Abstract
Background
The purpose of this study was to estimate the effects of a 3-year dietary intervention on age-related changes in triglyceride and apolipoprotein (apo A-V) levels in patients with impaired fasting glucose (IFG) or new-onset type 2 diabetes as a function of the APOA5 -1131 T > C polymorphism.
Methods
We genotyped the APOA5 -1131 T > C polymorphism in 203 Korean individuals with IFG or new-onset type 2 diabetes for the TT (n = 91), TC (n = 98), and CC (n = 14) alleles. Plasma apo A-V and triglyceride levels were evaluated at baseline and after a 3-year dietary intervention.
Results
Our results showed that HDL, glucose, insulin, HOMA-IR index, free fatty acids, and apo A-V decreased and brachial-ankle pulse wave velocity (ba-PWV) and malondialdehyde (MDA) increased at the 3-year follow-up visit compared with baseline. Plasma apo A-V levels were reduced in subjects with the C allele (TC or CC) (P = 0.036) and triglyceride levels were reduced in subjects with the TT allele (P = 0.047). Subjects with the C allele showed lower post-treatment apo A-V and higher post-treatment fasting triglyceride levels than subjects with the TT allele. Changes in apo A-V and triglyceride levels were negatively correlated in subjects with the TT allele and positively correlated in subjects with the C allele.
Conclusions
This study showed that the dietary intervention prevented an age-related increase in triglyceride levels in individuals with IFG or new-onset type 2 diabetes who possess the TT allele, but not the CT or CC allele, of the APOA5 -1131 T > C polymorphism.
Similar content being viewed by others
Background
Apolipoprotein A-V (apo A-V), which was discovered in 2001, is part of the apolipoprotein family (APOA1/C3/A4). Apo A-V is encoded by the APOA5 gene, which is located on human chromosome 11q23 and contains three introns and four exons [1–3]. Although apo A-V is mainly expressed in the liver in combination with the APO structure [2], apo A-V also exists in small amounts in the plasma and plays an important role in the regulation of triglyceride levels in both human and animal models. Pennacchio et al. found that triglyceride levels decreased approximately 66% in transgenic mice that overexpressed APOA5 and were four times higher in APOA5 knockout mice compared with control mice [1, 3, 4].
Elevated triglyceride levels are associated with abnormal lipid metabolism in patients with type 2 diabetes [1, 5]. However, the mechanism underlying the modulation of triglyceride levels by plasma apo A-V as well as the effect of APOA5 polymorphisms in humans has not been identified [4, 6]. Many studies have shown that genetic variations in the APOA5 gene are associated with hypertriglyceridemia and coronary heart disease [1, 2, 7]. However, few studies have explored genetic variations in the APOA5 gene in individuals with type 2 diabetes. Therefore, we tested the effects of a 3-year dietary intervention on triglyceride and apo A-V levels in patients with impaired fasting glucose (IFG) or new-onset type 2 diabetes as a function of their APOA5 polymorphisms.
The -1131 T > C single-nucleotide polymorphism (SNP) of the APOA5 gene has a significant impact on diseases [5–7]. A previous study has shown that among healthy, non-obese Korean men who were genotyped with the TT, TC, or CC allele of the APOA5 -1131 T > C polymorphism, triglyceride levels were higher in carriers of the C allele (TC or CC) compared with carriers of the TT allele. The levels of small dense low-density lipoprotein (LDL) cholesterol, high-sensitivity C-reactive protein, and lymphocyte DNA damage were also higher in carriers of the C allele compared with carriers of the TT allele. Therefore, the C allele of the APOA5 -1131 T > C polymorphism is significantly associated with risk factors for cardiovascular disease (CVD) [6]. Furthermore, Xu et al. performed a meta-analysis of the APOA5 -1131 T > C polymorphism among East Asian, European, and other ethnic populations and found that the C allele was associated with higher levels of fasting triglycerides, total cholesterol, and LDL cholesterol, and lower levels of high-density lipoprotein (HDL) cholesterol, especially in East Asian populations, in which metabolic syndrome was also increased by approximately 40% [8]. The C allele is more prevalent in Chinese (26-40%) than in Caucasian (8%) individuals [7]. Chandak et al. also found a higher prevalence of the C allele among Indians compared with Caucasians from the United Kingdom (20% vs. 4%, respectively; P < 0.001) [9]. Therefore, the C allele and disease-linked variants are common among the Asian population.
Carbohydrate-derived calories account for 65% of total caloric intake and refined rice is the primary source of carbohydrates among middle-aged adults, according the 2010 Korean National Health and Nutrition Examination Survey (KNHANES V-1). A high carbohydrate intake combined with a high prevalence of the APOA5 -1131C allele may contribute to the relatively high mean serum triglyceride concentration (142 mg/dL) in the Korean population (KNHANES V-1). Previous observational studies and intervention trials have shown that the APOA5 -1131 T > C polymorphism interacts with dietary factors, especially dietary fat, to determine triglyceride concentrations [10–14]. Lin et al. recently showed that elevated triglyceride levels were induced by a high carbohydrate diet in healthy individuals with the APOA5 -1131C allele [15]. A clear association exists between diabetic dyslipidemia and the APOA5 -1131 T > C polymorphism [16]. Furthermore, there is evidence that a high-legume, low glycemic index diet reduces fasting triglyceride levels in insulin-resistant subjects [17]. Therefore, we hypothesized that a 3-year dietary intervention with whole grains and legumes in a high-carbohydrate diet may mask the adverse effects of a high-carbohydrate intake among individuals with the C allele of the APOA5 -1131 T > C polymorphism. Therefore, we studied the effects of a 3-year high-carbohydrate diet with whole grains and legumes on circulating levels of apo A-V and triglycerides in patients with IFG or new-onset type 2 diabetes as a function of the APOA5 -1131 T > C polymorphism.
