Abstract
The production of veritable in-vitro models of bone tissue is essential to understand the biology of bone and its surrounding environment, to analyze the pathogenesis of bone diseases (e.g., osteoporosis, osteoarthritis, osteomyelitis, etc.), to develop effective therapeutic drug screening, and to test potential therapeutic strategies. Dysregulated interactions between vasculature and bone cells are often related to the aforementioned pathologies, underscoring the need for a bone model that contains engineered vasculature. Due to ethical restraints and limited prediction power of animal models, human stem cell-based tissue engineering has gained increasing relevance as a candidate approach to overcome the limitations of animals and to serve as preclinical models for drug testing. Since bone is a highly vascularized tissue, the concomitant development of vasculature and mineralized matrix requires a synergistic interaction between osteogenic and endothelial precursors. A number of experimental approaches have been used to achieve this goal, such as the combination of angiogenic factors and three-dimensional scaffolds, prevascularization strategies, and coculture systems. In this review, we present an overview of the current models and approaches to generate in-vitro stem cell-based vascularized bone, with emphasis on the main challenges of vasculature engineering. These challenges are related to the choice of biomaterials, scaffold fabrication techniques, and cells, as well as the type of culturing conditions required, and specifically the application of dynamic culture systems using bioreactors.
Similar content being viewed by others
Background
Bone architecture and development
Bones are hierarchically organized over multiple length scales. Macroscopically, bone can be divided into compact and trabecular tissues, each with very different mechanical strength and stiffness (Fig. 1a). The microscopic architecture of the former is characterized by osteons and Haversian channels containing nerves and blood supply, whereas the latter is comprised of interconnected trabeculae and the presence of the bone marrow [1] (Fig. 1b). At a molecular level, bone extracellular matrix (ECM) is composed largely of the fibrous macromolecule collagen type I and mineralized inorganic hydroxyapatite crystals [2] (Fig. 1c). Bone is primarily vascularized by an arterial network, but within the bone marrow cavity and the Haversian channels the vasculature branches into thin-walled capillaries, whose fundamental role is the exchange of nutrients and signals between blood and bone cells [3].
Bone fulfills a wide range of physiological functions. As essential structural and load-bearing elements, bones represent the foundation of physical locomotion and protect our internal organs. Due to the presence of the bone marrow, long bones serve as the tissue origin of the biological components required for hematopoiesis. Moreover, osseous tissues can trap potentially harmful metals (e.g., lead), as well as maintain the homeostasis of key electrolytes via calcium and phosphate ion storage [4]. Bone formation can occur through two distinct pathways, intramembranous and endochondral ossification. In both cases, the first step is the condensation of mesenchymal cells to produce a template for subsequent bone formation [5]. Intramembranous bone formation (typical of flat bones) involves the direct differentiation of mesenchymal progenitor cells into osteoblasts, whereas endochondral ossification (typical of long bones) involves the initial differentiation of the mesenchymal progenitor cells into chondrocytes, followed by their hypertrophy, matrix mineralization, and replacement by bone tissue [6]. Both intramembranous and endochondral ossification occur in close proximity to vascular ingrowth. During intramembranous ossification, capillaries invade the differentiating mesenchymal zone, whereas in endochondral ossification, hypertrophic chondrocytes recruit the infiltrating vasculature. Initial vascularization is followed by invasion of osteoclasts and osteoblasts, with coordinated resorption of hypertrophic cartilage and subsequent mineralization of the ECM and bone formation [7]. Hence, vascularization occurs from the ossification centers toward the growth plate and determines the rate of bone ossification [8]. It is interesting to note that many of the processes that occur during long bone formation are recapitulated during fracture healing [9], underscoring the importance of knowledge of bone development for the design of more effective strategies for bone repairing [6]. Vascular endothelial growth factor (VEGF) is a key regulator of angiogenesis and thus bone development [10]. For instance, VEGF-A gene depletion was shown to attenuate the resorption of hypertrophic chondrocytes and bone formation, highlighting the role of VEGF-dependent angiogenesis during bone formation [11]. VEGF-A levels were also found to be dependent on the oxygen-sensing hypoxia-inducible factor (HIF)-1α pathway in the modulation of bone mass, thus confirming the role of neoangiogenesis for the homing of osteoblast progenitor cells and for providing bone formation/promoting factors [12]. In fact, besides bone development, vasculature is also essential for bone remodeling, and fracture healing is also highly dependent on the vasculature. Dysregulated interactions between the vasculature and bone cells are the basis of a number of different pathologies [13]. Some of these, such as avascular necrosis and osteoporosis, are associated with a diminished vascular supply [14, 15], whereas others such as Gorham–Stout disease, a form of idiopathic osteolysis caused by abnormal proliferation of vascular structures originating in the bone [16], and Klippel-Trénaunay syndrome, characterized by vessel malformations and overgrowth of bones and soft tissues [17], are caused by excessive vascularization.
Bone and related pathologies
Bone diseases can cause loss of bone strength and density, and they may arise from nutrient deficiencies, abnormal development, genetic disorders, impaired vasculature, and other causes. Table 1 summarizes and compares the main pathologies affecting bone.
Osteoporosis refers to the loss of bone density resulting from an altered balance of the bone remodeling process, and affects approximately 10 million US adults 50 years of age and older [18]. The most widely used osteoporosis treatment is the administration of bisphosphonates, which shorten the osteoclast life span and inhibit bone resorption [19]. Although general risk factors of osteoporosis are well documented, little is known about the role of vasculature [20]. Some studies have revealed a connection between low bone mineral density and increased cardiovascular morbidity/mortality [21, 22]. Endothelial cells (ECs) are known regulators of vascular tone by releasing vasodilator molecules, such as nitric oxide (NO), and they have been addressed as a potential link between cardiovascular diseases and osteoporosis. Studies in rats showed that the inhibition of NO production or NO synthase (NOS) activity was followed by marked bone loss [23, 24], while human studies revealed lower NOS expression resulting from estrogen deficiency [25,26,27]. Since the presence of estrogen receptors has been found in human ECs [28, 29], it is possible that estrogen deficiency seen in postmenopausal women could alter the endothelial function of bone microcirculation. Although these studies suggest that endothelial dysfunction may play a role in the development of osteoporosis, the exact causal relationship has yet to be determined.
Osteoarthritis is the main cause of disability in the USA [30], and its hallmark is the progressive degeneration of cartilage. However, OA affects the whole joint and all tissues play a role in the disease [31]. In particular, the subchondral bone has been reported to be critical in the pathogenesis of OA [32]. During movement, there is continuous functional interaction across the osteochondral junction. Under the diseased state, altered mechanical loading in cartilage induces changes in bone and vice versa [33, 34]. The communication between the two tissues, however, is not limited to mechanical coupling and the associated mechanotransduction. Recent evidence indicates that the calcified cartilage and subchondral bone are not an impermeable barrier, and some molecules are capable of diffusing across the osteochondral junction [35,36,37,38]. Blood vessels and microchannels have been found to reach from the subchondral bone all the way to the uncalcified cartilage, and there is evidence of contact between uncalcified cartilage and subchondral bone and the marrow spaces [33, 39,40,41]. During OA, the osteochondral junction is significantly altered, allowing greater transport and cellular crosstalk between cartilage and bone [32, 38, 42]. Another hallmark change of the osteochondral junction occurring during OA is increased vascularization and neoangiogenesis [38, 43], which may further contribute to the molecular crosstalk between cartilage and bone. Part of this signaling involves an increase in the VEGF level in osteoarthritic chondrocytes compared to those in healthy cartilage [43], possibly contributing to the induction of vascular invasion as part of a proregenerative mechanism. In turn, ECs have recently been reported to enhance chondrogenic differentiation of mesenchymal stem cells (MSCs) [44], suggesting the potential of significant molecular interplay between subchondral bone vasculature and cartilage, an aspect that has not been much investigated. Overall, increased vascularity in the subchondral bone is associated with OA severity in cartilage and with clinical disease activity [33].
Another pathogenic bone condition with devastating consequences is osteomyelitis (OM). OM can be broadly defined as an infection within the bone and is classified by duration (acute or chronic), pathogenesis (trauma, contiguous spread, hematogeneous, surgical), site, extent, or type of patient [45]. Poor vascularity is a prime cause for both the development of an infection and resistance to antibiotics [46]. Acute OM can be eradicated before osteonecrosis occurs if the infection is treated promptly and aggressively with antibiotics and surgical debridement [46]. However, in an established/chronic infection, fibrous tissue and chronic inflammatory cells encapsulate the infected site, reducing vascular supply, inhibiting an effective inflammatory response, and limiting the action of antibiotics [46]. In addition, the bacteria become encapsulated within a biofilm, that both protects the bacteria from the body’s defenses and antibiotics and serves as a chronic source of new bacterial infection [45,46,47]. Several therapies intended to enhance blood flow to vascular or chronically infected areas have been tested in experimental models or clinical trials, including hyperbaric oxygen therapy, PRP, and VEGF delivery [47,48,49]. Recently, a VEGF gene-transfected muscle flap was shown to be effective as a treatment to supplement systemic antibiotic treatment in the management of experimental OM in a rodent model [50, 51]. These strategies seek to enhance the body’s own defense and the effectiveness of antibiotics by enhancing blood flow to avascular tissue.
Osteonecrosis, or avascular necrosis (AVN), occurs when the blood supply to the bone is disrupted, precipitating death of the bone cells, arthritis, and destruction of the hip joint. Common risk factors include long-term steroid treatment, alcohol, abuse, joint injury, arthritis, and cancer—all associated with altered blood supply [52]. The risk greatly increases with corticosteroid use (to treat pain and inflammation), bisphosphonate (to prevent bone loss), and anti-angiogenic therapy (in the treatment of some cancers or leprosy) [53, 54]. Areas susceptible to AVN have several common characteristics: they have limited routes of vascular supply, they undergo relatively high rates of bone turnover, and they may have higher than normal exposure to bacterial infections [55]. Evidence that reduced vascular supply causes AVN is indicated by the rise in AVN incidence following anti-angiogenic treatments used in cancer therapy, such as the VEGF-specific antibody bevacizumab, the tyrosine kinase inhibitor sunitinib (Sutent), and the mTOR inhibitor rapamycin, and new treatments for leprosy, such as lenalidomide. Osteoclasts and blood vessels are closely associated during bone remodeling, and recent studies indicate that osteoclasts promote angiogenesis through secretion of factors such as matrix metalloproteinase 9 (MMP9). Poor vascularization occurs in certain inflammatory and immunosuppressive states and in the presence of infection as well. While the pathogenesis of AVN is largely agreed upon, the cellular and molecular mechanisms are less understood, and thus counter-treatments to prevent the condition during anti-resorptive and anti-cancer treatments are not available.
Trauma-related injuries can lead to bone fractures, whose healing is critically dependent upon an adequate vascular supply. Breakage of the bone initiates the fracture healing process, which begins with an inflammatory reaction, followed by the processes typical of endochondral ossification for a period of up to 28 days. Neovascularization is critical for successful bone formation, since vascular endothelium interruption is the first event following trauma, which could lead to the formation of necrotic tissue. Experimental evidence showed impaired bone formation with administration of anti-angiogenic factors [56], whereas VEGF treatment enhanced fracture repair [57]. Clinical evidence showed that in the presence of decreased vascular perfusion to the fracture, the incidence of impaired healing (delayed union or nonunion) increases from 10–15% to 46% [58]. Standard treatment to augment bone healing and prevent delayed union or nonunion is represented by the use of autograft, allograft, or synthetic materials [59]. Bone marrow-derived endothelial progenitor cells (EPCs) participate in the generation of new blood vessels (vasculogenesis) at the site of injury [60, 61], and they have been shown, in combination with bone marrow-derived MSCs, to augment bone healing [62, 63]. Significant progress in improving fracture healing is hindered by the limited knowledge of the molecular mechanisms leading to nonunions; thus, a better understating of the biological pathways involved in this process would certainly benefit from the development of specific clinical therapies.
Bone also plays a role in pathologies related to ionic homeostasis, such as calcium and phosphate [64]. In particular, osteocytes are involved in phosphate homeostasis through the expression of different proteins, such as dentin matrix protein 1 (DMP1) [65], Phex, and fibroblast growth factor 23 (FGF-23) [66]. In addition to being key ions in systemic physiology, including muscle functions, calcium and phosphorous homeostasis is crucial for the development of the growth plate, which must be mineralized to promote proper vascular invasion and subsequent bone formation [67]. Bone may also act as a reservoir of lead, contributing to systemic toxicity [68], and it is the target tissue for the actions of a number of teratogens (e.g., valproic acid, thalidomide, etc.) [69, 70]. Studying the mechanism of how these toxins act at the cellular and molecular levels with bone is indeed crucial to developing new treatments.
