Abstract
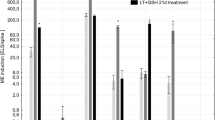
The impact of culture conditions and addition of antioxidants to media on microspore embryogenesis in rapeseed (Brassica napus cv. ‘PF704’) was investigated. Different concentrations of ascorbic acid (0, 5, 10, 20, 50, 100, and 200 mg l−1) and alpha (α)-tocopherol (0, 5, 10, 20, 50, 100, and 200 mg l−1) were evaluated along with two temperature pretreatments (18 d at 30°C; 2 d at 32.5°C followed by 16 d at 30°C). In addition, combinations of reduced glutathione (0, 10, 50, and 100 mg l−1) and ascorbic acid (5 and 10 mg l−1) were tested. Microspore embryogenesis was significantly enhanced using 10 mg l−1 ascorbic acid (334 embryos per Petri dish) compared with untreated cultures (184 embryos per Petri dish) at 30°C. α-Tocopherol (5 and 10 mg l−1) enhanced (312 and 314 embryos per Petri dish, respectively) microspore embryogenesis relative to untreated cultures (213 embryos per Petri dish) at 30°C, although there were no significant differences among cultures treated with 5–50 mg l−1 α-tocopherol. When 50 mg l−1 α-tocopherol was combined with 5 or 10 mg l−1 ascorbic acid, embryogenesis was significantly enhanced (308 and 328 embryos per Petri dish, respectively) relative to other ascorbic acid levels. Moreover, 10 mg l−1 of reduced glutathione and 5 mg l−l ascorbic acid enhanced microspore embryogenesis (335 embryos per Petri dish) compared to cultures without reduced glutathione (275 embryos per Petri dish). Microspore embryogenesis could be improved by adding ascorbic acid, α-tocopherol, and reduced glutathione when the appropriate combination and temperature pretreatment were selected.

Similar content being viewed by others
References
Ahmadi B.; Alizadeh K.; Teixeira da Silva J. A. Enhanced regeneration of haploid plantlets from microspores of Brassica napus L. using bleomycin, PCIB, and phytohormones. Plant Cell Tissue Organ Cult. 105: 525–533; 2012a.
Ahmadi B.; Ghadimzadeh M.; Moghaddam A. F.; Alizadeh K.; Teixeira da Silva J. A. Bud length, plating density, and incubation time on microspore embryogenesis in Brassica napus. Int. J. Veg. Sci. 18: 346–357; 2012b.
Ambrus H.; Darko É.; Király Z.; Barnabás B. Effect of ROS progenitors on the sporophytic development of maize microspores. Acta Biol. Szeged. 49: 25–28; 2005.
Athar H. R.; Khan A.; Ashraf M. Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environ. Exp. Bot. 63: 224–231; 2008.
Belmonte M. F.; Stasolla C.; Loukanina N.; Young E. C.; Thorpe T. A. Glutathione modulation of purine metabolism in cultured white spruce embryogenic tissue. Plant Sci. 165: 1377–1385; 2003.
Bhattacharjee S. Reactive oxygen species and oxidative burst: roles in stress, senescence and signal transduction in plant. Curr. Sci. 89: 1113–1121; 2005.
Chen Z.; Gallie D. R. Induction of monozygotic twinning by ascorbic acid in tobacco. PLoS One 7(6): e39147; 2012.
Coventry J.; Kott L. S. Doubled haploid technology for spring and winter Brassica napus (revised ed.) OAC Publication, University of Guelph, Ontario, Canada; Technol Bull.; 1998: 42 p.
de Carvalho M. H. C. Drought stress and reactive oxygen species. Plant Sig. Behav. 3: 156–165; 2008.
De Pinto M. C.; De Gara L. Changes in the ascorbate metabolism of apoplastic and symplastic space are associated with cell differentiation. J. Exp. Bot. 55: 2559–2569; 2004.
De Tullio M. C.; Paciolla C.; Vecchia F. D.; Rascio N.; D’Emerico S.; De Gara L.; Liso R.; Arrigoni O. Changes in onion root development induced the inhibition of peptidyl-prolyl hydroxylase and influence of the ascorbate system on cell division and elongation. Planta 209: 424–434; 1999.
Ferrie A. M. R.; Caswell K. L. Isolated microspore culture techniques and recent progress for haploid and doubled haploid plant production. Plant Cell Tissue Organ Cult. 104: 301–309; 2011.
Ferrie A. M. R.; Keller W. A. Optimization of methods for using polyethylene glycol as a non-permeating osmoticum for the induction of microspore embryogenesis in Brassicaceae. In Vitro Cell. Dev. Biol. Plant 43: 348–355; 2007.