Methods
Study design and participants
Study subjects were recruited from the Health Service Center (HSC) during routine check-ups at the National Health Insurance Corporation Ilsan Hospital in Goyang, Korea between August 2006 and February 2008. Subjects were sedentary and had not participated in weight-reduction programs within the previous 3 years. Based on the data screened from the HSC, subjects with IFG (fasting glucose between 100 and 126 mg/dL) or new-onset type 2 diabetes (fasting glucose ≥126 mg/dL) were referred to the Department of Family Medicine or Internal Medicine. Their health and lipid profiles were re-screened, and subjects who satisfied the study criteria were recommended to participate in the dietary intervention program. Exclusion criteria included a current and/or past history of CVD, including angina, cancer, abnormal liver or renal function, thyroid or pituitary disease, and use of any medications (including anti-hypertensive, lipid lowering, anti-platelets, and anti-diabetic drugs). The purpose of the study was carefully explained to all potential subjects and their written consent was obtained prior to their participation. During the recruiting period, total 244 subjects were enrolled (TT: 109, TC: 118, CC: 17). Approximately 83% of subjects achieved, and finally, a total of 203 subjects completed the study. The study protocol was approved by the Institutional Review Board of Yonsei University and performed in accordance with the Declaration of Helsinki.
Dietary intervention and assessment of dietary intake/physical activity level
Enrolled subjects visited the center one week before starting the intervention. Each subject’s habitual diet was obtained using both a 24-h recall method and a previously validated semi-quantitative food frequency questionnaire (SQFFQ) [18]. Analyses were conducted using the data from the 24-hour recall methods, and the SQFFQ was used to validate this data. All of the subjects were advised to continue their habitual diet for a week and were instructed to complete a 3-day food record (2 weekdays and 1 weekend day) as a baseline measurement. On the 3-day food record, subjects were instructed to weigh and record the amount of food before ingestion and any remaining food after ingestion. This 3-day record was compared with the 24-hour recall method to verify the habitual diet.
The dietary intervention program started one week after baseline measurements. The intervention program replaced each subject’s refined rice intake with equal parts of legumes, barley, and other whole grains three times per day as high-carbohydrate sources and increased their vegetable intake to at least 6 units (30–70 g/unit) per day to ensure a sufficient dietary fiber intake. To evaluate and reinforce compliance during the intervention period, dietitians interviewed the subjects bimonthly via telephone. Subjects were asked to report their 3-day food records at each visit. Subjects were also instructed to record their 24-hour physical activity in a diary every 4 weeks. Dietary energy values and nutrient content from the 3-day food records were calculated using the Computer-Aided Nutritional analysis program (CAN-pro 3.0, Korean Nutrition Society, Seoul, Korea). Total energy expenditure (kcal/day) was calculated from the 24-hour physical activity patterns [19], basal metabolic rate, and the specific dynamic actions of the food intake. Basal metabolic rate was calculated for each subject with the Harris–Benedict equation [20].
Anthropometric parameters and blood collection
Body weight and height were measured to calculate body mass index (BMI, kg/m2). Waist circumference was measured at the umbilical level with the subject in the standing position after normal expiration and hip girth was measured at the widest part of the hip to calculate the waist to hip ratio (WHR). Blood pressure (BP) was measured in the left arm of seated patients with an automatic BP monitor (TM-2654, A&D, Tokyo, Japan) after 20 minutes of rest. Venous blood specimens were collected after a 12-hour fasting period in EDTA-treated and plain tubes that were centrifuged to produce either plasma or serum and stored at −70°C.
Serum lipid profile, free fatty acids, fasting glucose, insulin concentration, and insulin resistance
Fasting serum concentrations of total cholesterol, triglycerides, and free fatty acids were measured using the Hitachi 7150 Autoanalyzer (Hitachi Ltd., Tokyo, Japan). Blood was precipitated using apoB-containing lipoproteins with dextran sulfate magnesium. Inter and intra-assay precision of total cholesterol was 1.73% and 1.52%, triglyceride was 2.52% and 1.28%, and free fatty acid was 3.73% and 0.99%, respectively. HDL concentrations in the supernatants were measured enzymatically. Inter and intra-assay precision was 1.61% and 1.37%. LDL was indirectly estimated in subjects with serum triglyceride concentrations less than 400 mg/dL using the Friedewald formula: LDL = total cholesterol - [HDL + (triglycerides/5)]. In subjects with serum triacylglycerol concentrations more than 400 mg/mL, LDL was measured directly using an enzymatic method on a Hitachi 7150 Autoanalyzer (Hitachi Ltd, Tokyo, Japan). Fasting glucose concentrations were measured with a glucose oxidase method from the Beckman Glucose Analyzer (Beckman Instruments, Irvine, CA). Inter and intra-assay precision was 1.14% and 0.94%. Insulin concentrations were measured by radioimmunoassay from the Immuno Nucleo Corporation (Stillwater, MN). Inter and intra-assay precision was 2.1% and 6.5%. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR = [fasting insulin (μIU/mL) × fasting glucose (mmol/L)] × 22.5.