Osteosarcoma is the most common bone malignancy affecting predominantly adolescents and young adults, with a 5-year survival rate of about 50–60% [71]. Osteosarcoma occurs mostly in the medullary cavity within the metaphysis of long bones, an active bone growing region, and then can propagate to the bone cortex and the surrounding soft tissues. The molecular pathogenesis of this tumor is quite complex and involves several elements, such as environmental factors (e.g., UV and ionizing radiation, methylcholanthrene and chromium salts, etc.), chromosomal abnormalities, p53 mutations, and so forth [72]. As for many other tumors, vasculature is a critical factor for the survival and proliferation of cancer cells; in fact, osteosarcoma generally involves downregulation of anti-angiogenic factors, such as thrombospondin-1 [73] and pigment epithelium-derived factor (PEDF) [74]. Although osteosarcoma is known as a vascular tumor, there are contradictory data about the correlation between the microvascular density/VEGF expression and the formation of metastasis [75, 76]. Emerging evidence suggests that the blood vessels of bone may play a role in the interactions between other tumors and the bone microenvironment in the pathogenesis of bone metastasis [77]. The extravasation of tumor cells can be related to the molecular receptors typical of a tissue/organ-specific blood vessel [78]. Interestingly, blood vessels located in the metaphysics of long bones express specific adhesion proteins, such as P-selectin and E-selectin, which have been shown to promote interaction and subsequent adhesion of tumor cells [79]. One widely studied example of this kind of interaction is the preferential spreading of breast cancer metastasis to bone. In fact, breast cancer cells express chemokine receptors, integrins, cadherins, and bone-remodeling factors that contribute to the successful and preferential spread of tumor to bone [79].
Due to the multifactorial nature of bone diseases, the aging population, and increased occurrence of trauma-related injuries, bone disorders represent a big concern and the current treatments do not provide optimal outcomes. Furthermore, any alteration in the vascular supply may lead to an increased susceptibility to osteoporosis, osteonecrosis, and osteomyelitis [8]. Animal models have been traditionally utilized in research to mimic these human pathologies. Nevertheless, on multiple occasions, animal models have been unsuccessful and unreliable in predicting the processes and development of human pathologies and the response to drug candidates, because their physiology is fundamentally different from that of humans. To overcome this deficiency, tissue engineering may represent an alternative platform by establishing in-vitro pathophysiological models based on human cells to study the causes and progression of specific diseases, and to develop and test candidate therapeutic strategies.
In-vitro modeling of 3D vascularized bone models through tissue engineering approaches
The successful development of in-vitro engineered bone is critically dependent on the ability to introduce a 3D vascular network that guarantees adequate oxygenation, mass transfer, nutrient delivery, and by-product removal [80]. Here, we summarize the challenges in generating in-vitro vascularized bone constructs and the main materials, scaffolds, and cells required to achieve this goal, with a focus on stem cell employment.
Challenges of in-vitro vascularized bone engineering
Bone is a highly vascularized organ and the development of vasculature and mineralized matrix requires a synergistic interaction between osteogenic and endothelial precursors [81]. These mechanisms have been studied in animal models, but they are still not understood due to the complexity of the in-vivo environment. In-vitro scale-up of bioengineered tissues is known to be limited by diffusion issues; therefore, the establishment of a functional vasculature within the construct could be essential to generate an accurate and large enough model capable of mimicking the native tissue. Current vascularization strategies comprise the use of angiogenic factors combined with 3D scaffolds (Fig. 2a), prevascularization strategies (Fig. 2b), and the use of coculture systems (Fig. 2c) [82, 83].
The first approach relies on the induction of vascularization by endothelial precursors using angiogenic factors such as VEGF. For instance, Braghirolli et al. [84] demonstrated that an electrospun poly(caprolactone) scaffold loaded with VEGF promoted the penetration and proliferation of EPCs within the 3D matrix. This process is primarily EC regulated and, in the context of TE, specific interactions with the scaffold material and other cell types are needed for optimal in-vitro vascularization. Thus, the current research trend is focused on the production of prevascularized constructs in-vitro. This approach proposes the generation of stable vasculature in-vitro using ECs and subsequent in-vivo implantation in the target site [85]. The principal drawback of this method, when utilized for in-vivo bone repair, is the difficulty of generating stable in-vitro vasculature prior to implantation. The use of coculture systems including different cell types is more complex and several parameters must be taken into account for the successful outcome of a vascularized bone construct, namely cell type (osteogenic and vasculogenic), media, seeding methodology, culture (static or dynamic), scaffolds, and microenvironment (e.g., oxygen tension) [83]. In the past decade, different attempts to generate vascularized bone constructs using osteogenic precursor cells and vascular progenitor cells have been made, but the majority of these studies were conducted through in-vivo models [86,87,88]. It has been difficult to create a veritable in-vitro model containing bone tissue and vasculature-like structures within the same construct. A recent attempt to introduce simultaneously osteogenic differentiation and vasculature development in-vitro was made by Tsigkou et al. [89], which combined human bone marrow MSCs and human umbilical vein endothelial cells (HUVECs) seeded on a polymeric scaffold and in a hydrogel, respectively. They observed the formation of capillary-like structures 4–7 days after implantation in a mouse model, and MSCs were found to be necessary for the development of a stable vasculature. The main outstanding issue is still the generation of accurate in-vitro models, due to the difficulties of obtaining quality mineralized bone matrix, further complicated by the introduction of the vasculature.
Bone mineralized matrix formation is provided by osteoblasts during embryonic development and postnatal growth, yet about 95% of the cellular population residing in the adult skeleton is represented by osteocytes. Osteocytes are the terminally differentiated osteoblasts embedded in the lacunae within the bone matrix. They are characterized by cellular processes radially spread toward the mineralized matrix inside tiny tunnels called canaliculi, filled by canalicular fluid [90]. The presence of osteocytes in an engineered bone construct is an indication of a mature developed osseous tissue. Although osteocytes play a secondary role in bone tissue formation, they are functionally important in its homeostasis, mechanosensation, and mechanotransduction [91, 92]. Osteocytes sense canalicular fluid flow-derived shear stress through their primary cilium [92] and specific ion channels and mechanotransduction proteins, releasing in response paracrine signaling molecules such as NO, ATP, and prostaglandins, which regulate osteoblast and osteoclast activity [93,94,95]. The lacuna–canalicular network connects the osteocytes to the vasculature and there is evidence linking osteocyte apoptosis and reduced VEGF production with consequent impaired vascularity and bone strength [96]. Taken together, these findings highlight the importance of the presence of mature osteocytes in an engineered bone. For this reason, many investigations are focused on the derivation of mature osteocytes from stem cells to better understand their physiology and assess their potential in bone engineering [97,98,99].
Bone is also innervated by sensory and sympathetic nerve fibers that, in addition to skeletal pain transmission, play a role in bone metabolism. Bone cells have been recently shown to express adrenoceptors, receptors for norepinephrine (NE), calcitonin gene-related peptide (CGRP), substance P, and vasoactive intestinal peptide (VIP) [100, 101]. NE reuptake influences bone remodeling by osteoclasts [102], CGRP deficiency was linked to decreased bone formation in mice [102], while SP has been shown to have both dose-dependent bone-resorbing and formation activity [102]. These are just a handful of examples on how innervation has been linked to bone production and turnover; thus, an in-vitro engineered bone construct would certainly benefit from the presence of nerve endings and related neurotransmitters. However, the generation of a vascularized and innervated bone construct clearly presents an additional level of complexity in terms of integrating and regulating such different tissue types [103].
Another parameter to consider when developing veritable in-vitro vascularized bone is the incorporation of osteoclast activity inside the engineered constructs. Osteoclasts are essential for bone remodeling by resorbing bone matrix and generating physical space for osteoblasts and ECs, thus allowing the formation of new bone tissue and vasculature [104]. The dissolution of the inorganic phase of bone matrix takes place by the secretion of hydrochloric acid, while the organic matrix is resorbed by secreted enzymes, like cathepsin K and metalloproteinase 9 [105]. The resorption activity as well as the area and depth of the resorption pits can be analyzed in-vitro using osteoclast-like cells derived from monocytes, for instance the RAW 264.7 cell line [106]; however, to date, there are considerable variations in the examined parameters. There is no specific, single functional osteoclast cell marker; thus, different parameters should be analyzed together, such as tartrate-resistant acid phosphatase 5b (TRAP 5b) [107], morphological changes from monocytes [104], and 3D volume characterization of pits [108]. Furthermore, as different materials can affect the response of osteoclasts, the choice of the scaffold is critical for an optimized bone engineering strategy. Different authors have studied the resorption ability of osteoclasts using different scaffolds/materials, from dentin to bone substitutes, as well as calcium phosphate and bioactive glasses [109,110,111,112], and have observed specific resorption rates depending on the material used. For example, Keller et al. [112] found that natural biomaterials were resorbed more rapidly than synthetic ones, while Badran et al. [111] showed an inhibition of osteoclast activity by increasing mineral density.
As highlighted by Alexander et al. in a recent minireview [31], a TE approach can be used for the development of in-vitro preclinical models of normal/pathological tissue function, but an effective high-throughput assay should consist of a minimal system with well-defined performance parameters. These systems should model the structure and function of human tissue, as well as the physiological response to different stimuli, such as the interaction with adjacent tissues [113,114,115]. Bone actively interacts with cartilage, muscles, ligaments, tendons, and many other tissues in its physiological function within the musculoskeletal system [116,117,118,119,120]. An ideal in-vitro model of bone should consider the possibility of studying its interaction with other tissues, thus posing significant challenges in accommodating different, tissue-specific microenvironments.
Due to their pluripotency/multipotency, stem cells represent an exceptional tool to achieve this complex integration between bone and other tissues. A good model to study the interaction between bone and cartilage was developed by Lin et al. [121], who produced a biphasic construct mimicking the nature of the osteochondral junction. This construct was developed using a 3D-printed dual-chamber bioreactor that allowed the creation of two separate yet communicating microenvironments [122]. Osseous and chondral phases were generated using one stem cell type, bone marrow-derived MSCs, encapsulated in a photocrosslinked gelatin methacrylate (gelMA) hydrogel. Osteogenic and chondrogenic media were used to differentiate MSCs toward bone and cartilage phenotype, respectively. The two constructs were independently controlled and tested by the introduction of bioactive agents or candidate drugs.
Although different models have been created to recapitulate the interaction between bone and other adjacent tissues, to the authors’ knowledge, an in-vitro engineered model coupling vascularized bone to another tissue has not yet been developed. Such capability would be particularly relevant when engineering the osteochondral junction, as only the simultaneous presence of bone matrix, vasculature, and cartilage can enhance or permit veritable behavior of the complex. In fact, the interactions between vasculature and cartilage are well known during in-vivo skeletogenesis [123, 124], and numerous in-vitro studies have documented the molecular exchanges driving the inhibition of chondrocyte differentiation, including the pivotal role exerted by VEGF secreted by ECs [125,126,127]. However, a model also containing subchondral bone would better mimic the complex interaction among the three tissues. For these reasons, a triphasic scaffold was produced by the authors using a recently developed 3D-printed microphysiological tissue system (MPS) bioreactor that allows the separate flow of specific media to the chondral and osseous components, while maintaining them in contact and allowing tissue–tissue communication [128] (Fig. 3). The cartilage construct was engineered incorporating human bone marrow-derived MSCs in a photocrosslinkable gelMA hydrogel, while the vascularized osseous construct was obtained by seeding MSCs and HUVECs in a poly(ε-caprolactone) (PCL) scaffold, produced through additive manufacturing as described by Puppi et al. [129]. Results from our preliminary assessments suggest a better differentiation of MSCs in the presence of the HUVECs, which form interconnected tubular-like structures in the osseous compartment.
a Cross-sectional bioreactor schematic. b Macroscopic and histological analysis of engineered osteochondral interface. c Live/dead staining of capillary-like network formed by HUVECs in bone compartment (GFP, green = HUVEC; Calcein Blue AM, blue = live cells; EthD-1, red = dead cells). gelMA gelatin methacrylate, PCL poly(ε-caprolactone), GFP green fluorescent protein, EthD-1 ethidium homodimer-1
Overall, the development of a successful in-vitro model of vascularized bone is heavily dependent on optimal selection of scaffold/matrix able to guide the formation of the different tissues, and on fine control of cell signaling.
Materials
Mineralized bone and associated vasculature are characterized by different morphological and structural properties, thus different biomaterials are needed for the engineering of both tissues to match their characteristics. Different materials have been investigated for the development of bone constructs, and evaluated both in-vitro and in-vivo. Calcium phosphates (CaPs) have been of great interest due to their osteoconductive properties. The majority of research has been focused on two main forms of CaPs, hydroxyapatite (HAp) and beta-tricalcium phosphate (β-TCP), or the combination of both [130]. Other inorganic materials that have been exploited in bone engineering are bioactive glasses [131], which have been shown to improve the formation of a hydroxycarbonate apatite layer by osteoblasts in-vitro and to support bone formation in-vivo [132]. The most commonly used polymeric materials for bone engineering include many natural polymers, such as collagen, fibrin, alginate, silk, hyaluronic acid, chitosan, and polyhydroxyalkanoates [133]. These biopolymers offer economic and environmental advantages, such as low manufacture/disposal costs and renewability, as well as biological advantages, such as supporting cell signaling, adhesion, and cell-mediated degradation and remodeling [134]. Synthetic polymers have also been thoroughly investigated as engineered bone scaffolds, by virtue of their controllable and reproducible chemical–physical and degradation properties, which can be tailored to the requirements of the target applications [135]. The different classes of polymers that have received significant attention include saturated poly(α-hydroxesters), such as poly(glycolic acid) (PGA), poly(lactic acid) (PLA), and PCL, and biodegradable polyurethanes, covering a wide range of properties, including mechanical strength, elasticity, biodegradability, hydrophilicity, and so forth [135]. When considering the development of a capillary-like network within a mineralized bone construct, the most current models include the embedding of endothelial-derived cells in hydrogels, such as Matrigel®, collagen, and fibronectin [136,137,138]. However, these materials have limitations in mechanical stability, durability, and immunogenicity when implanted [139, 140]. One recent example to overcome these limitations is represented by the use of methacrylated gelatin (gelMA), produced by conjugating methacrylate groups to the amine-containing side groups of gelatin, which becomes a photocrosslinkable hydrogel [141]. This material is characterized by the advantages of both natural and synthetic polymers, allowing the fine-tuning of mechanical properties, while preserving biological cues. Different studies in the past have reported the capability of creating vascular-like structures using gelMA and ECs [80, 89, 142].
