress and diseases. Furthermore, recent work in cell cultures and animal models has shed light on the molecular mechanisms potentially involved in the beneficial effects of resveratrol intake against the neurodegenerative process in Alzheimer's disease.
Similar content being viewed by others
Background
Alzheimer's disease (AD) is a progressive neurodegenerative disorder leading to the most common form of dementia in elderly people. Histopathological studies of the AD brain revealed dramatic ultra-structural changes triggered by two classical lesions, the senile plaques, mainly composed of amyloid-β (Aβ) peptides, and the neurofibrillary tangles, composed of hyperphosphorylated tau proteins [1, 2]. Although neurofibrillary tangles can occur independently, and cause neuronal death in frontotemporal dementia [3], the presence of both lesions in the neocortex is essential to the diagnosis of AD. The pathogenesis of the disease is complex and is driven by both environmental and genetic factors. Although most of the cases are sporadic with an obscure etiology, some forms of the disease are inherited and several genes were found to be clearly implicated in familial forms of the disease. The molecular identification and characterization of different genes associated with familial AD has provided strong support to the so-called amyloid cascade hypothesis as a causative event in the pathogenesis of AD [4]. This hypothesis states that Aβ generated from deregulated proteolysis of the amyloid precursor protein (APP) undergoes accelerated Aβ oligomerization, fibril formation, and amyloid deposition in a process that initiates the AD pathology [4].
In the past ten years, the large majority of the pharmacological research on AD has focused on understanding how Aβ is generated from APP via β- and γ-secretase cleavages, with the goal of designing specific inhibitors that will block Aβ production and the associated pathology. However, genetic and pharmacological approaches have recently demonstrated that these enzymes have additional molecular targets [5–7], and that secretase inhibitors can cause severe mechanism-based side effects in vivo, casting doubt over the potential of these therapeutic strategies [8]. In this context, approaches aimed at better understanding the molecular pathways involved in Aβ clearance have been gaining considerable attention over the last several years [9].
Mechanisms of amyloid-β proteolytic clearance
A number of different receptor-mediated or endoproteolytic pathways have been implicated in the process of Aβ degradation [10]. The major enzymes responsible for Aβ degradation in vivo are neprilysin (NEP), endothelin-converting enzyme (ECE)-1 and ECE-2, insulin-degrading enzyme (IDE), and plasmin. NEP, IDE, and ECEs are zinc metalloendopeptidases that hydrolyze bioactive peptides and several lines of evidence support a role for these enzymes in Aβ degradation in cell cultures and animal models (reviewed in [10]).
The plasminogen system
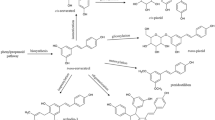
Plasmin is a serine protease released from the inactive zymogen plasminogen upon cleavage by the tissue-type plasminogen activator (t-PA) or by the urokinase-type plasminogen activator (u-PA) (Figure 1) [11]. The plasminogen system is negatively controlled by the action of soluble protein inhibitors that selectively bind plasminogen or plasmin. These include the plasminogen activator inhibitor-1 (PAI-1) and α2-plasmin inhibitor (α2-PI). Plasmin is involved in many pathophysiological processes primarily through its ability to degrade components of the extracellular matrix. In the brain, the plasminogen system was shown to be involved in numerous functions, including neuronal plasticity and long-term potentiation. Importantly, polymorphisms in the u-PA gene have been proposed to be associated with late-onset AD, suggesting that deregulation of the plasminogen system may be associated with the pathogenesis of the disease [12]. In addition, the t-PA-plasmin proteolytic cascade was found to promote the clearance of Aβ in vivo in two different mouse models of AD [13].