Serum high-sensitivity C-reactive protein, plasma malondialdehyde, LDL particle size, and brachial-ankle pulse wave velocity
Serum high-sensitivity C-reactive protein (hs-CRP) concentrations were measured by the Express Plus™ auto-analyzer (Chiron Diagnostics Co., Walpole, MA, USA) and high-sensitivity CRP-Latex(II) X2 kit (Seiken Laboratories Ltd., Tokyo, Japan). Inter and intra-assay precision was 2.08% and 2.46%. Malondialdehyde (MDA) was measured by the TBARS Assay kit with thiobarbituric acid-reactive substances (Zepto-Metrix Co., Buffalo, NY, USA). Inter and intra-assay precision was 1.9% and 1.0%. Particle size distribution of LDL isolated by sequential flotation ultracentrifugation was examined by a pore gradient lipoprotein system (CBS Scientific, CA, USA) on commercially available, non-denaturing polyacrylamide slab gels containing a linear gradient of 2-16% acrylamide (Alamo Gels Inc., San Antonio, TX, USA). Standards of latex beads (34 nm) thyroglobulin (17 nm), apoferritin (12.2 nm), and catalase (10.4 nm) were used to estimate the relative migration rates of each band. Gels were scanned using a GS-800 Calibrated Imaging Densitometer (Bio-Rad, Graz, Austria). Inter and intra-assay precision was 2.29% and 3.88%. Brachial-ankle pulse wave velocity (ba-PWV) was measured by the automatic waveform analyzer (model VP-1000; Nippon Colin Ltd., Komaki, Japan). Subjects were examined in the supine position after 5 minutes of rest.
Plasma apolipoprotein A-V and APOA5 -1131 T > C genotyping
Plasma apo A-V was measured by enzyme immunoassay (Human Apolipoprotein A ELISA kit, Millipore, MO). The results were read at 450 nm by the Victor2 (Perkin Elmer Life Sciences, Turka, Finland). Inter and intra-assay precision was 2.46% and 7.45%. Genomic DNA was extracted from 5 mL of whole blood using a commercially available DNA isolation kit (WIZARD® Genomic DNA purification kit, Promega Corp., Madison, WI, USA) according to the manufacturer’s instructions. Genotyping was performed by SNP-ITTM assays using single primer extension technology (SNPstream 25KTM System, Orchid BioSciences, NJ, USA). The colorimetric reactions were detected by an enzyme-linked immunosorbent assay (ELISA) reader, and the genotype was determined with QCReview™ software using methods that have been described previously [21].
Statistical analysis
Statistical analyses were performed using SPSS version 12.0 for Windows (Statistical Package for the Social Sciences, SPSS Inc., Chicago, IL). The Hardy-Weinberg Equilibrium (HWE) analysis was performed using Haploview version 3.32 (http://www.broadinstitute.org/scientific-community/science/programs/medical-and-population-genetics/haploview/haploview). Prior to statistical analysis, each variable was tested for normality. Logarithmic transformations were performed on skewed variables. However, for graphical and interpretation purposes, values are reported as the untransformed variables. The effects of the dietary intervention were evaluated using a paired t-test. The genotype effects were tested by either a one-way analysis of variance (ANOVA) with a Bonferroni correction for multiple comparisons or an independent t-test. The association between changes in apo A-V concentrations and other metabolic variables was computed with Pearson and partial correlation coefficients. A general linear model (GLM) analysis was performed to compare confounding factors. Multiple regressions were performed to determine the independent predictors of apo A-V changes. The results are reported as the mean ± SE. P < 0.05 was considered statistically significant.
Results
Distribution of the APOA5 -1131 T > C polymorphism
Among 203 Korean patients with IFG or new-onset type 2 diabetes, 91 were homozygous (TT) for the T allele, 98 were heterozygous for the C allele (TC), and 14 were homozygous (CC) for the C allele of the APOA5 -1131 T > C polymorphism. These frequencies did not deviate significantly from the Hardy-Weinberg equilibrium. The frequency of the C allele was 0.310, which is slightly higher than the frequency that was previously reported among a healthy Korean population (0.285) [22]. To increase statistical power, we pooled carriers of the less common allele (TC and CC).
Clinical characteristics and nutrient intake before and after a 3-year dietary intervention
At the 3-year follow-up visit, fasting HDL, glucose, insulin, HOMA-IR, free fatty acids, and apo A-V had decreased, and MDA and ba-PWV had increased from baseline across all subjects (Table 1). There were significant increases from baseline in both fiber intake and the ratio of polyunsaturated to saturated fatty acids as well as a slight decrease in total energy expenditure and total energy intake. The mean BMI of the subjects did not change, and no significant change was observed in other biochemical markers (Table 1).