There have been a number of investigations that explored a variety of biomaterials for the engineering of vascularized bone; however, identifying the most suitable biomaterials remains a difficult task, since each material has its inherent drawbacks. Ceramics are characterized by brittleness and the biodegradation rate not matching the formation of new bone tissue; natural polymers possess low mechanical, thermal, and chemical stability; and synthetic polymers lack biological cues. A logical path to overcome these limitations would be to combine different materials to obtain constructs with improved properties. An example of this paradigm is the development of nanocomposite materials based on biopolymers and ceramic nanofillers, in an attempt to exploit the biological activity of natural polymers as well as the osteoconductivity of ceramics [143].
Scaffolds
All of the biomaterials already described require multistep processing to develop 3D scaffolds/matrices capable of inducing and supporting osteoblast proliferation and differentiation, as well as vasculature development. Accordingly, in addition to osteoinductivity and osteoconductivity, an ideal scaffold should have functional physical properties such as interconnected macroporosity with pore size larger than 100 μm for optimal osteoblast differentiation and formation of new blood vessels, and biocompatible stiffness to match the mechanical properties of native bone [144]. Substrate mechanical properties greatly influence stem cell osteogenic differentiation, as well as EC behavior [145]; for instance, a scaffold Young’s modulus in the range 25–40 kPa directs MSCs toward osteoblastic differentiation [146, 147]. Current technologies do not allow the production of scaffolds with the exact mechanical properties of bone; however, a number of groups have explored development-inspired precursor templates to instruct stem cells to form a mature tissue [148,149,150,151]. Although such constructs do not possess the same mechanical properties as native bone initially, they are engineered in-vitro with sufficient stiffness to be implanted in a load-bearing region and are able to guide stem cells to develop mature bone. For instance, Daly et al. [152] produced an MSC-laden 3D-printed hypertrophic cartilage template, made of a reinforced alginate bioink, which developed into functional vascularized bone when implanted in mice.
Biologically inspired scaffolds should also harbor signals that act to induce the simultaneous development of bone and vasculature. Different biological scaffolds have been used for the development of in-vitro vascularized bone models, including decellularized bone and specific ECM preparations. Decellularized bone has been historically considered and applied as a biologically derived matrix, able to induce mineralized bone production by osteoprogenitor cells. A recent study by Correia et al. [81] showed the employment of decellularized bone plugs as scaffold for HUVECs and MSCs seeded in a fibrin carrier. Vasculature was able to grow inside the porosity of the scaffold both in-vitro and then in- vivo after subcutaneous implantation in mice. The use of decellularized ECM has been reported recently by Gao et al., who developed a vascular patch using MSCs in a decellularized human aortic matrix [153]. Another strategy involves the functionalization of “smart” scaffolds with angiogenic and osteogenic factors such as VEGF, providing a highly localized signal to control stem cell fate [154].
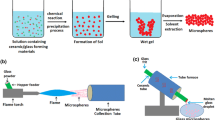
The need to control macrostructural and microstructural properties of a scaffold to meet key requirements of a specific application has led to the development of different manufacturing technologies, such as solvent casting/particulate leaching, freeze drying, phase separation, and combinations of these techniques [135], each conferring to the scaffold different properties. For instance, using a thermally induced phase separation (TIPS) technique, Mannella et al. [155] developed a porous scaffold with a pore size gradient able to mimic the porosity of the cancellous bone. Although several studies about the production of bone scaffolds using the aforementioned techniques have been published, these methodologies are lacking in control over the fine architecture of the structure, specifically in relation to pore morphology and interconnection, fundamental requirements for the introduction of vasculature. There are no precise values for specific bone and/or vascular ingrowth; pore sizes greater than 100 μm have been shown to favor bone formation [156], while capillary density is promoted when pore sizes are greater than 300 μm [130]. For these reasons, solid freeform fabrication (SFF) or additive manufacturing (AM) techniques are attracting great interest, owing to their capabilities of producing predefined interconnected porous structures, using natural and synthetic polymers, as well as ceramics as starting materials [157]. AM techniques comprise the layer-by-layer building of 3D structures, based on computer-aided design (CAD) and computer-aided manufacturing (CAM) processes. Depending on the specific working principles, AM can be classified into laser-based systems (e.g., selective laser sintering, stereolithography), printing-based systems (e.g., 3D printing), and nozzle/extrusion-based systems (e.g., fused deposition modeling, computer-aided wet-spinning), each allowing for specific scaffold macroarchitectural and microarchitectural features [158]. AM has significantly improved the technical ability to control key factors in bone scaffolds, such as composition, pore geometry, size, and interconnectivity, as well as scaffold mechanical performance. The fine-tuning of morphological parameters provided by AM allowed researchers to produce scaffolds for the concomitant development of osseous and vascular tissue. 3D printing has been thoroughly exploited for the generation of bone scaffolds with vascular integration, mostly using ceramics [159], but also synthetic polymers [160], natural polymers [160], and composites [160]. 3D-printed scaffolds have also been loaded with bioactive molecules, such as BMP and VEGF, to promote bone formation and vascularization respectively. Novel studies are exploring the incorporation of other molecules, such as KR-34893 indene compound that stimulates MSC differentiation and mineral deposition [160], oxygen-releasing agents like calcium peroxide to solve O2 diffusion issues [160], and platelet-rich fibrin to stimulate bone marrow-derived MSC differentiation [160]. AM and 3D-printing technologies have also produced great advancements in the field of microfluidics systems and organ-tissue chips that employ stem cells for in-vitro disease modeling and drug screening [161,162,163]. In-vitro vascularized bone models have steadily benefited from this technology. For instance, Jusoh et al. [164] recently reported a microfluidics platform based on HAp-loaded ECM to study angiogenesis and osteogenesis in a vascularized bone model. Furthermore, AM can be integrated with other scaffold fabrication methods to fabricate hybrid architectures with unique structural features, as described in detail by Giannitelli et al. [165]. Although AM techniques were widely studied for the development of bone scaffolds, to date, there are few studies employing them to develop in-vitro models of vascularized bone. A summary of the main scaffolds and fabrication techniques employed in bone engineering is presented in Table 2.
Another fabrication technology that is increasingly gaining attention is bioprinting, which refers to the predefined and precise dispense of cell-laden biomaterials for the construction of complex “living” 3D structures [166]. If the cell selection is represented by osteoprogenitor stem cells, the resulting structure will be composed of cells capable of producing bone matrix. The geometry and dimension of the construct can be controlled in an automated manner such that specific morphologies may be fabricated based on the application. Bioprinting, thus, represents another promising approach for the production of in-vitro models of bone [167]. Traditionally, bioprinting has been applied mainly to the formation of soft tissues and in-vitro vasculature. For instance, Norotte et al. [168] bioprinted single-layered and double-layered vascular tubes with a diameter ranging from 0.9 to 2.5 mm, using different vascular cell types, such as smooth muscle cells and fibroblasts. The identification of new stem cell sources as vascular precursors, coupled with the advancement in bioink and microfluidics technologies, has allowed bioprinting to create constructs that mimic the arrangement of the vasculature in bone for more precise organ modeling. Numerous studies of vasculature bioprinting and chips using stem cells have disclosed different aspects of blood vessel biology, such as the role of transforming growth factor beta on normal vascular function [169], the role of MSCs in promoting vasculature formation [170], regulation of the perivascular stem cell niche by MSCs [171], and the role of angiopoietin in MSC transition to mural cells in the presence of ECs [172]. Bone bioprinting requires the employment of materials with better mechanical properties, needed to mimic the stiffness of the bone matrix. One example of mechanically reinforced bioprinted construct has been made from alginate and PLA nanofibers, supporting adipose-derived MSC viability and differentiation [173]. Another approach is the use of stem cell-laden hydrogels in combination with thermoplastic fibers made of PCL or poly(vinyl alcohol) (PVA) [174, 175]. Gao et al. [176] developed a photopolymerized acrylated peptide, coprinted with poly(ethylene glycol) (PEG) which showed robust osteogenesis of MSCs. MSC osteogenesis has been also stimulated by combining low-intensity pulsed ultrasound (LIPUS), as mechanical stimulation, and a 3D-bioprinted PEG–RGD construct [177]. Other materials have been employed, in various formulations, for the development of bioprinted bone constructs, ranging from agarose and gelatin to PEG [178,179,180]. The integration of bioprinted vasculature and mechanically stiffer substrates would greatly improve the production of functional in-vitro vascularized bone. One example of this paradigm featured a dual 3D bioprinting system based on fused deposition modeling (FDM) and selective laser ablation (SLA) [181]. The authors used alternate deposition of stiff polylactide (PLA) fibers and MSC/HUVEC-laden gelMA hydrogel to achieve proper mechanical strength and biological cues of complex vascularized bone constructs. Another approach comprised the production of a mandible fragment using an integrated tissue-organ printer (ITOP), which was able to bioprint Pluronic-F127 hydrogel laden with human amniotic fluid-derived stem cells (hAFSCs), in combination with a PCL-based mechanical backbone, and featuring incorporated microchannels for nutrient diffusion [182]. Kolesky et al. bioprinted a thick vascularized tissue using different polymeric templates and fugitive inks. The construct was laden with MSCs in combination with HUVECs, as well as other parenchymal and stromal cells, showing robust osteogenesis and functional perfusion of the vasculature [183].
The great landscape of current scaffold fabrication technologies has provided a wealth of choices for the generation of in-vitro vascularized bone constructs. Future attention should be focused on high-throughput and high-resolution AM techniques that are able to produce functional vasculature within a mature bone construct. The key aspect is the biocompatibility of these methods with the chosen cells of interest in order to obtain their optimal functionality.
Osteogenic and vascular precursors
When considering the development of an in-vitro model of vascularized bone, the selection of suitable cell sources is crucial. A desired cell source must have little to no limitation in terms of availability and be easy to maintain and manipulate in-vitro. Adult MSCs are widely employed for somatic TE by virtue of their ability to differentiate into multiple lineages, such as cartilage, fat, muscle, and bone [184]. MSCs can be readily isolated from different tissue sources, such as adipose, muscle, bone, and in particular, bone marrow, which is the most widely used source [185]. In fact, bone marrow-derived MSCs have been benchmarked as one of the most appropriate cell sources for bone TE due to their well-defined osteogenic differentiation [186,187,188]. By using an appropriate culture medium, MSCs can be expanded without differentiation, and induced to undergo specific differentiation to a stable phenotype in culture [189]. In addition to bone marrow-derived MSCs, adipose-derived MSCs (ASCs) are now a widely accepted source for bone tissue engineering applications and have been employed also in numerous preclinical and clinical studies [190]. Induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) have also been studied as potential cell sources for bone regeneration [191,192,193,194]. Although pluripotent ESCs are a promising cell candidate for the development of fully functional vascularized bone in-vitro as they can form all specialized cell types constituting the human bone, including its vasculature [195], ESCs research also raises ethical and political controversies due to their derivation from early human embryos [196]. For these reasons, the use of iPSCs obtained by reprogramming of adult somatic cells, thus avoiding the ethical problems inherent in ESC research, has gained significant attention.
Current models of biomimetic, engineered vascularized bone, fabricated by culturing human MSCs on 3D scaffolds resembling the matrix of native bone [197], require a vascular compartment created using other cell sources. Various sources of stem cells, such as ESCs, MSCs, and iPSCs, have been identified as potential candidates for vascular engineering [198,199,200,201,202]. Endothelial cells have also been successfully differentiated from amniotic fluid stem cells [203, 204]. The choice of vascular precursor cells is crucial for functional production of a proper vasculature within the in-vitro bone model. Mature ECs have been traditionally used to stimulate angiogenesis [205], among them HUVECs, which represent one of the most commonly employed cells in vascularized bone engineering. These cells naturally form vessel-like structures when cultured in hydrogels and, most importantly, they were demonstrated to enhance MSC osteogenesis in-vitro [86, 206,207,208]. Another cell type that has gained attention are the EPCs, which were found to be 10 times more proliferative than HUVECs [209] and have been recently used, also in combination with MSCs, to improve vasculogenesis in-vivo [210,211,212,213] and in tissue engineering applications [214,215,216]. Blood vessel development was also achieved by employing iPSC-derived ECs [217].
As mentioned earlier, active osteoclasts are also a crucial component of in-vitro bone modeling; however, the exact optimal cell source for osteoclasts has yet to be defined. There are several cell types that can be differentiated into osteoclast-like cells, such as bone marrow and peripheral blood mononuclear cells [109, 218], and human mononuclear leukocytes isolated from umbilical cord blood [219]. Osteoclasts can be directly isolated from native bone tissue [220]. RAW 264.7, a mouse leukemic monocyte–macrophage cell line, has been frequently used to study osteoclastogenesis in-vitro [221]. Primary monocytes can also be differentiated into osteoclast-like cells and used to study osteoclastogenesis in-vitro [222]. Furthermore, osteoclasts have been obtained by differentiating macrophages derived from human iPSCs [99].
These well-established, strong relationships among the constituent cell types, taken together, underscore the important and fundamental requirement of these cells in the success for development of vascularized bone in-vitro and the production of a veritable bone model (Fig. 4).