Schematic representation of the plasminogen system. The proenzyme plasminogen (Plg) is converted to the active serine protease plasmin (Plm) by the soluble or membrane bound urokinase-type plasminogen activator (u-PA). Membrane bound u-PA binds to a cellular u-PA receptor (u-PAR). Inhibition of the plasminogen system may occur at the level of the plasminogen activators by plasminogen activator inhibitors (such as PAI-1), or at the level of plasmin by α2-plasmin inhibitor (α2-PI) (also called α2-antiplasmin (α2AP)).
Red wine intake and Alzheimer's disease
No specific environmental risk factor has been definitively identified as being associated with AD. However, the potentially important role for diet in the causation or prevention of AD is supported by several observations. For instance, there is evidence that homocysteine-related vitamins, fats, and red wine consumption have a role in the pathogenesis of AD [14]. The first study, published in 1997, reported that moderate to mild wine consumption was associated with a low risk of AD [15]. Later, a nested case-control study [16] and a cohort study [17] of individuals aged 65 years and older confirmed that intake of wine, but not other alcoholic drinks, was associated with a low risk of dementia, including AD. Furthermore, a prospective analysis of risk factors for AD in the Canadian population determined that wine consumption was the most protective variable against AD by reducing the risk of AD by 50% [18]. Interestingly, wine intake in this population was found to be even more protective than the use of nonsteroidal anti-inflammatory drugs (NSAIDs) [18]. Nevertheless, it should be noted that the notion that wine intake – and more specifically red wine intake – lowers AD risk is still controversial and remains to be clearly demonstrated. In line with the epidemiological data, however, Pasinetti and colleagues [19] have provided evidence that moderate consumption of red wine lowers Aβ levels and the associated neuropathology in the Tg2576 AD mouse model, implying that red wine intake may have a beneficial effect against AD pathology by promoting anti-amyloidogenic mechanisms.
Bioavailability and therapeutic potential of resveratrol
Resveratrol is a phytoalexin polyphenolic compound occurring in grapes and wine. The levels of resveratrol found in wine vary greatly, but is generally more abundant in red grapes and red wine. Numerous studies performed ex vivo and in animal models have provided information on the absorption, metabolism, and consequent bioavailability of this polyphenol [20]. The oral bioavailability of resveratrol is low due to rapid excretion and extensive metabolism into various glucuronide and sulfate conjugates of unknown potential biological activities. The major metabolites identified in the urine in human after oral dosing of synthetic resveratrol are: resveratrol monosulphate, two isomeric forms of resveratrol monoglucuronide, dihydroresveratrol monosulphate, and dihydroresveratrol monoglucuronide. Total sulphate conjugates account for more than one-third of the metabolites in the urine and total glucuronide conjugates represent about 20% [21]. These pharmacokinetic studies cast doubt on the therapeutic potential of unmodified resveratrol. Nevertheless, in vivo data from several studies have clearly demonstrated in various organisms that resveratrol intake has protective properties against multiple illnesses, including cancer, cardiovascular disease, and ischemia, and was also found to confer resistance to stress and to extend life span [22]. More recently, concordant studies have also revealed the beneficial effect of resveratrol in vivo on energy metabolism in diseases such as diet-induced obesity, insulin resistance, or aging-related syndromes [23–25].
The antioxidant functions of resveratrol
Resveratrol is believed to afford strong antioxidant functions in vitro and in cell culture models and, therefore, to contribute to the cardio-protective, anti-inflammatory, and neuroprotective properties of red wine intake [26]. Several lines of evidence underline the antioxidant and neuroprotective effects of resveratrol. The polyphenol was found to prevent membrane lipid peroxidation, internalization of oxidized lipoprotein, and to reduce the toxic effects of reactive oxygen intermediates in cultured cell lines [27, 28]. In addition, resveratrol delays Aβ-induced toxicity in different neuronal culture models [29–31]. Nevertheless, the mechanism by which resveratrol is able to achieve these effects remains to be clearly defined. Independent biochemical approaches have, however, demonstrated that the polyphenol has potent anti-amyloidogenic and anti-fibril effects in vitro [32–35], suggesting that resveratrol may act as an antioxidant by preventing the formation of toxic Aβ oligomers and protofibrillar intermediates.