Effects of a 3-year dietary intervention as a function of the APOA5 -1131 T > C genotype
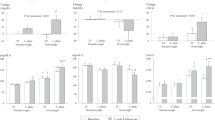
There were no significant differences in the distribution of sex, age, BMI, WHR, BP, total cholesterol, LDL, hs-CRP, or estimates of daily nutrient intake across the APOA5 -1131 T > C genotypes at baseline or the 3-year follow-up visit (data not shown). The mean (±SEs) fasting apo A-V and triglyceride levels at baseline and the 3-year follow-up visit across the APOA5 -1131 T > C genotypes are shown in Figure 1. At baseline, there was a significant association between apo A-V levels and the -1131 T > C genotype (P = 0.009) such that apo A-V levels were approximately 10% and 38% lower in TC and CC allele carriers, respectively, compared with TT allele carriers. Triglyceride levels were also approximately 17% and 34% higher in TC and CC allele carriers, respectively, compared with TT allele carriers at baseline. At the 3-year follow-up visit, plasma apo A-V levels were reduced in C allele carriers (P = 0.036) and fasting triglyceride levels were reduced in TT allele carriers (P = 0.047) (Table 2). After adjusting for baseline levels, significant associations were found between apo A-V changes and the APOA5 genotype (TT: −7.77 ± 10.79 ng/mL; TC: −23.99 ± 10.52; CC: −16.71 ± 16.11; P = 0.012) and between triglyceride changes and the APOA5 genotype (TT: −7.88 ± 5.44 mg/dL; TC: 14.38 ± 6.21; CC: 19.57 ± 18.58; P = 0.004) (Figure 1). TC or CC allele carriers had significantly lower post-treatment apo A-V levels and higher post-treatment fasting triglyceride levels than TT allele carriers.
Fasting apolipoprotein A-V and triglyceride levels at baseline (white square) and the 3-year follow-up visit (gray square) and mean changes as a function of the APOA5 -1131 T > C genotype. Data (mean ± SE) included 91 (TT), 98 (TC), and 14 (CC) subjects. §Log-transformed. P1, unadjusted P value; P2, baseline-adjusted P value. * P < 0.05 compared with baseline values for each genotype by paired t-test. † P < 0.05 compared with the TT allele; ‡ P < 0.05 compared with the TT allele after adjusting for baseline values; # P < 0.05 compared with the TC allele tested by a one-way ANOVA with a Bonferroni correction.
The mean (±SEs) fasting HDL, glucose, insulin, HOMA-IR index, free fatty acids, LDL particle size, MDA, and ba-PWV values at both baseline and the 3-year follow-up visit are reported in Table 2 as a function of the APOA5 -1131 T > C genotype. At baseline, C allele carriers had lower HDL levels (P = 0.024) than TT allele carriers. At the 3-year follow-up visit, fasting glucose (P < 0.001), insulin (P < 0.001), the HOMA-IR index (P < 0.001), and free fatty acids (P = 0.020) were reduced in TT allele carriers but not C allele carriers. C allele carriers had decreased HDL levels (P = 0.005) and LDL particle size (P = 0.048) and increased MDA (P < 0.001) and ba-PWV (P < 0.001) at the 3-year follow-up visit compared with baseline.
TT allele carriers had a greater reduction in glucose (P = 0.030) and the HOMA-IR index (P = 0.047) than C allele carriers after adjusting for baseline levels. C allele carriers had a greater reduction in LDL particle size (P = 0.006) and a greater increase in plasma MDA (P = 0.048) and ba-PWV (P = 0.046) than TT allele carriers after adjusting for baseline levels. C allele carriers also had lower post-treatment HDL levels (P = 0.001), smaller post-treatment LDL particle sizes (P = 0.008), and higher post-treatment MDA (P = 0.020) than TT allele carriers (Table 2).
Relationship between changes in plasma apo A-V levels and metabolic parameters
Correlations between the changes in apo A-V levels and metabolic parameters were determined after adjusting for age, sex, and changes in body weight (r 1 ), initial triglyceride levels (r 2 ), and initial apo A-V levels (r 3 ) (Table 3). Across all subjects, changes in apo A-V levels were negatively correlated with baseline apo A-V levels (r 1 = −0.543, r 2 = −0.555, all P < 0.001). In TT allele carriers, changes in apo A-V levels were negatively correlated with baseline apo A-V levels (r 1 = −0.535, r 2 = −0.552, all p < 0.001) and changes in triglyceride levels (r 3 = −0.299, p 3 = 0.006). In C allele carriers, changes in apo A-V levels were negatively correlated with baseline apo A-V levels (r 1 = −0.613, r 2 = −0.617, all of P < 0.001), but positively correlated with changes in triglyceride levels (r 1 = 0.207, p 1 = 0.038; r 2 = 0.221, p 2 = 0.028).