Primary cellular interactions between ECs, osteoblasts, and osteoclasts in production of vascularized bone. VEGF vascular endothelial growth factor, bFGF basic fibroblast growth factor, IL interleukin, CSF colony-stimulating factor, BMP bone morphogenetic protein, IGF insulin-like growth factor, MCP-1 monocyte chemoattractant protein-1, SDF stromal cell-derived factor-1, RANKL receptor activator of nuclear factor kappa-Β ligand, OSCAR osteoclast-associated receptor
Culture media
Based on the cells selected for the development of a vascularized bone construct, the choice of culture media and seeding methodology has to be carefully considered to avoid negative, undesired effects. Different medium formulations and supplements have been identified for optimal osteogenic differentiation of stem cells. Dexamethasone, β-glycerophosphate, ascorbic acid, 1,25-dihydroxyvitamin D3, and BMP-2 have significant osteogenic inductive activity on MSCs by influencing different aspects of their biology, such as activation of specific genes (osteopontin, core binding factor α1, alkaline phosphatase, osteocalcin) as well as signaling pathways like Wnt [184]. Regarding the culture of endothelial progenitors, VEGF, basic fibroblast growth factor (bFGF), and epidermal growth factor (EGF) are usually employed to stimulate endothelial progenitor expansion and differentiation/angiogenesis [223]. The use of individual differentiation medium, endothelial or osseous, generally yields satisfactory vasculature and mineralized matrix formation, albeit separately [224]. However, given the need for simultaneous development of bone and vasculature, a coculture system must be set up, and investigators have used either a combination of the two differentiation media [88, 206, 225,226,227], a single type of medium [88, 228], or osteogenic medium with some vascular growth factors [229]. In the case of a coculture system, while different cell types can be seeded simultaneously to avoid uneven distribution inside the construct and to allow communication between them, the choice of culture media is very important to assure cosurvival and differentiation. In another approach, different cell types can be expanded and differentiated separately and then seeded together, thus avoiding the media problem; however, some critical cell–cell interactions will be missing and, as discussed earlier, the generation of a veritable in-vitro model, which requires recapitulation of the interaction between bone cells and vasculature, may thus be compromised. Another parameter that needs to be considered is the cell ratio. Signaling between osteogenic and vascular precursors, as well as their adult counterparts in the mature organ, directs their functional integration (see Fig. 4). Altering the balance of this crosstalk compromises the engineering of the vascularized bone tissue. Investigators have studied varying combinations between osteogenic and vascular precursors, ranging from a 1:1 mix to unbalanced ratios in favor of osteogenic or endothelial precursors. Although a positive trend can be identified in the use of a 1:1 ratio, the results are also heterogeneous and an ideal ratio cannot be defined a priori [230]. Table 3 summarizes the main media and cell ratios that have been used in the engineering of vascularized bone and their major benefits and drawbacks.
A recently published review by Liu et al. [83] covered different aspects of the medium combinations and seeding methodologies used in coculture systems for vascularized bone tissue engineering. However, due to the heterogeneity of the experimental parameters used in coculture studies, a clear trend is difficult to establish.
Considering the tight interactions between osteogenic and vascular stem cells during development, and the functional integration between bone and blood vessels in the mature organ, the development of veritable in-vitro models of this organ should comprise both types of cells cultured in a mixed medium in order to not miss the key interplay between osseous and vascular precursors/mature cells.
Bioreactors
Bioreactors are generally defined as any device that is able to dynamically sustain biological and/or biochemical processes under precisely monitored and controlled experimental and operating conditions (e.g., pH, temperature, mechanical stimuli, time, nutrient supply, and waste removal) [231]. Different types of experimental setups have been developed to optimize cell seeding and mass transport, such as spinner flasks (Fig. 5a), rotating wall vessels (RWVs) (Fig. 5b), and perfusion bioreactors (Fig. 5c). Spinner flasks and RWVs minimize the nutrient gradient and metabolite concentration around the construct, while perfusion bioreactors directly perfuse media inside the scaffold, thus assuring mass transport within the porosity [232]. Using optimal material–scaffold–cell systems, coupled with the higher control over experimental parameters, has made bioreactors ideal means for the development of 3D tissues in-vitro. This is particularly true for the engineering of biological interfaces or complex tissues, like the osteochondral junction and vascularized bone. Bioreactors, in combination with MSCs, have in fact been used for the recreation of the osteochondral tissue interface, as reported in a number of studies [121, 233, 234]. In particular, culturing in flow perfusion bioreactors has been shown to upregulate expression of osteoblastic markers [235,236,237], and these bioreactors have been employed for the production of engineered bone [238,239,240]. To improve the amount and quality of bone beyond what has been produced by an in-vitro process, various studies have reported the use of “in-vivo bioreactor” systems to produce a clinically relevant amount of vascularized bone (Fig. 5d). These approaches were based on the body’s healing mechanism that supports the formation of neotissue in different kinds of scaffolds, ranging from calcium-alginate gel, to β-TCP, to natural bovine bone mineral-coated titanium meshes implanted subperiosteally in-vivo [241,242,243]. The engineered bone produced with this approach could be harvested from the “living bioreactor” host and subsequently transplanted successfully at a bone defect site. While in-vivo engineering surely ensures production of enhanced vascularized bone, high-throughput assays require a large number of identical samples that cannot be performed by in-vivo systems, thus necessitating the development of in-vitro platforms of vascularized bone engineering. In addition to improving in-vitro osteogenic differentiation of MSCs in the presence of ECs, several studies have reported better matrix mineralization by osteogenic MSCs cultured under dynamic conditions rather than static ones [237, 244,245,246]. However, the combination of these two strategies using a flow perfusion bioreactor did not significantly enhance osteogenic differentiation of MSCs in the presence of ECs [247, 248], likely because shear stress affects the function of ECs [249,250,251]. However, another approach was used by Nishi et al. [252], who cultured MSCs and ECs on a porous poly(lactic acid) scaffold in a rotating wall vessel bioreactor. The flow environment created by the RWV bioreactor, coupled with the interaction between the cell types, enhanced the distribution and differentiation of cells in the scaffold. To date, there are only a limited number of studies using a bioreactor in combination with bone and vascular progenitor cells, due to the technical and biological challenges of codifferentiating the two cell types together. The successful construction of a physiologically compatible bioreactor for engineered vascularized bone, to achieve fast and easy visualization of the target cells and their interactions, must take into account a number of design features, including noninvasive optical access, automation, uniform cell seeding, overall chamber dimensions, checkpoint markers, and mechanical stimuli [253, 254].
In utilizing engineered vascularized bone models in-vitro, instead of harvesting tissue samples and analyzing them at designated time points, the nondestructive tracking of cells and new matrix formation should result in a reduction of resources and permit real-time understanding of the processes. One example is represented by the work reported by Bersini et al. [255], who developed a biological 3D in-vitro microfluidic model to study breast cancer metastasis to bone. This microfluidics platform is based on hydrogel-containing chambers placed between surface-accessible microchannels, allowing high-resolution real-time imaging of single-cell behavior, cell–cell communication, cell–matrix interactions, and cell population dynamics [256]. Another useful high-throughput platform was developed by Moya et al. [257] to study vasculature in real time. They produced an in-vitro 3D metabolically active stroma (~ 1 mm3 volume) containing a living and dynamically perfused human capillary network, by combining human endothelial colony forming cell-derived ECs (ECFC-ECs) and a polydimethylsiloxane (PDMS) micromold. Although diffusion of oxygen was demonstrated to not be a limiting step in-vitro, thus allowing the development of tissue constructs in the order of centimeters in thickness [258], the major technical problem is nutrient diffusion. The density of the ECM plays an important role in the diffusion of nutrients and the morphogenesis of capillaries [259], and this is particularly true for mineralized bone matrix. In addition, real-time visualization of the process occurring inside the bone matrix is also limited by the opacity of the bone matrix itself, thus requiring the development of specific bioreactor or in-vitro models to overcome this limitation. X-ray microcomputed tomography (μCT) has been extensively used in the field of bone tissue engineering due to its ability to provide rapid, nondestructive 3D images of bone and bone scaffold microstructure at 1–50 μm resolution [260, 261]. Various groups have developed μCT-compatible bioreactors [262, 263], utilizing low radio-opacity materials, such as polysulfone, with dimensions matched to those of standard μCT chambers. Another requirement is that such systems should allow the study of the vasculature within in-vitro vascularized bone, which, however, is complicated by the 3D nature and limited access to the internal microvasculature due to the loss of optical access. While the analysis of thin sections of engineered vascularized bone could address this issue, it would obviate the purpose of recapitulating the 3D in-vivo structure. A more viable solution is the use of contrast-enhanced μCT for the investigation of microvasculature [264], which was previously studied in soft tissues, such as the kidney, heart, and liver [265,266,267]. This method is based on the injection of a radio-opaque contrast agent within the vasculature prior to imaging. However, again, the opacity and density of the bone matrix put some limitations on visualization of the vessel microstructure when this is performed in a bone model. It is thus not surprising that few studies have been published on the analysis of bone microvasculature using μCT [268, 269], illustrating the future potential of contrast-enhanced μCT for the evaluation of bone neovascularization in tissue-engineered constructs.
Perspectives and future directions
The definition of the parameters that must be accommodated in the design of in-vitro models of vascularized bone represents a challenging task for researchers. The complex interactions between blood vessels and bone have limited our ability to develop veritable in-vitro constructs of physiological systems representing human bone. First, from a biological point of view, the codifferentiation of endothelial precursors and osteogenic stem cells is hindered by the different mechanisms and signals to which these cells respond when recapitulating in-vivo development. As shown in numerous studies, a compromise between endothelial and osteogenic signals/cues must be considered, in order to minimize undesired differentiation of the cell types. On the other hand, predifferentiation of vasculature or bone cells does not permit the critical communications between the two physiological systems during development or repair, thus compromising the morphogenesis of the actual structure of the tissue complex and in a manner similar to native bone. This aspect is of high relevance when studying the effects of drugs and/or toxicants on the structure and development of bone, as well as screening for potential treatments. In other words, the engineered vascularized bone model must present advantages over the majority of studies that have been performed in-vitro on 2D cultures of separate endothelial and osteogenic cells or cocultures, and through in-vivo animal models [270, 271]. From an engineering point of view, the design of a system which could maintain the vitality and differentiation of different types of cells for long-term culture is also a significant challenge. Furthermore, experimental parameters must be controlled (i.e., system variables must be clearly defined) and, as described earlier, the system must be accessible for real-time imaging and evaluation. Bioreactors have partially solved the need to have in-vitro systems which could mimic the full physiological structure of bone and, at the same time, have allowed analysis of the constructs.
High-throughput screening of drugs or toxicant relies on a minimal system defined by precise parameters that can be stimulated by specific stimuli, such as mechanical, biochemical, genetic, and so forth. To address this, our group has developed a triphasic model composed of blood vessels, bone, and cartilage to study the interaction between these three different tissues [128]. Our 3D-printed microphysiological system, in combination with stem cells, allows for the codifferentiation of the three different tissues, and is also responsive to the introduction of different stimuli, including stress-inducing factors, such as IL-1β for the study of osteoarthritis, female hormones like estrogens for the study of osteoporosis [272], or teratogens to study their effect on skeletal development. The capabilities of additive manufacturing have enabled us to produce customized scaffolds and bioreactors to meet the requirements of vascularized bone engineering, coupled with a high-throughput platform for drug screening and toxicology assessment. In the future, bioprinting could represent a suitable on-demand platform for the versatile fabrication of specific cellular patterns at the micrometer scale for the production of vascularized bone, but also a broad range of other engineered tissues [166]. While still in its early stage, bioprinting can also benefit from the wide range of additive manufacturing techniques and bioreactor technologies to generate veritable in-vitro models of vascularized bone that could help understand the physiopathology of bone, and generate valuable treatment strategies. To become an economically viable resource, the production of in-vitro 3D models of vascularized bone should also consider the introduction of a certain level of automation, to reduce human intervention as well as product variability. An interesting and comprehensive review about this topic was recently published by Costa et al. [273], who reviewed the current automated tools and strategies for the production of bone substitutes.
Conclusions
In addition to applications in regenerative medicine, tissue-engineered constructs could be used as in-vitro preclinical models of normal or pathological tissues for applications in drug screening and toxicity assessment. For effective high-throughput assays, minimal systems and accurate modeling of the structure/function of the studied human tissue or organ are required. Bone pathologies present significant disease burden, underscoring the need to develop more effective treatment strategies. The development of in-vitro high-throughput microphysiological models that faithfully recapitulate the physiopathology of bone is thus highly relevant, particularly given the cost and intrinsic genetic difference of animal models. Different biological and engineering challenges must be overcome, including the right choice of stem cells, regulation of codifferentiation of vasculature and bone, control of system parameters and stimuli, access for real-time imaging, and functional evaluation. In the pursuit of this initiative, additive manufacturing techniques and bioreactor technologies have increased our ability to produce systems that integrate cells, scaffolds, and biological/environmental stimuli to create in-vitro models of native osseous tissue, and to study the processes regulating its biology.
Abbreviations
- EC:
-
Endothelial cell
- EPC:
-
Endothelial progenitor cell
- ESC:
-
Embryonic stem cell
- HUVEC:
-
Human umbilical vein endothelial cell
- iPSC:
-
Induced pluripotent stem cell
- MSC:
-
Mesenchymal stem cell
- μCT:
-
Microcomputed tomography
References
Thompson WR, Gottardi R, Stearns KM, Rubin J, Ambrosio F, Tuan RS. Biologics in cartilage, bone repair, and regeneration. In: Hughes CC, Childers M, editors. Appl Regen Med to Orthop Phys Ther. La Crosse: Orthopaedic Section: APTA; 2014. p. 1–24.
