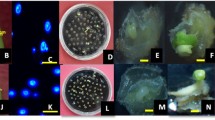
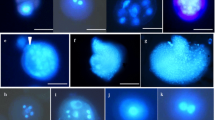
Abstract
A microspore culture protocol for Brassica oleracea of Indonesian origin (cv. ‘Kemeh’) has been successfully established. A high number of embryos formed with high microspore density i.e. 15 × 104 cells/ml. Embryo formation was improved by using flower buds (4.5–4.6 mm in length) as explants, a temperature treatment at 30.5°C for 48 h and then transfer to 25°C continuously until embryos formed. A total of 295 embryos were obtained from 189 buds, 30% of which were abnormal (i.e. with an abnormal cotyledon or lacking hypocotyls). All normal embryos that grew and survived, 165 in total, were successfully transferred to soil and grew well in plastic bags (15 cm in diameter) containing a mixture of burned-rice husk and organic manure (1:1, v/v).





Similar content being viewed by others
References
Abraha E, Bechyn M, Klíma M, Vyvadilová M (2008) Analysis of factors affecting embryogenesis in microspore cultures of Brassica carinata. Agricultura Tropica et Subtropica 41(2):53–59
Agarwal PK, Agarwal P, Custers JBM, Liu CM, Bhojwani SS (2006) PCIB, an antiauxin, enhances microspore embryogenesis in microspore culture of Brassica juncea. Plant Cell Tissue Organ Cult 86:201–210
Ali MM, Mian MAK, Custers JBM, Khuram MMH (2008) Microspore culture and the performance of microspore derived doubled haploid in Brassica juncea (L.). Bangladesh J Agric Res 33(3):571–578
Anandarajah K, Kott L, Beversdorf WD, McKersie BD (1991) Induction of desiccation tolerance in microspore-derived embryos of Brassica napus L. by thermal stress. Plant Sci 77:119–123
Ashihara H, Luit B, Belmonte M, Stasolla C (2008) Metabolism of nicotinamide, adenine and inosine in developing microspore-derived canola (Brassica napus) embryos. Plant Physiol Biochem 46(8–9):752–759
Chanana NP, Dhawan V, Bhojwani SS (2005) Morphogenesis in isolated microspore cultures of Brassica juncea. Plant Cell Tissue Organ Cult 83:169–177
Chuong PV, Beversdorf WD (1985) High frequency embryogenesis through isolated microspore culture in Brassica napus L. and B. carinata Braun. Plant Sci 39:219–226
Coventry J, Kott L, Beversdorf WD (1988) Manual for microspore culture technique for Brassica napus. University of Guelph, Guelph
Custers JBM (2003) Microspore culture in rapeseed (Brassica napus L.). In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants: a manual. Kluwer Academic Publishers, Dordrecht, Boston, London, pp 185–194
Dias JS (2001) Effect of incubation temperature regimes and culture medium on broccoli microspore culture embryogenesis. Euphytica 199:389–394
Dias JS, Correia MC (2002) Effect of medium renovation and incubation temperature regimes on tronchuda cabbage microspore culture embryogenesis. Sci Hort 93(3–4):205–214
Elhiti M, Tahir M, Gulden RH, Khamiss K, Stasolla C (2010) Modulation of embryo-forming capacity in culture through the expression of Brassica genes involved in the regulation of the shoot apical meristem. J Exp Bot 61(14):4069–4085
Ferrie AMR, Caswell KL (2010) Isolated microspore culture techniques and recent progress for haploid and doubled haploid plant production. Plant Cell Tissue Organ Cult 104(3):301–309
Ferrie AMR, Möller C (2010) Haploids and doubled haploids in Brassica spp. for genetic and genomic research. Plant Cell Tissue Organ Cult 104(3):375–386
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Guo YD, Pulli S (1996) High-frequency embryogenesis in Brassica campestris microspore culture. Plant Cell Tissue Organ Cult 46:219–225
Hadfi K, Speth V, Neuhaus G (1998) Auxin-induced developmental patterns in Brassica juncea embryos. Development 125:879–887
Huang B, Bird S, Kemble R, Simmonds D, Keller WA, Miki B (1990) Effects of culture density, conditioned medium and feeder cultures on microspore embryogenesis in Brassica napus. Plant Cell Rep 8:594–597
Huang B, Bird S, Kemble R, Miki B, Keller W (1991) Plant regeneration from microspore-derived embryos of Brassica napus: effect of embryo age, culture temperature, osmotic pressure, and abscisic acid. In Vitro Cell Dev Biol Plant 27:28–31
Kott LS, Polsoni L, Ellis B, Beversdorf WD (1988) Autotoxicity in isolated microspore cultures of Brassica napus. Can J Bot 66:1665–1670
Lemonnier-Le Penhuizic C, Chatelet C, Kloareg B, Potin P (2001) Carrageenan oligosaccharides enhance stress-induced microspore embryogenesis in Brassica oleracea var italica. Plant Sci 160(6):1211–1220
Lichter R (1982) Induction of haploid plants from isolated pollen of Brassica napus. Z Planzenphysiol 105:427–433
Pechan PM, Keller WA (1988) Identification of potentially embryogenic microspores in Brassica napus. Physiol Plant 74:377–384
Prem D, Gupta K, Agnihotri A (2005) Effect of various endogenous and exogenous factors on microspore embryogenesis in Indian mustard (Brassica juncea (L.) Cern and Coss). In Vitro Cell Dev Biol Plant 41:266–273