Independent predictors of apo A-V and triglyceride changes
Based on these results, we performed a multiple regression to determine independent predictors of apo A-V changes (Table 4). The APOA5 -1131 T > C genotype, age, baseline BMI, baseline apo A-V levels, baseline triglyceride levels, change in triglyceride levels, baseline HDL, change in HDL, baseline glucose, change in glucose, baseline HOMA-IR, change in HOMA-IR, baseline free fatty acids, and change in free fatty acids were tested. The APOA5 -1131 T > C genotype emerged as an independent predictor of changes in apo A-V levels (β = −24.532 ± 11.686, P = 0.037) along with baseline apo A-V levels (β = −107.870 ± 12.445, P < 0.001) across all subjects. Baseline apo A-V levels and changes in triglyceride levels and changes in HDL cholesterol levels also emerged as independent predictors of changes in apo A-V levels (β = −112.767 ± 19.550, P < 0.001; β = −0.404 ± 0.191, P = 0.038; β = 3.280 ± 1.007, P = 0.002, respectively) in both TT and C allele carriers (β = −101.958 ± 16.096; P < 0.001, β = 0.349 ± 0.133, P = 0.010; β = 2.239 ± 0.666, P = 0.001, respectively). To figure out the independent predictors of triglyceride changes, APOA5 -1131 T > C genotype, age, baseline BMI, baseline apo A-V levels, baseline triglyceride levels, change in triglyceride levels, change in HDL, baseline glucose, change in glucose, baseline HOMA-IR, and change in HOMA-IR were tested (data not shown). The APOA5 -1131 T > C genotype emerged as an independent predictor of changes in triglyceride levels (β = 17.668 ± 8.200, P = 0.033) along with baseline triglyceride levels (β = −35.455 ± 8.401, P < 0.001) and baseline apo A-V levels (β = −20.856 ± 10.025, P = 0.039) across all subjects. Baseline triglyceride levels and changes in apo A-V levels also emerged as independent predictors of changes in triglyceride levels (β = −36.641 ± 11.743, P = 0.003 and β = −0.179 ± 0.068, P = 0.010, respectively) in both TT and C allele carriers (β = −29.611 ± 12.206, P = 0.017 and β = 0.182 ± 0.078, P = 0.001, respectively). However, baseline apo A-V levels had shown as predictors of changes in triglyceride levels only in TT allele (β = −31.465 ± 13.668, P = 0.024).
Discussion
This study shows an effect of the APOA5 -1131 T > C polymorphism in patients with IFG or type 2 diabetes among the Korean population. The frequencies of the APOA5 -1131 T > C alleles (TT: 44.8%, TC: 48.3%, CC: 6.9%) did not deviate from the Hardy-Weinberg equilibrium. Our data showed that the TT allele is associated with lower triglyceride levels compared with the C allele, which agrees with a study by Xu et al. that demonstrated higher levels of fasting triglycerides, total cholesterol, LDL, and HDL, as well as a 33% increased risk of metabolic syndrome, in 51,868 Chinese subjects with the C allele [8]. In our previous research, CC allele carriers showed lower apo A-V levels than TT allele carriers, and showed association with greater triglyceride levels, smaller LDL particle size, and lower HDL-cholesterol [23]. Similarly, Li et al. found a higher C allele frequency among individuals with high total cholesterol compared with individuals with normal total cholesterol levels in both ethnic populations (Hei Yi Zhuang and Han Chinese; P < 0.05) [24]. However, a 15-year follow-up study of healthy men in the United Kingdom (the Northwick Park Heart Study II) showed no effects of the APOA5 -1131 T > C polymorphism on the risk of type 2 diabetes [25]. This discordance across studies may be related to the differences among ethnic groups. The C allele of the APOA5 -1131 T > C is more common among Asian populations (34% frequency in Japanese, 28-30% in Koreans, and 7.4% in Caucasians) [26, 27]. The living conditions and progression of the disease as well as other subject characteristics may also affect the results.
The number of people diagnosed with diabetes worldwide is approximately 171 million, with a predicted increase to 366 million people by 2030 [28]. Elevated triglyceride levels as well as changes in plasma lipoproteins are associated with diabetes [24]. In the Framingham Heart Study, high VLDL levels which prompted the consideration of high triglyceride levels were associated with the development of diabetes, especially among subjects who were obese [29]. The relationship between obesity and diabetes is interdependent. Therefore, diet and exercise are extremely important for patients with diabetes.
Whole grain and legume consumption is known to decrease insulin resistance or insulin demand and improve lipid profiles, including decreasing triglyceride levels [30]. In particular, soy intake has been associated with increased HDL and decreased triglyceride levels [31]. This association is consistent with our finding that TT allele carriers who consumed whole grains and legumes had a significant decrease in fasting triglyceride levels. In contrast, C allele carriers had an increase in fasting triglyceride levels despite adhering to the same diet. This observation suggests that the magnitude of the effect of the APOA5 gene on triglyceride levels is masked when metabolic conditions improved as a consequence of the whole grain and legume diet. This result is consistent with previous reports that lifestyle modification attenuates the biological effect of a genetic predisposition on metabolic profiles and diabetes risk [32].
Exercise has also been shown to increase insulin sensitivity in patients with type 2 diabetes [33]. Another study showed that exercise increased insulin sensitivity in both type 2 diabetes and healthy control subjects with the same age, BMI, and initial triglyceride levels. However, although exercise had little effect on triglyceride levels in control subjects, exercise significantly decreased triglyceride levels in patients with type 2 diabetes. Therefore, exercise may be therapeutic for abnormal lipid metabolism [34]. This result suggests that if exercise is therapeutic only for abnormal lipid metabolism, then an innate human genome sequence for this effect may not exist. Previous research has suggested that the promoter variant -1131 T > C is in strong linkage disequilibrium with APOA5 -3A > G, which occurs in the ‘Kozak’ sequence in APOA5, and disruption of this sequence has been shown in other genes to severely disrupt ribosomal translation efficiency, leading to lower levels of apo A-V from this mRNA. Therefore, the C allele may itself not be functional, but is acting as a marker for the -3A > G that may contribute to lower apo A-V levels [35]. More studies are needed to determine whether diet and exercise can improve apo A-V levels in individuals with the C allele.