Resveratrol, SIRT1, and amyloid pathology
Recent data provide interesting insights into the effect of resveratrol on longevity and its potential protective role in age-related human diseases, including AD. Resveratrol mimics caloric restriction by extending the lifespan of different organisms via activation of deacetylases from the sirtuin family [36]. Indeed, resveratrol binds to and activates the deacetylase activity of several sirtuin members, including the mammalian ortholog, SIRT1, by lowering the Michaelis constants of these enzymes [37]. Sirtuins are nicotinamide adenine dinucleotide-dependent deacetylases required for the increased longevity due to caloric restriction in yeast, Drosophila, and mice [36, 38]. Sirtuins deacetylate and control the activity of several transcription factors, including peroxisome proliferator-activated receptor (PPAR)γ cofactors, through a mechanism that regulates lifespan in mice [39, 40]. Importantly, Kim and colleagues [41] recently reported that intracerebroventricular injection of resveratrol reduced neurodegeneration in the hippocampus and prevented learning impairment in the p25 transgenic AD mouse model by a mechanism that may involve a decrease in the acetylation of known SIRT1 substrates, for example, peroxisome proliferator-activated receptor gamma coactivator (PGC-1)α and p53. In addition, caloric restriction was found to attenuate amyloid deposition and Aβ-associated neuropathology in different animal models [42, 43], and SIRT1 activation may contribute to the anti-amyloidogenic properties of this particular intervention [44, 45]. In line with these studies, resveratrol appears to be protective against the dysregulation of energy homeostasis observed in mouse models for metabolic syndromes by a mechanism implicating the activation of SIRT1, PGC-1α, and the energy sensor protein kinase AMPK (AMP-activated protein kinase) [23–25].
Resveratrol, SIRT1, and plasmin activation
Our recent work indicates that resveratrol reduces Aβ accumulation in cell cultures [46]. Resveratrol does not inhibit Aβ production, since it has no effect on the Aβ-producing enzymes β- and γ-secretases, but promotes instead the proteolytic clearance of Aβ by a mechanism that does not implicate NEP, ECE-1 and ECE-2, or IDE [46]. Several groups have obtained evidence that resveratrol treatment increases the expression of the plasminogen activators t-PA and u-PA, suggesting that resveratrol could lead to plasminogen endoproteolysis and plasmin activation (Figure 1) [47, 48]. Additional evidence indicates that plasminogen activation is positively controlled by PPARγ that appears to be a transcriptional target of SIRT1 [39, 40, 49]. This raises the intriguing possibility that resveratrol controls plasmin activation by promoting SIRT1-mediated transcription. This last observation may further explain the anti-amyloidogenic effect of PPARγ activation recently shown by several groups, including our own [50–53]. Additional observations argue against a direct effect of resveratrol on a protease degrading Aβ, but instead point toward the implication of a transcriptional mechanism in the regulation of Aβ degradation. There is, indeed, no data indicating that resveratrol binds to and directly stimulates a known Aβ-degrading protease. Moreover, the long incubation periods required for resveratrol action [46] clearly suggest the implication of gene expression regulation in the mechanism of Aβ clearance. Therefore, it is possible that resveratrol acts by interacting with SIRT1-mediated transcription in a pathway of Aβ degradation.
Conclusion
Although unmodified resveratrol appears to have a weak bioavailability, several studies have clearly demonstrated the in vivo neuroprotective properties of the red wine-derived polyphenol, strongly supporting the notion that natural metabolites of resveratrol may have biological activities. Furthermore, recent findings have shed light on the potential role of resveratrol in transcription- and degradation-dependent anti-amyloidogenic mechanisms, suggesting that natural metabolites or potent synthetic analogues of resveratrol have a therapeutic potential in AD.