Prem D, Gupta K, Sarkar G, Agnihotri A (2008) Activated charcoal induced high frequency microspore embryogenesis and efficient doubled haploid production in Brassica juncea. Plant Cell Tissue Organ Cult 93:269–282
Shi Y, Xu G, Warrington TB, Murdoch GK, Kazala EC, Snyder CL, Weselake RJ (2008) Microspore-derived cell suspension cultures of oilseed rape as a system for studying gene expression. Plant Cell Tissue Organ Cult 92:131–139
Smýkalová I, Větrovcová M, Klíma M, Macháčková I, Griga M (2006) Efficiency of microspore culture for doubled haploid production in the breeding project “Czech Winter Rape”. Czech J Genet Plant Breed 42(2):58–71
Supena EDJ, Suharsono S, Jacobsen E, Custers JBM (2006) Successful development of a shed-microspore culture protocol for doubled haploid production in Indonesian hot pepper (Capsicum annuum L.). Plant Cell Rep 25:1–10
Supena EDJ, Winarto B, Riksen T, Dubas E, van Lammeren A, Offringa R, Boutilier K, Custers JBM (2008) Regeneration of zygotic-like microspore-derived embryos suggests an important role for the suspensor in early embryo patterning. J Exp Bot 59(4):803–814
Takahata Y, Keller WA (1991) High frequency embryogenesis and plant regeneration in isolated microspore culture of Brassica oleracea L. Plant Sci 74(2):235–242
Takahata Y, Takani Y, Kaizuma N (1993) Determination of microspore population to obtain high frequency embryogenesis in broccoli (Brassica oleracea L.). Plant Tissue Cult Lett 10(1):49–53
Takahira J, Cousin A, Nelson MN, Cowling WA (2010) Improvement in efficiency of microspore culture to produce doubled haploid canola (Brassica napus L.) by flow cytometry. Plant Cell Tissue Organ Cult (Published Online 30 July 2010)
Taylor DC, Weber N, Underhill EW, Pomeroy MK, Keller WA, Scowcroft WR, Wilen RW, Moloney MM, Holbrook LA (1990) Storage protein regulation and lipid accumulation in microspore embryos of Brassica napus L. Planta 181:18–25
Telmer CA, Simmonds DH, Newcomb W (1992) Determination of developmental stage to obtain high frequencies of embryogenic microspores in Brassica napus. Physiol Plant 84:417–424
Tian H, Yao CY, Sun MX (2004) High frequency conversion of microspore-derived embryos of Brassica napus cv. Topas by supplemental calcium and vitamins. Plant Cell Tissue Organ Cult 76:159–165
Touraev A, Vincente O, Heberle-Bors E (1997) Initiation of microspore embryogenesis by stress. Trends Plant Sci 2:297–302
Wan GL, Naeem MS, Geng XX, Xu L, Li B, Jilani G, Zhou WJ (2011) Optimization of microspore embryogenesis and plant regeneration protocols for Brassica napus. Int J Agric Biol 13:83–88
Wang TT, Li HX, Zhang J, Ouyang B, Lu Y, Ye Z (2009) Initiation and development of microspore embryogenesis in recalcitrant purple flowering stalk (Brassica campestris ssp. chinensis var. purpurea Hort) genotypes. Sci Hort 121(4):419–424
Westfall PH, Tobias RD, Rom D, Wolfinger RD, Hochberg Y (1999) Multiple comparisons and multiple tests: using the SAS system. SAS Publishing, SAS Institute Inc, Cary, NC
Xu L, Najeeb U, Tang GX, Gu HH, Zhang GQ, He Y, Zhou WJ (2007) Haploid and doubled haploid technology. In: Gupta SK, Delseny M, Kader JC (eds) Rapeseed breeding. Adv Bot Res 45:181–216
Yeung EC (1995) Structural and developmental patterns in somatic embryogenesis. In: Thorpe TA (ed) In vitro embryogenesis in plants. Kluwer, Dordrecht, The Netherlands, pp 205–247
Yeung EC (2002) The canola microspore-derived embryo as a model system to study developmental process in plants. J Plant Biol 45(3):119–133
Zaki M, Dickinson H (1995) Modification of cell development in vitro: the effect of colchicine on anther and isolated microspore culture in Brassica napus. Plant Cell Tissue Organ Cult 40:255–270
Zhang W, Fu Q, Dai X, Bao M (2008) The culture of isolated microspores of ornamental kale (Brassica oleracea var. acephala) and the importance of genotype to embryo regeneration. Sci Hort 117(1):69–72
Acknowledgments
Budi Winarto would like to express his gratitude to Dr. Jan B. M. Custers and his assistant Tjitske Rikson for their invaluable guidance and encouragement during his training on Brassica microspore culture in conjunction with transfer of haploid technology to Indonesia at Plant Research International, Wageningen, The Netherlands. Dr. Winarto is also thankful to the Agency of The Dutch Ministry of Economic Affairs and International Agricultural Centre (IAC), Wageningen, The Netherlands for financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Winarto, B., Teixeira da Silva, J.A. Microspore culture protocol for Indonesian Brassica oleracea . Plant Cell Tiss Organ Cult 107, 305–315 (2011). https://doi.org/10.1007/s11240-011-9981-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-011-9981-z