Apo A-V would increase the binding of TG-rich lipoproteins to glycosaminoglycans present on the surface of endothelial cells, thus rendering these lipoproteins more accessible to LPL [36]. Therefore, decreased apo A-V levels may reduce LPL-mediated TG hydrolysis of chylomicrons and VLDL and delay the clearance of lipoprotein remnants by the liver [37]. After hydrolysis of TG-rich lipoproteins by LPL, surface components are detached to form native HDL of discoidal form [38]. Thus, the delayed TG hydrolysis, attributable to apo A-V deficiency, reduces the availability of surface components of triglyceride-rich lipoproteins, thereby leading to a decreased formation of HDL-cholesterol. In this way, apo A-V is associated with HDL formation, and this may result in significant contribution of HDL as independent predictors for changes in apo A-V levels.
There are several limitations in our study. First, dietary intake during the 3-year dietary intervention was based on self-reports obtained from weighed food. However, measurement errors from self-reported dietary intake and lifestyle variables are relatively small [39]. Second, the euglycemic glucose clamp technique and 2-hour glucose tolerance test were not performed. Finally, we focused on individuals with IFG or new-onset type 2 diabetes; therefore, our results cannot be generalized to the greater population. Despite these limitations, we found that the APOA5 -1131 T > C polymorphism plays an important role in the metabolic response to a 3-year dietary intervention in patients with IFG or new-onset type 2 diabetes. Whole-grain ingestion prevented the age-related increase in triglyceride levels in patients with IFG or new-onset type 2 diabetes who carried the TT allele but not the C allele of the APOA5 -1131 T > C polymorphism. Our results suggest that the C allele contributes to the increased triglyceride levels among the Korean population. In addition, a positive relationship between triglyceride levels and the C allele may affect the onset of diabetes. However, long-term case–control studies and studies of the underlying mechanisms are needed to determine the relationship between the APOA5 -1131 T > C polymorphism and human health.
Conclusions
This study showed that the dietary intervention prevented an age-related increase in triglyceride levels in individuals with IFG or new-onset type 2 diabetes who possess the TT allele, but not the CT or CC allele, of the APOA5 -1131 T > C polymorphism.
Abbreviations
- apo A-V:
-
Apolipoprotein A-V
- IFG:
-
Impaired fasting glucose
- SNP:
-
Single-nucleotide polymorphism
- CVD:
-
Cardiovascular disease
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- BMI:
-
Body mass index
- WHR:
-
Waist hip ratio
- BP:
-
Blood pressure
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- hs-CRP:
-
High-sensitivity C-reactive protein
- MDA:
-
Malondialdehyde
- ba-PWV:
-
Brachial-ankle pulse wave velocity
- PUFA:
-
Polyunsaturated fatty acids
- SFA:
-
Saturated fatty acids.
References
Li GP, Wang JY, Yan SK, Chen BS, Xue H, Wu G: Genetic effect of two polymorphisms in the apolipoprotein A5 gene and apolipoprotein C3 gene on serum lipids and lipoproteins levels in a Chinese population. Clin Genet. 2004, 65: 470-476. 10.1111/j.1399-0004.2004.00251.x.
Li YY, Wu XY, Xu J, Qian Y, Zhou CW, Wang B: ApoA5–1131 T/C, FgB -455G/A, −148C/T, and CETP TaqIB gene polymorphisms and coronary artery disease in the Chinese population: a meta-analysis of 15,055 subjects. Mol Biol Rep. 2013, 40: 1997-2014. 10.1007/s11033-012-2257-9.
Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC, Krauss RM, Rubin EM: An Apolipoprotein influencing triglycerides in Humans and mice revealed by comparative sequencing. Science. 2001, 294: 169-173. 10.1126/science.1064852.
Baum L, Tomlinson B, Thomas GN: APOA5-1131 T > C polymorphism is associated with triglyceride levels in Chinese men. Clin Genet. 2003, 63: 377-379. 10.1034/j.1399-0004.2003.00063.x.
Yang Y, Huacong D, Long J, Yanxin S, Yongling L, Gongpu M: Associations of apolipoprotein A5 with triglyceride, adiponectin and insulin resistance in patients with impaired glucose regulation and type 2 diabetes mellitus. Int J Diabetes Dev Ctries. 2013, 33: 13-17. 10.1007/s13410-012-0102-2.
Dallinga-Thie GM, Van Tol A, Hattori H, der Zee LC VV-v, Jansen H, Sijbrands EJ, DALI study group: Plasma apolipoprotein A5 and triglyceride in type 2 diabetes. Diabetologia. 2006, 49: 1505-1511. 10.1007/s00125-006-0261-0.
Jang Y, Kim JY, Kim OY, Lee JE, Cho H, Ordovas JM, Lee JH: The -1131 T → C polymorphism in the apolipoprotein A5 gene is associated with postprandial hypertriacylglycerolemia; elevated small, dense LDL concentrations; and oxidative stress in nonobese Korean men. Am J Clin Nutr. 2004, 80: 832-840.