Ferrie A. M. R.; Taylor D. C.; MacKenzie S. L.; Keller W. A. Microspore embryogenesis of high sn-2 erucic acid Brassica oleracea germplasm. Plant Cell Tissue Organ Cult. 57: 79–84; 1999.
Gallie D. R. l-ascorbic acid: a multifunctional molecule supporting plant growth and development. Scientifica 2013: 1–24; 2013.
Gamborg O. L.; Miller R. A.; Ojima L. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50: 151–158; 1968.
Gill S. S.; Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 42: 909–930; 2010.
Habibi N.; Suthar P. K.; Purohit S. D. Role of PGRs and inhibitors in induction and control of somatic embryogenesis in Themeda quadrivalvis. Indian J. Exp. Biol. 47: 198–203; 2009.
Huang B.; Bird S.; Kemble R.; Miki B.; Keller W. Plant regeneration from microspore-derived embryos of Brassica napus: effect of embryo age, culture temperature, osmotic pressure, and abscisic acid. In Vitro Cell. Dev. Biol. Plant 27: 28–31; 1991.
Jiang K.; Ballinger T.; Li D.; Zhang S.; Feldman L. J. A role for mitochondria in the establishment and maintenance of the maize root quiescent center. Plant Physiol. 140: 1118–1125; 2006.
Li S.; Xue L.; Xu S.; Feng H.; An L. Hydrogen peroxide involvement in formation and development of adventitious roots in cucumber. Plant Growth Regul. 52: 173–180; 2007.
Li S.; Xue L.; Xu S.; Feng H.; An L. Hydrogen peroxide acts as a signal molecule in the adventitious root formation of mung bean seedlings. Environ. Exp. Bot. 65: 63–71; 2009a.
Li S.; Xue L.; Xu S.; Feng S.; An L. IBA-induced changes in antioxidant enzymes during adventitious rooting in mung bean seedlings: the role of H2O2. Environ. Exp. Bot. 66: 442–450; 2009b.
Liao W.; Xiao H.; Zhang M. Role and relationship of nitric oxide and hydrogen peroxide in adventitious root development of marigold. Acta Physiol. Plant. 31: 1279–1289; 2009.
Lichter R. Induction of haploid plants from isolated pollens of Brassica napus. Z. Pflanzenphysiol. 105: 427–434; 1982.
Liso R.; Innocenti A. M.; Bitonti M. B.; Arrigoni O. Ascorbic acid induced progression of quiescent centre cells from G1 to S phase. New Phytol. 110: 469–471; 1988.
Liszkay A.; van der Yalm E.; Schopfer P. Production of reactive oxygen intermediates (O2 •–, H2O2 and •OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 135: 3114–3123; 2004.
Miyake C.; Asada K. Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product, monodehydroascorbate radicals in the thylakoids. Plant Cell Physiol. 33: 541–553; 1992.
Pagnussat G. C.; Simontacchi M.; Puntarulo S.; Lamattina L. Nitric oxide is required for root organogenesis. Plant Physiol. 129: 954–956; 2002.
Palmer C. E.; Keller W. A.; Kasha K. J. Haploids in crop improvement II. Springer, Berlin; 2005.
Prem D.; Gupta K.; Agnihotri A. Effect of various exogenous and endogenous factors on microspore embryogenesis in Indian mustard (Brassica juncea L. Czern and Coss). In Vitro Cell. Dev. Biol. Plant 41: 266–273; 2005.
Prem D.; Solís M.-T.; Bárány I.; Rodríguez-Sanz H.; Risueño M. C.; Testillano P. S. A new microspore embryogenesis system under low temperature which mimics zygotic embryogenesis initials, expresses auxin and efficiently regenerates doubled-haploid plants in Brassica napus. BMC Plant Biol. 12: 127; 2012.
Rodríguez-Serrano M.; Bárány I.; Prem D.; Coronado M. J.; Risueño M. C.; Testilano P. S. NO, ROS, and cell death associated with caspase-like activity increase in stress-induced microspore embryogenesis of barley. J. Exp. Bot. 63: 2007–2024; 2011.
Sánchez-Fernández R.; Flicker M.; Corben L. B.; White N. S.; Sheard N. S.; Leaver C. J.; Van Montagu M.; Inzé D.; May M. J. Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proc. Natl. Acad. Sci. U. S. A. 94: 2745–2750; 1997.
Schopfer P.; Liszkay A.; Bechtold M.; Frahry G.; Wagner A. Evidence that hydroxyl radicals mediate auxin-induced extension growth. Plant J. 28: 679–688; 2002.
Seguí-Simarro J. M.; Nuez F. How microspores transform into haploid embryos: changes associated with embryogenesis induction and microspore-derived embryogenesis. Physiol. Plant. 134: 1–12; 2008.