Abbreviations
- Aβ:
-
amyloid-β
- AD:
-
Alzheimer's disease
- APP:
-
amyloid precursor protein
- ECE:
-
endothelin-converting enzyme
- IDE:
-
insulin-degrading enzyme
- NEP:
-
neprilysin
- PGC:
-
peroxisome proliferator-activated receptor gamma coactivator
- PPAR:
-
peroxisome proliferator-activated receptor
- t-PA:
-
tissue-type plasminogen activator
- u-PA:
-
urokinase-type plasminogen activator.
References
Selkoe DJ: Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001, 81: 741-766.
Davies P: A very incomplete comprehensive theory of Alzheimer's disease. Ann N Y Acad Sci. 2000, 924: 8-16. 10.1111/j.1749-6632.2000.tb05553.x.
Goedert M, Spillantini MG: Tau mutations in frontotemporal dementia FTDP-17 and their relevance for Alzheimer's disease. Biochim Biophys Acta. 2000, 1502: 110-121.
Hardy J, Selkoe DJ: The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002, 297: 353-356. 10.1126/science.1072994.
Marambaud P, Robakis NK: Genetic and molecular aspects of Alzheimer's disease shed light on new mechanisms of transcriptional regulation. Genes Brain Behav. 2005, 4: 134-146. 10.1111/j.1601-183X.2005.00086.x.
Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, et al: A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002, 21: 1948-1956. 10.1093/emboj/21.8.1948.
Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK: A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003, 114: 635-645. 10.1016/j.cell.2003.08.008.
Harrison T, Churcher I, Beher D: gamma-Secretase as a target for drug intervention in Alzheimer's disease. Curr Opin Drug Discov Devel. 2004, 7: 709-719.
Tanzi RE, Moir RD, Wagner SL: Clearance of Alzheimer's Abeta peptide: the many roads to perdition. Neuron. 2004, 43: 605-608.
Turner AJ, Fisk L, Nalivaeva NN: Targeting amyloid-degrading enzymes as therapeutic strategies in neurodegeneration. Ann N Y Acad Sci. 2004, 1035: 1-20. 10.1196/annals.1332.001.
Collen D: Ham-Wasserman lecture: role of the plasminogen system in fibrin-homeostasis and tissue remodeling. Hematology (Am Soc Hematol Educ Program). 2001, 1-9.
Ertekin-Taner N, Ronald J, Feuk L, Prince J, Tucker M, Younkin L, Hella M, Jain S, Hackett A, Scanlin L, et al: Elevated amyloid beta protein (Abeta42) and late onset Alzheimer's disease are associated with single nucleotide polymorphisms in the urokinase-type plasminogen activator gene. Hum Mol Genet. 2005, 14: 447-460. 10.1093/hmg/ddi041.
Melchor JP, Pawlak R, Strickland S: The tissue plasminogen activator-plasminogen proteolytic cascade accelerates amyloid-beta (Abeta) degradation and inhibits Abeta-induced neurodegeneration. J Neurosci. 2003, 23: 8867-8871.
Luchsinger JA, Mayeux R: Dietary factors and Alzheimer's disease. Lancet Neurol. 2004, 3: 579-587. 10.1016/S1474-4422(04)00878-6.
Orgogozo JM, Dartigues JF, Lafont S, Letenneur L, Commenges D, Salamon R, Renaud S, Breteler MB: Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997, 153: 185-192.
Truelsen T, Thudium D, Gronbaek M: Amount and type of alcohol and risk of dementia: the Copenhagen City Heart Study. Neurology. 2002, 59: 1313-1319.
Luchsinger JA, Tang MX, Siddiqui M, Shea S, Mayeux R: Alcohol intake and risk of dementia. J Am Geriatr Soc. 2004, 52: 540-546. 10.1111/j.1532-5415.2004.52159.x.
Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I: Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002, 156: 445-453. 10.1093/aje/kwf074.