Xu C, Bai R, Zhang D, Li Z, Zhu H, Lai M, Zhu Y: Effects of APOA5–1131 T > C (rs662799) on fasting plasma lipids and risk of metabolic syndrome: evidence from a case–control study in China and a meta-analysis. PLoS One. 2013, 8: e56216-10.1371/journal.pone.0056216.
Chandak GR, Ward KJ, Yajnik CS, Pandit AN, Bavdekar A, Joglekar CV, Fall CH, Mohankrishna P, Wilkin TJ, Metcalf BS, Weedon MN, Frayling TM, Hattersley AT: Triglyceride associated polymorphisms of the APOA5 gene have very different allele frequencies in Pune, India compared to Europeans. BMC Med Genet. 2006, 7: 76-81. 10.1186/1471-2350-7-76.
Sánchez-Moreno C, Ordovás JM, Smith CE, Baraza JC, Lee YC, Garaulet M: APOA5 gene variation interacts with dietary fat intake to modulate obesity and circulating triglycerides in a Mediterranean population. J Nutr. 2011, 141: 380-385. 10.3945/jn.110.130344.
Kim JY, Kim OY, Koh SJ, Jang Y, Yun SS, Ordovas JM, Lee JH: Comparison of low-fat meal and high-fat meal on postprandial lipemic response in non-obese men according to the -1131 T > C polymorphism of the apolipoprotein A5 (APOA5) gene (randomized cross-over design). J Am Coll Nutr. 2006, 25: 340-347. 10.1080/07315724.2006.10719544.
Corella D, Lai CQ, Demissie S, Cupples LA, Manning AK, Tucker KL, Ordovas JM: APOA5 gene variation modulates the effects of dietary fat intake on body mass index and obesity risk in the Framingham Heart Study. J Mol Med (Berl). 2007, 85: 119-128. 10.1007/s00109-006-0147-0.
Mattei J, Demissie S, Tucker KL, Ordovas JM: Apolipoprotein A5 polymorphisms interact with total dietary fat intake in association with markers of metabolic syndrome in Puerto Rican older adults. J Nutr. 2009, 139: 2301-2308. 10.3945/jn.109.109900.
Lai CQ, Corella D, Demissie S, Cupples LA, Adiconis X, Zhu Y, Parnell LD, Tucker KL, Ordovas JM: Dietary intake of n-6 fatty acids modulates effect of apolipoprotein A5 gene on plasma fasting triglycerides, remnant lipoprotein concentrations, and lipoprotein particle size: the Framingham Heart Study. Circulation. 2006, 113: 2062-2070. 10.1161/CIRCULATIONAHA.105.577296.
Lin J, Fang DZ, Du J, Shigdar S, Xiao LY, Zhou XD, Duan W: Elevated levels of triglyceride and triglyceride-rich lipoprotein triglyceride induced by a high-carbohydrate diet is associated with polymorphisms of APOA5-1131 T > C and APOC3-482C > T in Chinese healthy young adults. Ann Nutr Metab. 2011, 58: 150-157. 10.1159/000327913.
Charriere S, Bernard S, Aqallal M, Merlin M, Billon S, Perrot L, Le Coquil E, Sassolas A, Moulin P, Marcais C: Association of APOA5–1131 T > C and S19W gene polymorphisms with both mild hypertriglyceridemia and hyperchylomicronemia in type 2 diabetic patients. Clin Chim Acta. 2008, 394: 99-103. 10.1016/j.cca.2008.04.013.
Zhang Z, Lanza E, Kris-Etherton PM, Colburn NH, Bagshaw D, Rovine MJ, Ulbrecht JS, Bobe G, Chapkin RS, Hartman TJ: A high legume low glycemic index diet improves serum lipid profiles in men. Lipids. 2010, 45: 765-775. 10.1007/s11745-010-3463-7.
Shim JS, Oh KW, Suh I, Kim MY, Shon CY, Lee EJ, Nam CM: A study on validity of a 299 semiquantitative food frequency questionnaire of Korean adults. Korean J Community Nutr. 2002, 7: 484-494.
Christian JL, Greger JL: Energy Sources and Uses (4th eds). Nutrition for Living. 1994, Redwood city, CA: Benjamin/Cummings Publishing Co, 242-266.
Butte NF, Caballero B: Energy Needs: Assessment and Requirements (10th eds). Modern Nutrition In Health And Disease. Edited by: Shils ME, Shike M, Ross AC, Cousins RJ. 2006, Philadelphia: Lippincott Williams & Wilkins, 136-148.
Hyun YJ, Jang Y, Chae JS, Kim JY, Paik JK, Kim SY, Yang JY, Ordovas JM, Ko YG, Lee JH: Association of apolipoprotein A5 concentration with serum insulin and triglyceride levels and coronary artery disease in Korean men. Atherosclerosis. 2009, 205: 568-573. 10.1016/j.atherosclerosis.2008.12.035.
Jang Y, Paik JK, Hyun YJ, Chae JS, Kim JY, Choi JR, Lee SH, Shin DJ, Ordovas JM, Lee JH: The apolipoprotein A5–1131 T > C promoter polymorphism in Koreans: association with plasma APOA5 and serum triglyceride concentrations, LDL particle size and coronary artery disease. Clin Chim Acta. 2009, 402: 83-87. 10.1016/j.cca.2008.12.024.