Laurencin CT, Ambrosio AMA, Borden MD, Cooper JA. Tissue engineering: Orthopedic applications. Annu Rev Biomed Eng. 1999;1:19–46.
Cowin SC, Cardoso L. Blood and interstitial flow in the hierarchical pore space architecture of bone tissue. J Biomech. 2015;48:842–54.
Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40:363–408.
Yang Y. Skeletal morphogenesis during embryonic development. Crit Rev Eukaryot Gene Expr. 2009;19:197–218.
Fröhlich M, Grayson WL, Wan LQ, Marolt D, Drobnic M, Vunjak-Novakovic G. Tissue engineered bone grafts: biological requirements, tissue culture and clinical relevance. Curr Stem Cell Res Ther. 2008;3:254–64.
Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol. 2011;27:563–84.
Kanczler JM, Oreffo ROC. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008;15:100–14.
Hu DP, Ferro F, Yang F, Taylor AJ, Chang W, Miclau T, et al. Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes. Development. 2017;144:221–34.
Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76.
Haigh JJ, Gerber HP, Ferrara N, Wagner EF. Conditional inactivation of VEGF-A in areas of collagen2a1 expression results in embryonic lethality in the heterozygous state. Development. 2000;127:1445–53.
Schipani E, Maes C, Carmeliet G, Semenza GL. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J Bone Miner Res. 2009;24:1347–53.
Carulli C, Innocenti M, Brandi ML. Bone vascularization in normal and disease conditions. Front Endocrinol. 2013;4:106.
Burkhardt R, Kettner G, Böhm W, Schmidmeier M, Schlag R, Frisch B, et al. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone. 1987;8:157–64.
Smith DW. Is avascular necrosis of the femoral head the result of inhibition of angiogenesis? Med Hypotheses. 1997;49:497–500.
Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37–A:985–1004.
Funayama E, Sasaki S, Oyama A, Furukawa H, Hayashi T, Yamamoto Y. How do the type and location of a vascular malformation influence growth in Klippel-Trénaunay syndrome? Plast Reconstr Surg. 2011;127:340–6.
Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520–6.
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–81.
Alagiakrishnan K, Juby A, Hanley D, Tymchak W, Sclater A. Role of vascular factors in osteoporosis. J Gerontol A Biol Sci Med Sci. 2003;58:M362–6.
Browner WS, Pressman AR, Nevitt MC, Cauley JA, Cummings SR. Association between low bone density and stroke in elderly women. The study of osteoporotic fractures. Stroke. 1993;24:940–6.
Samelson EJ, Kiel DP, Broe KE, Zhang Y, Cupples LA, Hannan MT, et al. Metacarpal cortical area and risk of coronary heart disease: the Framingham Study. Am J Epidemiol. 2004;159:589–95.
Wimalawansa SJ, De Marco G, Gangula P, Yallampalli C. Nitric oxide donor alleviates ovariectomy-induced bone loss. Bone. 1996;18:301–4.
Kasten TP, Collin-Osdoby P, Patel N, Osdoby P, Krukowski M, Misko TP, et al. Potentiation of osteoclast bone-resorption activity by inhibition of nitric oxide synthase. Proc Natl Acad Sci U S A. 1994;91:3569–73.
MacRitchie AN, Jun SS, Chen Z, German Z, Yuhanna IS, Sherman TS, et al. Estrogen upregulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ Res. 1997;81:355–62.
Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002;23:665–86.
García-Durán M, de Frutos T, Díaz-Recasens J, García-Gálvez G, Jiménez A, Montón M, et al. Estrogen stimulates neuronal nitric oxide synthase protein expression in human neutrophils. Circ Res. 1999;85:1020–6.
Gargett CE, Bucak K, Zaitseva M, Chu S, Taylor N, Fuller PJ, et al. Estrogen receptor-alpha and -beta expression in microvascular endothelial cells and smooth muscle cells of myometrium and leiomyoma. Mol Hum Reprod. 2002;8:770–5.
Toutain CE, Filipe C, Billon A, Fontaine C, Brouchet L, Guery JC, et al. Estrogen receptor expression in both endothelium and hematopoietic cells is required for the accelerative effect of estradiol on reendothelialization. Arterioscler Thromb Vasc Biol. 2009;29:1543–50.
Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr Cartil. 2013;21:1145–53.
Alexander PG, Gottardi R, Lin H, Lozito TP, Tuan RS. Three-dimensional osteogenic and chondrogenic systems to model osteochondral physiology and degenerative joint diseases. Exp Biol Med (Maywood). 2014;239:1080–95.
Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15:223.
Mahjoub M, Berenbaum F, Houard X. Why subchondral bone in osteoarthritis? The importance of the cartilage bone interface in osteoarthritis. Osteoporos Int. 2012;23:841–6.
Coughlin TR, Kennedy OD. The role of subchondral bone damage in post-traumatic osteoarthritis. Ann N Y Acad Sci. 2016;1383:58–66.
Arkill KP, Winlove CP. Solute transport in the deep and calcified zones of articular cartilage. Osteoarthr Cartil. 2008;16:708–14.
Lories RJ, Luyten FP. The bone–cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7:43–9.
Pan J, Zhou X, Li W, Novotny JE, Doty SB, Wang L. In situ measurement of transport between subchondral bone and articular cartilage. J Orthop Res. 2009;27:1347–52.
Funck-Brentano T, Cohen-Solal M. Crosstalk between cartilage and bone: when bone cytokines matter. Cytokine Growth Factor Rev. 2011;22:91–7.
Imhof H, Breitenseher M, Kainberger F, Rand T, Trattnig S. Importance of subchondral bone to articular cartilage in health and disease. Top Magn Reson Imaging. 1999;10:180–92.
Berry JL, Thaeler-Oberdoerster DA, Greenwald AS. Subchondral pathways to the superior surface of the human talus. Foot Ankle. 1986;7:2–9.
Lyons TJ, McClure SF, Stoddart RW, McClure J. The normal human chondro-osseous junctional region: evidence for contact of uncalcified cartilage with subchondral bone and marrow spaces. BMC Musculoskelet Disord. 2006;7:52.
Botter SM, van Osch GJVM, Clockaerts S, Waarsing JH, Weinans H, van Leeuwen JPTM. Osteoarthritis induction leads to early and temporal subchondral plate porosity in the tibial plateau of mice: an in vivo microfocal computed tomography study. Arthritis Rheum. 2011;63:2690–9.
Walsh DA, Bonnet CS, Turner EL, Wilson D, Situ M, McWilliams DF. Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthr Cartil. 2007;15:743–51.
Tsai T-L, Wang B, Squire MW, Guo L-W, Li W-J. Endothelial cells direct human mesenchymal stem cells for osteo- and chondro-lineage differentiation through endothelin-1 and AKT signaling. Stem Cell Res Ther. 2015;6:88.
Calhoun J, Manring MM, Shirtliff M. Osteomyelitis of the long bones. Semin Plast Surg. 2009;23:59–72.
Brem H, Jacobs T, Vileikyte L, Weinberger S, Gibber M, Gill K, et al. Wound-healing protocols for diabetic foot and pressure ulcers. Surg Technol Int. 2003;11:85–92.
Fang RC, Galiano RD. Adjunctive therapies in the treatment of osteomyelitis. Semin Plast Surg. 2009;23:141–7.
Namazi H. Platelet-rich plasma as a novel therapeutic agent in osteomyelitis. Med Hypotheses. 2007;69:706.
Ross JJ. Angiogenic gene therapy as a potential therapeutic agent in chronic osteomyelitis. Med Hypotheses. 2006;67:161–3.
Aliyev M, Aykan A, Eski M, Arslan N, Kurt B, Şengezer M. Effects of transpositional muscle flaps transfected with vascular endothelial growth factor gene in the treatment of experimental osteomyelitis. Ulus Travma Acil Cerrahi Derg. 2016;22:205–14.
Ghali S, Bhatt KA, Dempsey MP, Jones DM, Singh S, Arabi S, et al. Treating chronic wound infections with genetically modified free flaps. Plast Reconstr Surg. 2009;123:1157–68.
Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World J Orthop. 2015;6:590–601.
Kubo T, Ueshima K, Saito M, Ishida M, Arai Y, Fujiwara H. Clinical and basic research on steroid-induced osteonecrosis of the femoral head in Japan. J Orthop Sci. 2016;21:407–13.
Fantasia JE. The role of antiangiogenic therapy in the development of osteonecrosis of the jaw. Oral Maxillofac Surg Clin North Am. 2015;27:547–53.
Aghaloo T, Hazboun R, Tetradis S. Pathophysiology of osteonecrosis of the jaws. Oral Maxillofac Surg Clin North Am. 2015;27:489–96.
Hausman M, Schaffler M, Majeska R. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone. 2001;29:560–4.
Street J, Bao M, DeGuzman L, Bunting S, Peale FV, Ferrara N, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99:9656–61.
Bahney CS, Hu DP, Miclau T, Marcucio RS. The multifaceted role of the vasculature in endochondral fracture repair. Front Endocrinol. 2015;6:4.
Gómez-Barrena E, Rosset P, Lozano D, Stanovici J, Ermthaller C, Gerbhard F. Bone fracture healing: cell therapy in delayed unions and nonunions. Bone. 2015;70:93–101.
Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, et al. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood. 2005;105:1068–77.
Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8.
Li R, Atesok K, Nauth A, Wright D, Qamirani E, Whyne CM, et al. Endothelial progenitor cells for fracture healing: a microcomputed tomography and biomechanical analysis. J Orthop Trauma. 2011;25:467–71.
Keramaris NC, Kaptanis S, Moss HL, Loppini M, Pneumaticos S, Maffulli N. Endothelial progenitor cells (EPCs) and mesenchymal stem cells (MSCs) in bone healing. Curr Stem Cell Res Ther. 2012;7:293–301.
Marenzana M, Shipley AM, Squitiero P, Kunkel JG, Rubinacci A. Bone as an ion exchange organ: evidence for instantaneous cell-dependent calcium efflux from bone not due to resorption. Bone. 2005;37:545–54.
Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–5.
Pajevic PD. Regulation of bone resorption and mineral homeostasis by osteocytes. IBMS Bonekey. 2009;6:63–70.
Hunter WL, Arsenault AL, Hodsman AB. Rearrangement of the metaphyseal vasculature of the rat growth plate in rickets and rachitic reversal: a model of vascular arrest and angiogenesis renewed. Anat Rec. 1991;229:453–61.
Pounds JG, Long GJ, Rosen JF. Cellular and molecular toxicity of lead in bone. Environ Health Perspect. 1991;91:17–32.
Vargesson N. Thalidomide-induced teratogenesis: history and mechanisms. Birth Defects Res C Embryo Today. 2015;105:140–56.
Ornoy A. Valproic acid in pregnancy: how much are we endangering the embryo and fetus? Reprod Toxicol. 2009;28:1–10.
Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–90.
Broadhead ML, Clark JCM, Myers DE, Dass CR, Choong PFM. The molecular pathogenesis of osteosarcoma: a review. Sarcoma. 2011;2011:959248.
Ren B, Yee KO, Lawler J, Khosravi-Far R. Regulation of tumor angiogenesis by thrombospondin-1. Biochim Biophys Acta. 2006;1765:178–88.
Cai J, Parr C, Watkins G, Jiang WG, Boulton M. Decreased pigment epithelium-derived factor expression in human breast cancer progression. Clin Cancer Res. 2006;12:3510–7.
Mantadakis E, Kim G, Reisch J, McHard K, Maale G, Leavey PJ, et al. Lack of prognostic significance of intratumoral angiogenesis in nonmetastatic osteosarcoma. J Pediatr Hematol Oncol. 2001;23:286–9.
Kaya M, Wada T, Akatsuka T, Kawaguchi S, Nagoya S, Shindoh M, et al. Vascular endothelial growth factor expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis. Clin Cancer Res. 2000;6:572–7.
Raymaekers K, Stegen S, van Gastel N, Carmeliet G. The vasculature: a vessel for bone metastasis. Bonekey Rep. 2015;4:742.
Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84.
Glinsky VV. Intravascular cell-to-cell adhesive interactions and bone metastasis. Cancer Metastasis Rev. 2006;25:531–40.
Chen Y-C, Lin R-Z, Qi H, Yang Y, Bae H, Melero-Martin JM, et al. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv Funct Mater. 2012;22:2027–39.
Correia C, Grayson WL, Park M, Hutton D, Zhou B, Guo XE, et al. In vitro model of vascularized bone: synergizing vascular development and osteogenesis. PLoS One. 2011;6:e28352.
Bayer EA, Gottardi R, Fedorchak MV, Little SR. The scope and sequence of growth factor delivery for vascularized bone tissue regeneration. J Control Release. 2015;219:129–40.
Liu Y, Chan JKY, Teoh S-H. Review of vascularised bone tissue-engineering strategies with a focus on co-culture systems. J Tissue Eng Regen Med. 2015;9:85–105.
Braghirolli DI, Helfer VE, Chagastelles PC, Dalberto TP, Gamba D, Pranke P. Electrospun scaffolds functionalized with heparin and vascular endothelial growth factor increase the proliferation of endothelial progenitor cells. Biomed Mater. 2017;12:25003.
Liu Y, Teoh S-H, Chong MSK, Lee ESM, Mattar CNZ, Randhawa NK, et al. Vasculogenic and osteogenesis-enhancing potential of human umbilical cord blood endothelial colony-forming cells. Stem Cells. 2012;30:1911–24.
