Abstract
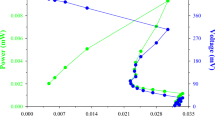
An efficient biosurfactant-producing native Pseudomonas aeruginosa RS29 has been isolated from crude oil contaminated soil. Isolation was followed by optimization of different factors to achieve maximum production of biosurfactant in terms of surface tension reduction (STR) and emulsification index (E24). The isolated strain produced highest biosurfactant in the presence of glycerol after 48 h of incubation at 37.5°C, with pH range of 7–8 and at salinity <0.8% (w/v). The extent of STR and the E24 of medium with different nitrogen sources were investigated and found to be maximal for sodium nitrate (26.3 mN/m, E24 = 80%) and potassium nitrate (26.4 mN/m, E24 = 79%). The production of biomass by the designated strain was found to be maximal in ammonium-nitrate-containing medium as compared to the other nitrogen sources. A kinetic study revealed that biosurfactant production is positively correlated with growth of P. aeruginosa, and highest STR was achieved (27.0 mN/m) after 44 h of growth. The biosurfactant was produced as a primary metabolite and 6 g/L crude biosurfactant was extracted by chloroform:methanol (2:1). The critical micelle concentration of the biosurfactant was 90 mg/L. The absorption bands of the FTIR spectra confirmed the rhamnolipid nature of the biosurfactant. The biosurfactant was thermostable (up to 121°C for 15 min) and could withstand a wide range of pH (2–10) and NaCl concentration (2%–10% w/v). The extracted biosurfactant had good foaming and emulsifying activities and was of satisfactory quality in terms of stability (temperature, pH and salinity) and foaming activity.








Similar content being viewed by others
References
Abdel-Mawgoud AM, Aboulwafa MM, Hassouna NA (2009) Characterization of Rhamnolipid Produced by Pseudomonas aeruginosa Isolate Bs20. Appl Biochem Biotechnol 157:329–345
Abouseoud M, Maachi R, Amrane A, Boudergua S, Nabi A (2008a) Evaluation of different carbon and nitrogen sources in production of biosurfactant by Pseudomonas fluorescens. Desalination 223:143–151
Abouseoud M, Yataghene A, Amrane A, Maachi R (2008b) Biosurfactant production by free and alginate entrapped cells of Pseudomonas fluorescens. J Ind Microbiol Biotechnol 35:1303–1308
Al-Araji Y, Issa L (2004) Biosurfactant production by Pseudomonas aeruginosa 181. PhD thesis, University Putra Malaysia
Babu PS, Vaidya AN, Bal AS, Kapur R, Juwarkar A, Khanna P (1996) Kinetics of biosurfactant production by Pseudomonas aeruginosa strain BS2 from industrial waste. Biotechnol Lett 18:263–268
Banat IM (1995) Biosurfactant production and possible uses in microbial enhanced oil recovery and oil pollution remediation: review. Bioresour Technol 55:1–12
Banat IM, Makkar RS, Cameotra SS (2000) Potential commercial applications of microbial surfactants. Appl Environ Microbiol 53:495–508
Bonilla M, Olivaro C, Corona M, Vazquez A, Soubes M (2005) Production and characterization of a new bioemulsifier from Pseudomonas putida ML2. J Appl Microbiol 98:456–463
Bordoloi NK, Konwar BK (2008) Microbial surfactant-enhanced mineral oil recovery under laboratory conditions. Colloids Surf B Biointerfaces 63:73–82
Bordoloi NK, Konwar BK (2009) Bacterial biosurfactant in enhancing solubility and metabolism of petroleum hydrocarbons. J Hazard Mater 170:495–505
Cameotra SS, Makkar RS (2004) Recent applications of biosurfactants as biological and immunological molecules. Curr Opin Microbiol 7:262–266
Cappuccino JG, Sherman N (1999) Microbiology—a laboratory manual. Addison-Wesley Longman, Harlow, pp 417–421
Cooper DG (1986) Biosurfactants. Microbiol Sci 3:145–149
Cooper DG, Goldenberg BG (1987) Surface-active agents from two Bacillus species. Appl Environ Microbiol 53(2):224–229
Cooper DG, Zajic JE (1980) Surface-active compounds from microorganism. Adv Appl Microbiol 26:229–256