Kim JY, Kim OY, Paik JK, Lee S-H, Lee JH: Association of apolipoprotein A-V concentration with apolipoprotein A5 gene -1131 T > C polymorphism and fasting triglyceride levels. J Clin Lipid. 2013, 1782: 447-452.
Li YY, Yin RX, Lai CQ, Li M, Long XJ, Li KL, Liu WY, Zhang L, Wu JZ: Association of apolipoprotein A5 gene polymorphisms and serum lipid levels. Nutr Metab Cardiovasc Dis. 2011, 21: 947-956. 10.1016/j.numecd.2010.04.004.
Talmud PJ, Cooper JA, Hattori H, Miller IP, Miller GJ, Humphries SE: The apolipoprotein A-V genotype and plasma apolipoprotein A-V and triglyceride levels: prospective risk of type 2 diabetes. Results from the Northwick Park Heart Study II. Diabetologia. 2006, 49: 2337-2340. 10.1007/s00125-006-0387-0.
Kim DH, Lee SH, Han KH, Kim CB, Song KY, Cho S, Lee KH: APOA5 polymorphism is associated with metabolic syndrome in Korean postmenopausal women. Endocrinol Metab. 2012, 27: 276-281. 10.3803/EnM.2012.27.4.276.
Yamada Y, Kato K, Hibino T, Yokoi K, Matsuo H, Segawa T, Watanabe S, Ichihara S, Yoshida H, Satoh K, Nozawa Y: Prediction of genetic risk for metabolic syndrome. Atherosclerosis. 2007, 191: 298-304. 10.1016/j.atherosclerosis.2006.05.035.
Wild S, Roglic G, Green A, Sicree R, King H: Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care. 2004, 27: 1047-1053. 10.2337/diacare.27.5.1047.
Castelli WP: The triglyceride issue: a view from Framingham. Am Heart J. 1986, 112: 432-436. 10.1016/0002-8703(86)90296-6.
Katcher HI, Legro RS, Kunselman AR, Gillies PJ, Demers LM, Bagshaw DM, Kris-Etherton PM: The effects of a whole grain-enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am J Clin Nutr. 2008, 87: 79-90.
Zhan S, Ho SC: Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am J Clin Nutr. 2005, 81: 397-408.
Temelkova-Kurktschiev T, Stefanov T: Lifestyle and genetics in obesity and type 2 diabetes. Exp Clin Endocrinol Diabetes. 2012, 120: 1-6.
Dela F, Larsen JJ, Mikines KJ, Ploug T, Petersen LN, Galbo H: Insulin-stimulated muscle glucose clearance in patients with NIDDM. Effects of one-legged physical training. Diabetes. 1995, 44: 1010-1020. 10.2337/diab.44.9.1010.
Bruce CR, Kriketos AD, Cooney GJ, Hawley JA: Disassociation of muscle triglyceride content and insulin sensitivity after exercise training in patients with type 2 diabetes. Diabetologia. 2004, 47: 23-30. 10.1007/s00125-003-1265-7.
Martin S, Nicaud V, Humphries SE, Talmud PJ: Contribution of APOA5 gene variants to plasma triglyceride determination and to the response to both fat and glucose tolerance challenges. Biochim Biophys Acta. 2003, 1637: 217-225. 10.1016/S0925-4439(03)00033-4.
Merkel M, Loeffler B, Kluger M, Fabig N, Geppert G, Pennacchio LA, Laatsch A, Heeren J: Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J Biol Chem. 2005, 280: 21553-21560. 10.1074/jbc.M411412200.
Priore Oliva C, Carubbi F, Schaap FG, Bertolini S, Calandra S: Hypertriglyceridaemia and low plasma HDL in a patient with apolipoprotein A-V deficiency due to a novel mutation in the APOA5 gene. J Intern Med. 2008, 263: 450-458. 10.1111/j.1365-2796.2007.01912.x.
Benlian P: Genes of Lipoprotein Metabolism. Genetics of Dyslipidemia, Volume 7. 2001, Norwell, MA, USA: Kluwer Academic Publishers, 125-127.
Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC: Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992, 135: 1114-1126.
Acknowledgements
We sincerely thank those who participated in the studies described in this report. This research was supported by the Bio & Medical Technology Development Program (2006–2005306 and 2012M3A9C4048762) and Mid-career Researcher Program (2010–0015017) of the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning, Republic of Korea”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare they have no competing interests.
Authors’ contributions
Conceived and designed the experiments: MK, JSC, MK, S-HL, and JHL. Performed the experiments: MK, JSC, and MK. Analyzed the data: MK and MK. Contributed reagents/materials/analysis tools: S-HL and JHL. Wrote the paper: JHL and MK. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kim, M., Chae, J.S., Kim, M. et al. Effects of a 3-year dietary intervention on age-related changes in triglyceride and apolipoprotein A-V levels in patients with impaired fasting glucose or new-onset type 2 diabetes as a function of the APOA5 -1131 T > C polymorphism. Nutr J 13, 40 (2014). https://doi.org/10.1186/1475-2891-13-40
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2891-13-40