Grellier M, Granja PL, Fricain J-C, Bidarra SJ, Renard M, Bareille R, et al. The effect of the co-immobilization of human osteoprogenitors and endothelial cells within alginate microspheres on mineralization in a bone defect. Biomaterials. 2009;30:3271–8.
Moioli EK, Clark PA, Chen M, Dennis JE, Erickson HP, Gerson SL, et al. Synergistic actions of hematopoietic and mesenchymal stem/progenitor cells in vascularizing bioengineered tissues. Giannobile W, editor. PLoS One. 2008;3:e3922.
Usami K, Mizuno H, Okada K, Narita Y, Aoki M, Kondo T, et al. Composite implantation of mesenchymal stem cells with endothelial progenitor cells enhances tissue-engineered bone formation. J Biomed Mater Res A. 2009;90A:730–41.
Tsigkou O, Pomerantseva I, Spencer JA, Redondo PA, Hart AR, O’Doherty E, et al. Engineered vascularized bone grafts. Proc Natl Acad Sci U S A. 2010;107:3311–6.
Wittkowske C, Reilly GC, Lacroix D, Perrault CM. In vitro bone cell models: impact of fluid shear stress on bone formation. Front Bioeng Biotechnol. 2016;4:87.
Alliston T. Biological regulation of bone quality. Curr Osteoporos Rep. 2014;12:366–75.
Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell … and more. Endocr Rev. 2013;34:658–90.
Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, et al. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16:3100–6.
Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212:207–14.
Zaman G, Pitsillides AA, Rawlinson SC, Suswillo RF, Mosley JR, Cheng MZ, et al. Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res. 1999;14:1123–31.
Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616–26.
Ehnes DD, Price FD, Shrive NG, Hart DA, Rancourt DE, zur Nieden NI. Embryonic stem cell-derived osteocytes are capable of responding to mechanical oscillatory hydrostatic pressure. J Biomech. 2015;48:1915–21.
Thompson WR, Uzer G, Brobst KE, Xie Z, Sen B, Yen SS, et al. Osteocyte specific responses to soluble and mechanical stimuli in a stem cell derived culture model. Sci Rep. 2015;5:11049.
Jeon OH, Panicker LM, Lu Q, Chae JJ, Feldman RA, Elisseeff JH. Human iPSC-derived osteoblasts and osteoclasts together promote bone regeneration in 3D biomaterials. Sci Rep. 2016;6:26761.
Grässel S, Muschter D. Peripheral nerve fibers and their neurotransmitters in osteoarthritis pathology. Int J Mol Sci. 2017;18:931.
Grässel SG. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res Ther. 2014;16:485.
Aitken SJ, Landao-Bassonga E, Ralston SH, Idris AI. Beta2-adrenoreceptor ligands regulate osteoclast differentiation in vitro by direct and indirect mechanisms. Arch Biochem Biophys. 2009;482:96–103.
Marrella A, Lee TY, Lee DH, Karuthedom S, Syla D, Chawla A, et al. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater Today. 2017; https://doi.org/10.1016/j.mattod.2017.10.005. In Press.
Detsch R, Boccaccini AR. The role of osteoclasts in bone tissue engineering. J Tissue Eng Regen Med. 2015;9:1133–49.
Bruzzaniti A, Baron R. Molecular regulation of osteoclast activity. Rev Endocr Metab Disord. 2006;7:123–39.
Detsch R, Schaefer S, Deisinger U, Ziegler G, Seitz H, Leukers B. In vitro: osteoclastic activity studies on surfaces of 3D printed calcium phosphate scaffolds. J Biomater Appl. 2011;26:359–80.
Halleen JM, Alatalo SL, Suominen H, Cheng S, Janckila AJ, Väänänen HK. Tartrate-resistant acid phosphatase 5b: a novel serum marker of bone resorption. J Bone Miner Res. 2000;15:1337–45.
Goldberg SR, Georgiou J, Glogauer M, Grynpas MD. A 3D scanning confocal imaging method measures pit volume and captures the role of Rac in osteoclast function. Bone. 2012;51:145–52.
Tortelli F, Pujic N, Liu Y, Laroche N, Vico L, Cancedda R. Osteoblast and osteoclast differentiation in an in vitro three-dimensional model of bone. Tissue Eng Part A. 2009;15:2373–83.
Midha S, van den Bergh W, Kim TB, Lee PD, Jones JR, Mitchell CA. Bioactive glass foam scaffolds are remodelled by osteoclasts and support the formation of mineralized matrix and vascular networks in vitro. Adv Healthc Mater. 2013;2:490–9.
Badran Z, Pilet P, Verron E, Bouler J-M, Weiss P, Grimandi G, et al. Assay of in vitro osteoclast activity on dentine, and synthetic calcium phosphate bone substitutes. J Mater Sci Mater Med. 2012;23:797–803.
Keller J, Brink S, Busse B, Schilling AF, Schinke T, Amling M, et al. Divergent resorbability and effects on osteoclast formation of commonly used bone substitutes in a human in vitro-assay. PLoS One. 2012;7:e46757.
Grayson WL, Bhumiratana S, Grace Chao PH, Hung CT, Vunjak-Novakovic G. Spatial regulation of human mesenchymal stem cell differentiation in engineered osteochondral constructs: effects of pre-differentiation, soluble factors and medium perfusion. Osteoarthr Cartil. 2010;18:714–23.
Lam J, Lu S, Meretoja VV, Tabata Y, Mikos AG, Kasper FK. Generation of osteochondral tissue constructs with chondrogenically and osteogenically predifferentiated mesenchymal stem cells encapsulated in bilayered hydrogels. Acta Biomater. 2014;10:1112–23.
Rodrigues MT, Lee SJ, Gomes ME, Reis RL, Atala A, Yoo JJ. Bilayered constructs aimed at osteochondral strategies: the influence of medium supplements in the osteogenic and chondrogenic differentiation of amniotic fluid-derived stem cells. Acta Biomater. 2012;8:2795–806.
Rothrauff BB, Tuan RS. Cellular therapy in bone-tendon interface regeneration. Organ. 2014;10:13–28.
Cooper JO, Bumgardner JD, Cole JA, Smith RA, Haggard WO. Co-cultured tissue-specific scaffolds for tendon/bone interface engineering. J Tissue Eng. 2014;5:2041731414542294.
Spalazzi JP, Dagher E, Doty SB, Guo XE, Rodeo SA, Lu HH. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J Biomed Mater Res A. 2008;86:1–12.
Font Tellado S, Balmayor ER, Van Griensven M. Strategies to engineer tendon/ligament-to-bone interface: biomaterials, cells and growth factors. Adv Drug Deliv Rev. 2015;94:126–40.
Spalazzi JP, Doty SB, Moffat KL, Levine WN, Lu HH. Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng. 2006;12:3497–508.
Lin H, Lozito TP, Alexander PG, Gottardi R, Tuan RS. Stem cell-based microphysiological osteochondral system to model tissue response to interleukin-1β. Mol Pharm. 2014;11:2203–12.
Iannetti L, D’Urso G, Conoscenti G, Cutrì E, Tuan RS, Raimondi MT, et al. Distributed and lumped parameter models for the characterization of high throughput bioreactors. PLoS One. 2016;11:e0162774.
Colnot C. Cellular and molecular interactions regulating skeletogenesis. J Cell Biochem. 2005;95:688–97.
Colnot C, Lu C, Hu D, Helms JA. Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Dev Biol. 2004;269:55–69.
Bittner K, Vischer P, Bartholmes P, Bruckner P. Role of the subchondral vascular system in endochondral ossification: endothelial cells specifically derepress late differentiation in resting chondrocytes in vitro. Exp Cell Res. 1998;238:491–7.
Carlevaro MF, Cermelli S, Cancedda R, Descalzi CF. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J Cell Sci. 2000;113(Pt1):59–69.
Babarina AV, Möllers U, Bittner K, Vischer P, Bruckner P. Role of the subchondral vascular system in endochondral ossification: endothelial cell-derived proteinases derepress late cartilage differentiation in vitro. Matrix Biol. 2001;20:205–13.
Gottardi R, Pirosa A, Alexander PG, Manner PA, Puppi D, Chiellini F, et al. An in vitro chondro-osteo-vascular triphasic model of the osteochondral complex for studying osteochondral biology and for drug screening. Orlando: Orthopaedic Research Society Annual Meeting; 2016.
Puppi D, Mota C, Gazzarri M, Dinucci D, Gloria A, Myrzabekova M, et al. Additive manufacturing of wet-spun polymeric scaffolds for bone tissue engineering. Biomed Microdevices. 2012;14:1115–27.
Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–91.
Jones JR, Ehrenfried LM, Hench LL. Optimising bioactive glass scaffolds for bone tissue engineering. Biomaterials. 2006;27:964–73.
San Miguel B, Kriauciunas R, Tosatti S, Ehrbar M, Ghayor C, Textor M, et al. Enhanced osteoblastic activity and bone regeneration using surface-modified porous bioactive glass scaffolds. J Biomed Mater Res A. 2010;94:1023–33.
Lee S-H, Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev. 2007;59:339–59.
Morelli A, Puppi D, Chiellini F. Polymers from renewable resources. J Renew Mater. 2013;1:83–112.
Puppi D, Chiellini F, Piras AM, Chiellini E. Polymeric materials for bone and cartilage repair. Prog Polym Sci. 2010;35:403–40.
Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428:138–9.
Rao RR, Vigen ML, Peterson AW, Caldwell DJ, Putnam AJ, Stegemann JP. Dual-phase osteogenic and vasculogenic engineered tissue for bone formation. Tissue Eng Part A. 2015;21:530–40.
Ma J, Yang F, Both SK, Prins H-J, Helder MN, Pan J, et al. In vitro and in vivo angiogenic capacity of BM-MSCs/HUVECs and AT-MSCs/HUVECs cocultures. Biofabrication. 2014;6:15005.
Helary C, Bataille I, Abed A, Illoul C, Anglo A, Louedec L, et al. Concentrated collagen hydrogels as dermal substitutes. Biomaterials. 2010;31:481–90.
Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109–16.
Van Den Bulcke AI, Bogdanov B, De Rooze N, Schacht EH, Cornelissen M, Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1:31–8.
Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–44.
Pina S, Oliveira JM, Reis RL. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: a review. Adv Mater. 2015;27:1143–69.
Scaglione S, Ilengo C, Fato M, Quarto R. Hydroxyapatite-coated polycaprolacton wide mesh as a model of open structure for bone regeneration. Tissue Eng Part A. 2009;15:155–63.
Kohn JC, Zhou DW, Bordeleau F, Zhou AL, Mason BN, Mitchell MJ, et al. Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biophys J. 2015;108:471–8.
Mao AS, Shin J-W, Mooney DJ. Effects of substrate stiffness and cell-cell contact on mesenchymal stem cell differentiation. Biomaterials. 2016;98:184–91.
Lv H, Li L, Sun M, Zhang Y, Chen L, Rong Y, et al. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res Ther. 2015;6:103.
Visser J, Gawlitta D, Benders KEM, Toma SMH, Pouran B, van Weeren PR, et al. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials. 2015;37:174–82.
Sheehy EJ, Vinardell T, Toner ME, Buckley CT, Kelly DJ. Altering the architecture of tissue engineered hypertrophic cartilaginous grafts facilitates vascularisation and accelerates mineralisation. Leach K, editor. PLoS One. 2014;9:e90716.
Scotti C, Tonnarelli B, Papadimitropoulos A, Scherberich A, Schaeren S, Schauerte A, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A. 2010;107:7251–6.
Scotti C, Piccinini E, Takizawa H, Todorov A, Bourgine P, Papadimitropoulos A, et al. Engineering of a functional bone organ through endochondral ossification. Proc Natl Acad Sci U S A. 2013;110:3997–4002.
Daly AC, Cunniffe GM, Sathy BN, Jeon O, Alsberg E, Kelly DJ. 3D bioprinting of developmentally inspired templates for whole bone organ engineering. Adv Healthc Mater. 2016;5:2353–62.
Gao LP, Du MJ, Lv JJ, Schmull S, Huang RT, Li J. Use of human aortic extracellular matrix as a scaffold for construction of a patient-specific tissue engineered vascular patch. Biomedical Materials. 2017;12:065006.
Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8:153–70.
Mannella GA, Conoscenti G, Carfì Pavia F, La Carrubba V, Brucato V. Preparation of polymeric foams with a pore size gradient via Thermally Induced Phase Separation (TIPS). Mater Lett. 2015;160:31–3.
Jones AC, Arns CH, Sheppard AP, Hutmacher DW, Milthorpe BK, Knackstedt MA. Assessment of bone ingrowth into porous biomaterials using MICRO-CT. Biomaterials. 2007;28:2491–504.
Hutmacher DW, Sittinger M, Risbud MV. Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004;22:354–62.
Mota C, Puppi D, Chiellini F, Chiellini E. Additive manufacturing techniques for the production of tissue engineering constructs. J Tissue Eng Regen Med. 2015;9:174–90.
Fielding G, Bose S. SiO2 and ZnO dopants in three-dimensionally printed tricalcium phosphate bone tissue engineering scaffolds enhance osteogenesis and angiogenesis in vivo. Acta Biomater. 2013;9:9137–48.
Seyednejad H, Gawlitta D, Kuiper RV, de Bruin A, van Nostrum CF, Vermonden T, et al. In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly(ε-caprolactone). Biomaterials. 2012;33:4309–18.
Geraili A, Jafari P, Hassani MS, Araghi BH, Mohammadi MH, Ghafari AM, et al. Controlling differentiation of stem cells for developing personalized organ-on-chip platforms. Adv Healthc Mater. 2018;7:1700426.