Wang J, Ho L, Zhao Z, Seror I, Humala N, Dickstein DL, Thiyagarajan M, Percival SS, Talcott ST, Pasinetti GM: Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer's disease. FASEB J. 2006, 20: 2313-2320. 10.1096/fj.06-6281com.
Wenzel E, Somoza V: Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res. 2005, 49: 472-481. 10.1002/mnfr.200500010.
Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK: High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004, 32: 1377-1382. 10.1124/dmd.104.000885.
Baur JA, Sinclair DA: Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006, 5: 493-506. 10.1038/nrd2060.
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al: Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006, 127: 1109-1122. 10.1016/j.cell.2006.11.013.
Dasgupta B, Milbrandt J: Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA. 2007, 104 (17): 7217-7222. 10.1073/pnas.0610068104.
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al: Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006, 444: 337-342. 10.1038/nature05354.
Pervaiz S: Resveratrol: from grapevines to mammalian biology. FASEB J. 2003, 17: 1975-1985. 10.1096/fj.03-0168rev.
Sun AY, Chen YM, James-Kracke M, Wixom P, Cheng Y: Ethanol-induced cell death by lipid peroxidation in PC12 cells. Neurochem Res. 1997, 22: 1187-1192. 10.1023/A:1021968526696.
Draczynska-Lusiak B, Doung A, Sun AY: Oxidized lipoproteins may play a role in neuronal cell death in Alzheimer disease. Mol Chem Neuropathol. 1998, 33: 139-148. 10.1007/BF02870187.
Han YS, Zheng WH, Bastianetto S, Chabot JG, Quirion R: Neuroprotective effects of resveratrol against beta-amyloid-induced neurotoxicity in rat hippocampal neurons: involvement of protein kinase C. Br J Pharmacol. 2004, 141: 997-1005. 10.1038/sj.bjp.0705688.
Jang JH, Surh YJ: Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radic Biol Med. 2003, 34: 1100-1110. 10.1016/S0891-5849(03)00062-5.
Savaskan E, Olivieri G, Meier F, Seifritz E, Wirz-Justice A, Muller-Spahn F: Red wine ingredient resveratrol protects from beta-amyloid neurotoxicity. Gerontology. 2003, 49: 380-383. 10.1159/000073766.
Ahn JS, Lee JH, Kim JH, Paik SR: Novel method for quantitative determination of amyloid fibrils of alpha-synuclein and amyloid beta/A4 protein by using resveratrol. Anal Biochem. 2007, 367: 259-265. 10.1016/j.ab.2007.05.023.
Riviere C, Richard T, Vitrac X, Merillon JM, Valls J, Monti JP: New polyphenols active on beta-amyloid aggregation. Bioorg Med Chem Lett. 2008, 18: 828-831. 10.1016/j.bmcl.2007.11.028.
Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M: Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro : implications for the prevention and therapeutics of Alzheimer's disease. J Neurochem. 2003, 87: 172-181. 10.1046/j.1471-4159.2003.01976.x.
Riviere C, Richard T, Quentin L, Krisa S, Merillon JM, Monti JP: Inhibitory activity of stilbenes on Alzheimer's beta-amyloid fibrils in vitro. Bioorg Med Chem. 2007, 15: 1160-1167. 10.1016/j.bmc.2006.09.069.
Guarente L: Calorie restriction and SIR2 genes – towards a mechanism. Mech Ageing Dev. 2005, 126: 923-928. 10.1016/j.mad.2005.03.013.
Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, et al: Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003, 425: 191-196. 10.1038/nature01960.
Chen D, Steele AD, Lindquist S, Guarente L: Increase in activity during calorie restriction requires Sirt1. Science. 2005, 310: 1641-10.1126/science.1118357.
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P: Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005, 434: 113-118. 10.1038/nature03354.
Nemoto S, Fergusson MM, Finkel T: SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1alpha. J Biol Chem. 2005, 280: 16456-16460. 10.1074/jbc.M501485200.
Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, et al: SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007, 26: 3169-3179. 10.1038/sj.emboj.7601758.
Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, Finch CE: Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol Aging. 2005, 26: 995-1000. 10.1016/j.neurobiolaging.2004.09.014.
Wang J, Ho L, Qin W, Rocher AB, Seror I, Humala N, Maniar K, Dolios G, Wang R, Hof PR, et al: Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer's disease. FASEB J. 2005, 19: 659-661. 10.1096/fj.04-2370com.
Qin W, Chachich M, Lane M, Roth G, Bryant M, de Cabo R, Ottinger MA, Mattison J, Ingram D, Gandy S, et al: Calorie restriction attenuates Alzheimer's disease type brain amyloidosis in Squirrel monkeys (Saimiri sciureus). J Alzheimers Dis. 2006, 10: 417-422.
Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, et al: Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006, 281: 21745-21754. 10.1074/jbc.M602909200.
Marambaud P, Zhao H, Davies P: Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J Biol Chem. 2005, 280: 37377-37382. 10.1074/jbc.M508246200.
Abou-Agag LH, Aikens ML, Tabengwa EM, Benza RL, Shows SR, Grenett HE, Booyse FM: Polyphyenolics increase t-PA and u-PA gene transcription in cultured human endothelial cells. Alcohol Clin Exp Res. 2001, 25: 155-162. 10.1111/j.1530-0277.2001.tb02193.x.
Yang SH, Kim JS, Oh TJ, Kim MS, Lee SW, Woo SK, Cho HS, Choi YH, Kim YH, Rha SY, et al: Genome-scale analysis of resveratrol-induced gene expression profile in human ovarian cancer cells using a cDNA microarray. Int J Oncol. 2003, 22: 741-750.
Nicholas SB, Kawano Y, Wakino S, Collins AR, Hsueh WA: Expression and function of peroxisome proliferator-activated receptor-gamma in mesangial cells. Hypertension. 2001, 37: 722-727.
Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, Citron M, Landreth G: Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer's disease. J Neurosci. 2003, 23: 7504-7509.
d'Abramo C, Massone S, Zingg JM, Pizzuti A, Marambaud P, Dalla Piccola B, Azzi A, Marinari UM, Pronzato MA, Ricciarelli R: Role of peroxisome proliferator-activated receptor gamma in amyloid precursor protein processing and amyloid beta-mediated cell death. Biochem J. 2005, 391: 693-698. 10.1042/BJ20050560.
Camacho IE, Serneels L, Spittaels K, Merchiers P, Dominguez D, De Strooper B: Peroxisome-proliferator-activated receptor gamma induces a clearance mechanism for the amyloid-beta peptide. J Neurosci. 2004, 24: 10908-10917. 10.1523/JNEUROSCI.3987-04.2004.
Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, O'Banion K, Klockgether T, Van Leuven F, Landreth GE: Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1–42 levels in APPV717I transgenic mice. Brain. 2005, 128: 1442-1453. 10.1093/brain/awh452.
Acknowledgements
This work is supported in part by the Alzheimer's Association, the Institute for the Study of Aging (Alzheimer's Drug Discovery Foundation), and the National Center for Complementary and Alternative Medicine (NCCAM, NIH) PO1 Award No. AT004511-01 Project 2.
This article has been published as part of BMC Neuroscience Volume 9 Supplement 2: 2008 Proceedings of the 8th International Conference on Alzheimer's Disease Drug Discovery The full contents of the supplement are available online at http://www.biomedcentral.com/1471-2202/9?issue=S2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
PM, VV, UDW, HZ and PD conceived the project and performed the work. PM wrote the manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Vingtdeux, V., Dreses-Werringloer, U., Zhao, H. et al. Therapeutic potential of resveratrol in Alzheimer's disease. BMC Neurosci 9 (Suppl 2), S6 (2008). https://doi.org/10.1186/1471-2202-9-S2-S6
Published:
DOI: https://doi.org/10.1186/1471-2202-9-S2-S6