Shanker A. K.; Djanaguiraman M.; Sudhagar R.; Chandrashekar C. N.; Pathmanabhan G. Differential antioxidative response of ascorbate glutathione pathway enzymes and metabolites to chromium speciation stress in green gram (Vigna radiata (L.) R. Wilczek. cv CO 4) roots. Plant Sci. 166: 1035–1043; 2004.
Shi Y.; Xu G.; Warrington T. B.; Murdoch G. K.; Kazala E. C.; Snyder C. L.; Weselake R. J. Microspore-derived cell suspension cultures of oilseed rape as a system for studying gene expression. Plant Cell Tissue Organ Cult. 92: 131–139; 2008.
Shohael A.; Ali M.; Hahn E.; Paek K. Glutathione metabolism and antioxidant responses during Eleutherococcus senticosus somatic embryo development in a bioreactor. Plant Cell Tissue Organ Cult. 89: 121–129; 2007.
Smirnoff N. Ascorbate, tocopherol and carotenoids: metabolism, pathway engineering and functions. In: Smirnoff N. (ed) Antioxidants and reactive oxygen species in plants. Blackwell Publishing Ltd, Oxford, pp 53–86; 2005.
Stasolla C.; Yeung E. C. Cellular ascorbic acid regulates the activity of major peroxidase in the apical poles of germinating white spruce (Picea glauca) somatic embryos. Plant Physiol. Biochem. 45: 188–198; 2007.
Teixeira da Silva J. A. Impact of paper bridges, activated charcoal, and antioxidants on growth and development of protocorm-like bodies of hybrid Cymbidium. In Vitro Cell. Dev. Biol. Plant 49: 414–420; 2013.
Traber M. G.; Stevens J. F. Vitamin C and E: beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 51: 1000–1013; 2011.
Tuteja N. Mechanisms of high salinity tolerance in plants. Methods Enzymol. 428: 419–438; 2007.
Vernoux T.; Wilson R. C.; Seeley K. A.; Reichheld J. P.; Muroy S.; Brown S.; Maughan S. C.; Cobbett C. S.; Van Montagu M.; Inzé D.; May M. J.; Sung Z. R. The ROOT MERISTEMLESS 1/CADMIUM SENSITIVE 2 gene defines a glutathione-dependant pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12: 97–109; 2000.
Vieira L. D. N.; Santa-Catarina C.; Fraga H. P. D. F.; Santos A. L. W. D.; Steinmacher D. A.; Schlogl P. S.; Silveira V.; Steiner N.; Floh E. I. S.; Guerra M. P. Glutathione improves early somatic embryogenesis in Araucaria angustifolia (Bert) O. Kuntze by alteration in nitric oxide emission. Plant Sci. 195: 80–87; 2012.
Wang X.; Quinn P. J. Vitamin E and its function in membranes. Prog. Lipid Res. 38: 309–336; 1999.
Weselake R. J. Storage lipids. In: Murphy D. J. (ed) Plant lipids: biology, utilization, and manipulation. Blackwell Publishing, Oxford, pp 162–221; 2005.
Winarto B.; Teixeira da Silva J. A. Microspore culture protocol for Indonesian Brassica oleracea. Plant Cell Tissue Organ Cult. 107: 305–315; 2011.
Zeng X.; Weng J.; Wan Z.; Yi B.; Shen J.; Ma C.; Tu J.; Fu T. Effects of bleomycin on microspore embryogenesis in Brassica napus and detection of somaclonal variation using AFLP molecular marker. Plant Cell Tissue Organ Cult. 101: 23–29; 2010.
Zhang G. Q.; Zhang D. Q.; Tang G. X.; He Y.; Zhou W. J. Plant development from microspore-derived embryos in oilseed rape as affected by chilling, desiccation and cotyledon excision. Biol. Plant. 50: 180–186; 2006.
Zur L.; Dubas F.; Golemier F.; Szechynska-Hebda M.; Golehiowska G.; Wedzony M. Stress-related variation in antioxidative enzymes activity and cell metabolism efficiency associated with embryogenesis induction in isolated microspore culture of triticale (× Triticosecale Wittm.). Plant Cell Rep. 28: 1279–1287; 2009.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: John Chen
Rights and permissions
About this article
Cite this article
Hoseini, M., Ghadimzadeh, M., Ahmadi, B. et al. Effects of ascorbic acid, alpha-tocopherol, and glutathione on microspore embryogenesis in Brassica napus L.. In Vitro Cell.Dev.Biol.-Plant 50, 26–35 (2014). https://doi.org/10.1007/s11627-013-9579-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-013-9579-8