Ahadian S, Civitarese R, Bannerman D, Mohammadi MH, Lu R, Wang E, et al. Organ-on-a-chip platforms: a convergence of advanced materials, cells, and microscale technologies. Adv Healthc Mater. 2018;7:1700506.
Nichols DA, Sondh IS, Litte SR, Zunino P, Gottardi R. Design and validation of an osteochondral bioreactor for the screening of treatments for osteoarthritis. Biomed Microdevices. 2018;20:18.
Jusoh N, Oh S, Kim S, Kim J, Jeon NL. Microfluidic vascularized bone tissue model with hydroxyapatite-incorporated extracellular matrix. Lab Chip. 2015;15:3984–8.
Giannitelli SM, Mozetic P, Trombetta M, Rainer A. Combined additive manufacturing approaches in tissue engineering. Acta Biomater. 2015;24:1–11.
Mandrycky C, Wang Z, Kim K, Kim D-H. 3D bioprinting for engineering complex tissues. Biotechnol Adv. 2016;34:422–34.
Skardal A. Bioprinting essentials of cell and protein viability. In: Atala A, Yoo JJ, editors. Essentials of 3D Biofabrication and Translation. Winston-Salem: Elsevier; 2015. p. 1–17.
Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30:5910–7.
van der Meer AD, Orlova VV, ten Dijke P, van den Berg A, Mummery CL. Three-dimensional co-cultures of human endothelial cells and embryonic stem cell-derived pericytes inside a microfluidic device. Lab Chip. 2013;13:3562–8.
Khan OF, Chamberlain MD, Sefton MV. Toward an in vitro vasculature: differentiation of mesenchymal stromal cells within an endothelial cell-seeded modular construct in a microfluidic flow chamber. Tissue Eng Part A. 2012;18:744–56.
Carrion B, Huang CP, Ghajar CM, Kachgal S, Kniazeva E, Jeon NL, et al. Recreating the perivascular niche ex vivo using a microfluidic approach. Biotechnol Bioeng. 2010;107:1020–8.
Jeon JS, Bersini S, Whisler JA, Chen MB, Dubini G, Charest JL, et al. Generation of 3D functional microvascular networks with human mesenchymal stem cells in microfluidic systems. Integr Biol. 2014;6:555–63.
Narayanan LK, Huebner P, Fisher MB, Spang JT, Starly B, Shirwaiker RA. 3D-bioprinting of polylactic acid (PLA) nanofiber–alginate hydrogel bioink containing human adipose-derived stem cells. ACS Biomater Sci Eng. 2016;2:1732–42.
Visser J, Peters B, Burger TJ, Boomstra J, Dhert WJA, Melchels FPW, et al. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication. 2013;5:35007.
Schuurman W, Khristov V, Pot MW, van Weeren PR, Dhert WJA, Malda J. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication. 2011;3:21001.
Gao G, Yonezawa T, Hubbell K, Dai G, Cui X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol J. 2015;10:1568–77.
Zhou X, Castro NJ, Zhu W, Cui H, Aliabouzar M, Sarkar K, et al. Improved human bone marrow mesenchymal stem cell osteogenesis in 3D bioprinted tissue scaffolds with low intensity pulsed ultrasound stimulation. Sci Rep. 2016;6:32876.
Jia W, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu W, et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. 2016;106:58–68.
Huang J, Fu H, Wang Z, Meng Q, Liu S, Wang H, et al. BMSCs-laden gelatin/sodium alginate/carboxymethyl chitosan hydrogel for 3D bioprinting. RSC Adv. 2016;6:108423–30.
Daly AC, Critchley SE, Rencsok EM, Kelly DJ. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication. 2016;8:45002.
Cui H, Zhu W, Nowicki M, Zhou X, Khademhosseini A, Zhang LG. Hierarchical fabrication of engineered vascularized bone biphasic constructs via dual 3D bioprinting: integrating regional bioactive factors into architectural design. Adv Healthc Mater. 2016;5:2174–81.
Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34:312–9.
Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A. 2016;113:3179–84.
Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5:32–45.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7.
Frank O, Heim M, Jakob M, Barbero A, Schäfer D, Bendik I, et al. Real-time quantitative RT-PCR analysis of human bone marrow stromal cells during osteogenic differentiation in vitro. J Cell Biochem. 2002;85:737–46.
Qi H, Aguiar DJ, Williams SM, La Pean A, Pan W, Verfaillie CM. Identification of genes responsible for osteoblast differentiation from human mesodermal progenitor cells. Proc Natl Acad Sci U S A. 2003;100:3305–10.
Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113:1161–6.
Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–94.
Minteer DM, Marra KG, Rubin JP. Adipose stem cells: biology, safety, regulation, and regenerative potential. Clin Plast Surg. 2015;42:169–79.
de Peppo GM, Marcos-Campos I, Kahler DJ, Alsalman D, Shang L, Vunjak-Novakovic G, et al. Engineering bone tissue substitutes from human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2013;110:8680–5.
de Peppo GM, Vunjak-Novakovic G, Marolt D. Cultivation of human bone-like tissue from pluripotent stem cell-derived osteogenic progenitors in perfusion bioreactors. Methods Mol Biol. 2014;1202:173–84.
Marolt D, Campos IM, Bhumiratana S, Koren A, Petridis P, Zhang G, et al. Engineering bone tissue from human embryonic stem cells. Proc Natl Acad Sci U S A. 2012;109:8705–9.
Sundaram S, One J, Siewert J, Teodosescu S, Zhao L, Dimitrievska S, et al. Tissue-engineered vascular grafts created from human induced pluripotent stem cells. Stem Cells Transl Med. 2014;3:1535–43.
Barberi T, Willis LM, Socci ND, Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:e161.
Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30:204–13.
Grayson WL, Fröhlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, et al. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A. 2010;107:3299–304.
Kusuma S, Gerecht S. Recent progress in the use of induced pluripotent stem cells in vascular regeneration. Expert Rev Cardiovasc Ther. 2013;11:661–3.
Kaupisch A, Kennedy L, Stelmanis V, Tye B, Kane NM, Mountford JC, et al. Derivation of vascular endothelial cells from human embryonic stem cells under GMP-compliant conditions: towards clinical studies in ischaemic disease. J Cardiovasc Transl Res. 2012;5:605–17.
Kusuma S, Facklam A, Gerecht S. Characterizing human pluripotent-stem-cell-derived vascular cells for tissue engineering applications. Stem Cells Dev. 2015;24:451–8.
Huang NF, Li S. Mesenchymal stem cells for vascular regeneration. Regen Med. 2008;3:877–92.
Lowenthal J, Gerecht S. Stem cell-derived vasculature: a potent and multidimensional technology for basic research, disease modeling, and tissue engineering. Biochem Biophys Res Commun. 2016;473:733–42.
Benavides OM, Petsche JJ, Moise KJ, Johnson A, Jacot JG. Evaluation of endothelial cells differentiated from amniotic fluid-derived stem cells. Tissue Eng Part A. 2012;18:1123–31.
Tancharoen W, Aungsuchawan S, Pothacharoen P, Markmee R, Narakornsak S, Kieodee J, et al. Differentiation of mesenchymal stem cells from human amniotic fluid to vascular endothelial cells. Acta Histochem. 2017;119:113–21.
Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–94.
Bidarra SJ, Barrias CC, Barbosa MA, Soares R, Amédée J, Granja PL. Phenotypic and proliferative modulation of human mesenchymal stem cells via crosstalk with endothelial cells. Stem Cell Res. 2011;7:186–97.
Saleh FA, Whyte M, Genever PG. Effects of endothelial cells on human mesenchymal stem cell activity in a three-dimensional in vitro model. Eur Cell Mater. 2011;22:242–57. discussion 257
Pedersen TO, Blois AL, Xing Z, Xue Y, Sun Y, Finne-Wistrand A, et al. Endothelial microvascular networks affect gene-expression profiles and osteogenic potential of tissue-engineered constructs. Stem Cell Res Ther. 2013;4:52.
Murohara T. Cord blood-derived early outgrowth endothelial progenitor cells. Microvasc Res. 2010;79:174–7.
Zigdon-Giladi H, Bick T, Lewinson D, Machtei EE. Co-transplantation of endothelial progenitor cells and mesenchymal stem cells promote neovascularization and bone regeneration. Clin Implant Dent Relat Res. 2015;17:353–9.
Zigdon-Giladi H, Bick T, Lewinson D, Machtei EE. Mesenchymal stem cells and endothelial progenitor cells stimulate bone regeneration and mineral density. J Periodontol. 2014;85:984–90.
Zigdon-Giladi H, Bick T, Morgan EF, Lewinson D, Machtei EE. Peripheral blood-derived endothelial progenitor cells enhance vertical bone formation. Clin Implant Dent Relat Res. 2015;17:83–92.
Zigdon-Giladi H, Michaeli-Geller G, Bick T, Lewinson D, Machtei EE. Human blood-derived endothelial progenitor cells augment vasculogenesis and osteogenesis. J Clin Periodontol. 2015;42:89–95.
Fedorovich NE, Haverslag RT, Dhert WJA, Alblas J. The role of endothelial progenitor cells in prevascularized bone tissue engineering: development of heterogeneous constructs. Tissue Eng Part A. 2010;16:2355–67.
Li Q, Wang Z. Influence of mesenchymal stem cells with endothelial progenitor cells in co-culture on osteogenesis and angiogenesis: an in vitro study. Arch Med Res. 2013;44:504–13.
Amini AR, Laurencin CT, Nukavarapu SP. Differential analysis of peripheral blood- and bone marrow-derived endothelial progenitor cells for enhanced vascularization in bone tissue engineering. J Orthop Res. 2012;30:1507–15.
Belair DG, Whisler JA, Valdez J, Velazquez J, Molenda JA, Vickerman V, et al. Human vascular tissue models formed from human induced pluripotent stem cell derived endothelial cells. Stem Cell Rev. 2015;11:511–25.
Severson AR. Differentiation of mononuclear cells into multinucleated osteoclast-like cells. Exp Cell Biol. 1983;51:267–74.
Fong D, Bisson M, Laberge G, McManus S, Grenier G, Faucheux N, et al. Bone morphogenetic protein-9 activates Smad and ERK pathways and supports human osteoclast function and survival in vitro. Cell Signal. 2013;25:717–28.
Monchau F, Lefèvre A, Descamps M, Belquin-myrdycz A, Laffargue P, Hildebrand HF. In vitro studies of human and rat osteoclast activity on hydroxyapatite, beta-tricalcium phosphate, calcium carbonate. Biomol Eng. 2002;19:143–52.
Wei S, Teitelbaum SL, Wang MW, Ross FP. Receptor activator of nuclear factor-kappa b ligand activates nuclear factor-kappa b in osteoclast precursors. Endocrinology. 2001;142:1290–5.
Cody JJ, Rivera AA, Liu J, Liu JM, Douglas JT, Feng X. A simplified method for the generation of human osteoclasts in vitro. Int J Biochem Mol Biol. 2011;2:183–9.
Goodwin AM. In vitro assays of angiogenesis for assessment of angiogenic and anti-angiogenic agents. Microvasc Res. 2007;74:172–83.
Kolbe M, Xiang Z, Dohle E, Tonak M, Kirkpatrick CJ, Fuchs S. Paracrine effects influenced by cell culture medium and consequences on microvessel-like structures in cocultures of mesenchymal stem cells and outgrowth endothelial cells. Tissue Eng Part A. 2011;17:2199–212.
Geuze RE, Wegman F, Oner FC, Dhert WJA, Alblas J. Influence of endothelial progenitor cells and platelet gel on tissue-engineered bone ectopically in goats. Tissue Eng Part A. 2009;15:3669–77.
Rouwkema J, de Boer J, Van Blitterswijk CA. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 2006;12:2685–93.
Kaigler D, Krebsbach PH, West ER, Horger K, Huang Y-C, Mooney DJ. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005;19:665–7.
Yu H, VandeVord PJ, Mao L, Matthew HW, Wooley PH, Yang S-Y. Improved tissue-engineered bone regeneration by endothelial cell mediated vascularization. Biomaterials. 2009;30:508–17.
Rouwkema J, Westerweel PE, de Boer J, Verhaar MC, van Blitterswijk CA. The use of endothelial progenitor cells for prevascularized bone tissue engineering. Tissue Eng Part A. 2009;15:2015–27.
Ma J, van den Beucken JJJP, Yang F, Both SK, Cui F-Z, Pan J, et al. Coculture of osteoblasts and endothelial cells: optimization of culture medium and cell ratio. Tissue Eng Part C Methods. 2011;17:349–57.
Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22:80–6.
Gaspar DA, Gomide V, Monteiro FJ. The role of perfusion bioreactors in bone tissue engineering. Biomatter. 2012;2:167–75.
Vunjak-Novakovic G, Meinel L, Altman G, Kaplan D. Bioreactor cultivation of osteochondral grafts. Orthod Craniofac Res. 2005;8:209–18.
Yoshioka T, Mishima H, Ohyabu Y, Sakai S, Akaogi H, Ishii T, et al. Repair of large osteochondral defects with allogeneic cartilaginous aggregates formed from bone marrow-derived cells using RWV bioreactor. J Orthop Res. 2007;25:1291–8.
Grayson WL, Bhumiratana S, Cannizzaro C, Chao P-HG, Lennon DP, Caplan AI, et al. Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue Eng Part A. 2008;14:1809–20.
Goldstein AS, Juarez TM, Helmke CD, Gustin MC, Mikos AG. Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds. Biomaterials. 2001;22:1279–88.
Bjerre L, Bünger CE, Kassem M, Mygind T. Flow perfusion culture of human mesenchymal stem cells on silicate-substituted tricalcium phosphate scaffolds. Biomaterials. 2008;29:2616–27.
Janssen FW, van Dijkhuizen-Radersma R, Van Oorschot A, Oostra J, de Bruijn JD, Van Blitterswijk CA. Human tissue-engineered bone produced in clinically relevant amounts using a semi-automated perfusion bioreactor system: a preliminary study. J Tissue Eng Regen Med. 2010;4:12–24.
Janssen FW, Oostra J, van Oorschot A, van Blitterswijk CA. A perfusion bioreactor system capable of producing clinically relevant volumes of tissue-engineered bone: in vivo bone formation showing proof of concept. Biomaterials. 2006;27:315–23.
Gardel LS, Serra LA, Reis RL, Gomes ME. Use of perfusion bioreactors and large animal models for long bone tissue engineering. Tissue Eng Part B Rev. 2014;20:126–46.
Stevens MM, Marini RP, Schaefer D, Aronson J, Langer R, Shastri VP. In vivo engineering of organs: the bone bioreactor. Proc Natl Acad Sci U S A. 2005;102:11450–5.
Han D, Dai K. Prefabrication of a vascularized bone graft with Beta tricalcium phosphate using an in vivo bioreactor. Artif Organs. 2013;37:884–93.
Liu Y, Möller B, Wiltfang J, Warnke PH, Terheyden H. Tissue engineering of a vascularized bone graft of critical size with an osteogenic and angiogenic factor-based in vivo bioreactor. Tissue Eng Part A. 2014;20:3189–97.
Qian X, Yuan F, Zhimin Z, Anchun M. Dynamic perfusion bioreactor system for 3D culture of rat bone marrow mesenchymal stem cells on nanohydroxyapatite/polyamide 66 scaffold in vitro. J Biomed Mater Res B Appl Biomater. 2013;101:893–901.
Stiehler M, Bünger C, Baatrup A, Lind M, Kassem M, Mygind T. Effect of dynamic 3-D culture on proliferation, distribution, and osteogenic differentiation of human mesenchymal stem cells. J Biomed Mater Res A. 2009;89:96–107.
Cartmell SH, Porter BD, García AJ, Guldberg RE. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 2003;9:1197–203.
Liu Y, Teoh S-H, Chong MSK, Yeow C-H, Kamm RD, Choolani M, et al. Contrasting effects of vasculogenic induction upon biaxial bioreactor stimulation of mesenchymal stem cells and endothelial progenitor cells cocultures in three-dimensional scaffolds under in vitro and in vivo paradigms for vascularized bone tissue engine. Tissue Eng Part A. 2013;19:893–904.
Dahlin RL, Gershovich JG, Kasper FK, Mikos AG. Flow perfusion co-culture of human mesenchymal stem cells and endothelial cells on biodegradable polymer scaffolds. Ann Biomed Eng. 2014;42:1381–90.
Viggers RF, Wechezak AR, Sauvage LR. An apparatus to study the response of cultured endothelium to shear stress. J Biomech Eng. 1986;108:332–7.
Davies PF, Remuzzi A, Gordon EJ, Dewey CF, Gimbrone MA. Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci U S A. 1986;83:2114–7.
Yin W, Shanmugavelayudam SK, Rubenstein DA. The effect of physiologically relevant dynamic shear stress on platelet and endothelial cell activation. Thromb Res. 2011;127:235–41.
Nishi M, Matsumoto R, Dong J, Uemura T. Engineered bone tissue associated with vascularization utilizing a rotating wall vessel bioreactor. J Biomed Mater Res A. 2013;101:421–7.
Kluge JA, Leisk GG, Cardwell RD, Fernandes AP, House M, Ward A, et al. Bioreactor system using noninvasive imaging and mechanical stretch for biomaterial screening. Ann Biomed Eng. 2011;39:1390–402.
Salter E, Goh B, Hung B, Hutton D, Ghone N, Grayson WL. Bone tissue engineering bioreactors: a role in the clinic? Tissue Eng Part B Rev. 2012;18:62–75.
Bersini S, Jeon JS, Dubini G, Arrigoni C, Chung S, Charest JL, et al. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials. 2014;35:2454–61.
Shin Y, Han S, Jeon JS, Yamamoto K, Zervantonakis IK, Sudo R, et al. Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nat Protoc. 2012;7:1247–59.
Moya ML, Hsu Y-H, Lee AP, Hughes CCW, George SC. In vitro perfused human capillary networks. Tissue Eng Part C Methods. 2013;19:730–7.
Griffith CK, Miller C, Sainson RCA, Calvert JW, Jeon NL, Hughes CCW, et al. Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng. 2005;11:257–66.
Ghajar CM, Chen X, Harris JW, Suresh V, Hughes CCW, Jeon NL, et al. The effect of matrix density on the regulation of 3-D capillary morphogenesis. Biophys J. 2008;94:1930–41.
Peyrin F. Evaluation of bone scaffolds by micro-CT. Osteoporos Int. 2011;22:2043–8.
Altman AR, Tseng W-J, de Bakker CMJ, Chandra A, Lan S, Huh BK, et al. Quantification of skeletal growth, modeling, and remodeling by in vivo micro computed tomography. Bone. 2015;81:370–9.
Porter B, Lin A, Peister A, Hutmacher D, Guldberg R. Noninvasive image analysis of 3D construct mineralization in a perfusion bioreactor. Biomaterials. 2007;28:2525–33.
Hagenmüller H, Hitz M, Merkle HP, Meinel L, Müller R. Design and validation of a novel bioreactor principle to combine online micro-computed tomography monitoring and mechanical loading in bone tissue engineering. Rev Sci Instrum. 2010;81:14303.
Prajapati SI, Keller C. Contrast enhanced vessel imaging using microCT. J Vis Exp. 2011;47:e2377. https://doi.org/10.3791/2377.
Puls R, Hosten N, Lemke M, Teichgraber UK, Steinkamp HK, Felix R. Perfusion abnormalities of kidney parenchyma: microvascular imaging with contrast-enhanced color and power Doppler ultrasonography—preliminary results. J Ultrasound Med. 2000;19:817–21.
Shufelt CL, Thomson LEJ, Goykhman P, Agarwal M, Mehta PK, Sedlak T, et al. Cardiac magnetic resonance imaging myocardial perfusion reserve index assessment in women with microvascular coronary dysfunction and reference controls. Cardiovasc Diagn Ther. 2013;3:153–60.
Sboros V, Tang M-X. The assessment of microvascular flow and tissue perfusion using ultrasound imaging. Proc Inst Mech Eng H. 2010;224:273–90.
Arkudas A, Beier JP, Pryymachuk G, Hoereth T, Bleiziffer O, Polykandriotis E, et al. Automatic quantitative micro-computed tomography evaluation of angiogenesis in an axially vascularized tissue-engineered bone construct. Tissue Eng Part C Methods. 2010;16:1503–14.
Young S, Kretlow JD, Nguyen C, Bashoura AG, Baggett LS, Jansen JA, et al. Microcomputed tomography characterization of neovascularization in bone tissue engineering applications. Tissue Eng Part B Rev. 2008;14:295–306.
Reinwald S, Burr D. Review of nonprimate, large animal models for osteoporosis research. J Bone Miner Res. 2008;23:1353–68.
Kuyinu EL, Narayanan G, Nair LS, Laurencin CT. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res. 2016;11:19.
Gottardi R, Lin H, D’urso G, Iannetti L, Zunino P, Lozito TP, et al. Validation of an osteochondral microphysiological system applied to study the protective role of sex hormones. Orlando: Orthopaedic Research Society Annual Meeting; 2016.
Costa PF, Martins A, Neves NM, Gomes ME, Reis RL. Automating the processing steps for obtaining bone tissue-engineered substitutes: from imaging tools to bioreactors. Tissue Eng Part B Rev. 2014;20:567–77.
Sandell LJ. Etiology of osteoarthritis: genetics and synovial joint development. Nat Rev Rheumatol. 2012;8:77–89.
Pelletier J-P, Martel-Pelletier J, Raynauld J-P. Most recent developments in strategies to reduce the progression of structural changes in osteoarthritis: today and tomorrow. Arthritis Res Ther. 2006;8:206.
Karim AR, Cherian JJ, Jauregui JJ, Pierce T, Mont MA. Osteonecrosis of the knee: review. Ann Transl Med. 2015;3:6.
Shah KN, Racine J, Jones LC, Aaron RK. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med. 2015;8:201–9.
Goulding A. Risk factors for fractures in normally active children and adolescents. Med Sport Sci. 2007;51:102–20.
Handforth C, D’Oronzo S, Coleman R, Brown J. Cancer treatment and bone health. Calcif Tissue Int. 2018;102:251–64.
Jackuliak P, Payer J. Osteoporosis, fractures, and diabetes. Int J Endocrinol. 2014;2014:820615.
Sakamoto A, Iwamoto Y. Current status and perspectives regarding the treatment of osteo-sarcoma: chemotherapy. Rev Recent Clin Trials. 2008;3:228–31.
Chen K, Lin X, Zhang Q, Ni J, Li J, Xiao J, et al. Decellularized periosteum as a potential biologic scaffold for bone tissue engineering. Acta Biomater. 2015;19:46–55.
Marcos-Campos I, Marolt D, Petridis P, Bhumiratana S, Schmidt D, Vunjak-Novakovic G. Bone scaffold architecture modulates the development of mineralized bone matrix by human embryonic stem cells. Biomaterials. 2012;33:8329–42.
Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43.
Lu T, Li Y, Chen T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int J Nanomed. 2013;8:337–50.
Hutmacher DW. Scaffold design and fabrication technologies for engineering tissues—state of the art and future perspectives. J Biomater Sci Polym Ed. 2001;12:107–24.
Badylak SF, Weiss DJ, Caplan A, Macchiarini P. Engineered whole organs and complex tissues. Lancet. 2012;379:943–52.
Hoshiba T, Lu H, Kawazoe N, Chen G. Decellularized matrices for tissue engineering. Expert Opin Biol Ther. 2010;10:1717–28.
Cheng CW, Solorio LD, Alsberg E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol Adv. 2014;32:462–84.
Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today. 2013;16:496–504.
Lin L, Tong A, Zhang H, Hu Q, Fang M, Li K, Li X, Irwin GW, He G, editors. Life System Modeling and Simulation: International Conference, LSMS 2007; Shanghai, China, September 14–17, 2007; Proceedings, vol. 2007. Berlin, Heidelberg: Springer-Verlag. p. 146–52.
Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74:782–8.
Sabree I, Gough JE, Derby B. Mechanical properties of porous ceramic scaffolds: influence of internal dimensions. Ceram Int. 2015;41:8425–32.
Roohani-Esfahani SI, Dunstan CR, Li JJ, Lu Z, Davies B, Pearce S, et al. Unique microstructural design of ceramic scaffolds for bone regeneration under load. Acta Biomater. 2013;9:7014–24.
Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–43.
Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–85.
Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM, et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35:4026–34.
Choong CSN, Hutmacher DW, Triffitt JT. Co-culture of bone marrow fibroblasts and endothelial cells on modified polycaprolactone substrates for enhanced potentials in bone tissue engineering. Tissue Eng. 2006;12:2521–31.
Hofmann A, Ritz U, Verrier S, Eglin D, Alini M, Fuchs S, et al. The effect of human osteoblasts on proliferation and neo-vessel formation of human umbilical vein endothelial cells in a long-term 3D co-culture on polyurethane scaffolds. Biomaterials. 2008;29:4217–26.
Fuchs S, Hofmann A, Kirkpatrick CJ. Microvessel-like structures from outgrowth endothelial cells from human peripheral blood in 2-dimensional and 3-dimensional co-cultures with osteoblastic lineage cells. Tissue Eng. 2007;13:2577–88.
Villars F, Guillotin B, Amédée T, Dutoya S, Bordenave L, Bareille R, et al. Effect of HUVEC on human osteoprogenitor cell differentiation needs heterotypic gap junction communication. Am J Physiol Physiol. 2002;282:C775–85.
Xue Y, Xing Z, Hellem S, Arvidson K, Mustafa K. Endothelial cells influence the osteogenic potential of bone marrow stromal cells. Biomed Eng Online. 2009;8:34.
Yu H, VandeVord PJ, Gong W, Wu B, Song Z, Matthew HW, et al. Promotion of osteogenesis in tissue-engineered bone by pre-seeding endothelial progenitor cells-derived endothelial cells. J Orthop Res. 2008;26:1147–52.
Acknowledgements
The authors acknowledge funding support from the sources indicated.
Funding
This work was supported by the Commonwealth of Pennsylvania, Department of Health, the Environmental Protection Agency (R835736), the US Department of Defense (W81XWH-14-1-0217 and W81XWH-14-2-0003), CASIS (GA-2016-236), the Ri.MED Foundation, and the National Institutes of Health (1UG3 TR002136).
Availability of data and materials
Not applicable to this article, as no data were generated or analyzed.
Author information
Authors and Affiliations
Contributions
All authors participated in the writing and review of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Pirosa, A., Gottardi, R., Alexander, P.G. et al. Engineering in-vitro stem cell-based vascularized bone models for drug screening and predictive toxicology. Stem Cell Res Ther 9, 112 (2018). https://doi.org/10.1186/s13287-018-0847-8
Published:
DOI: https://doi.org/10.1186/s13287-018-0847-8