Das K, Mukherjee AK (2005) Characterization of biochemical properties and biological activities of biosurfactants produced by Pseudomonas aeruginosa mucoid and non-mucoid strains isolated from hydrocarbon-contaminated soil samples. Appl Microbiol Biotechnol 69:192–199
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol R 61(1):47–64
Deziel E, Lepine F, Dennie D, Boismenu D, Mamer OA, Villemur R (1999) Liquid chromatography/mass spectrometry analysis of mixture of rhamnolipids produced by P.aeruginosa strain 57RP grown on mannitol or naphthalene. Biochim Biophys Acta 1440:244–252
Díaz de Villegas ME, Villa P, Frías A (2002) Evaluation of the siderophores production by Pseudomonas aeruginosa PSS. Rev Latinoam Microbiol 44:112–117
Dubois M, Gills KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugar and related substances. Anal Chem 28:350–356
Guerra-Santos L, Kappeli O, Fiechter A (1983) Growth and biosurfactant production of a bacterium in continuous culture. In: Donaldson EC, Clark JB (eds) International conference on microbial enhancement of oil recovery. US Department of Energy, Washington DC, pp 12–14
Guerra-Santos LH, Kappeli O, Fiechter A (1986) Dependence of Pseudomonas aeruginosa continuous culture biosurfactant production on nutritional and environmental factors. Appl Microbiol Biotechnol 24:443–448
Hisatsuka K, Nakahara T, Sano N, Yamada K (1971) Formation of rhamnolipid by Pseudomonas aeruginosa: its function in hydrocarbon fermentation. Agric Biol Chem 35:686–692
Itoh S, Suzuki T (1972) Effect of rhamnolipids on growth of Pseudomonas aeruginosa mutant deficient in n-paraffinutilizing ability. Agric Biol Chem 36:2233–2235
Jenny K, Deltrieu V, Kappelli O (1993) Lipopeptide production by Bacillus licheniformis. In: Kosaric N (ed) Biosurfactants. Production, property, application. Dekker, New York, pp 135–156
Karsa DR, Bailey RM, Shelmerdine B, McCann SA (1999) Overview: a decade of change in the surfactant industry. In: Karsa DR (ed) Industrial applications of surfactants, vol 4. Royal Society of Chemistry, London, pp 1–22
Kim H, Yoon B, Lee C, Suh H, Oh H, Katsuragi T, Tani Y (1997) Production and properties of a lipopeptide biosurfactant from Bacillus subtilis C9. J Ferment Bioeng 84(1):41–46
Kosaric N (1993) Biosurfactants. Production, property, application, surfactant sciences series, 48. Dekker, New York
Lang S, Wullbrandt D (1999) Rhamnose lipids, biosynthesis, microbial production and application potential. Appl Microbiol Biotechnol 50:22–32
Lin SC, Sharma MM, Georgiou G (1993) Production and deactivation of biosurfactant by Bacillus licheniformis JF-2. Biotechnol Prog 9(2):138–145
Mata-Sandoval JC, Karns J, Torrents A (1999) High performance liquid chromatography method for the characterization of rhamnolipid mixtures produces by Pseudomonas aeruginosa UG2 on corn oil. J Chromatogr A 864:211–220
Makkar RS, Cameotra SS (2002) Effects of Various Nutritional Supplements on Biosurfactant Production by a Strain of Bacillus subtilis at 45°C. J Surfactants Deterg 5:11–17
Meylheuc T, Heary JM, Bellon-Fontaine MN (2001) Les biosurfactants, des biomolécules à forte potentialité d’application. Sci Aliments 21:591–649
Nair A, Juwarkar AA, Singh SK (2007) Production and characterization of siderophores and its application in arsenic removal from contaminated soil. Water Air Soil Pollut 180:199–212
Noudeh GD, Noodeh AD, Moshafi MH, Behravan E, Afzadi MA, Sodagar M (2010) Investigation of cellular hydrophobicity and surface activity effects of biosynthesed biosurfactant from broth media of PTCC 1561. Afr J Microbiol Res 4(17):1814–1822
Patel RM, Desai AJ (1997) Surface-active properties of rhamnolipids from Pseudomonas aeruginosa GS3. J Basic Microbiol 37:281–286
Perfumo A, Banat IM, Canganella F, Marchant R (2006) Rhamnolipid production by a novel thermophilic hydrocarbon-degrading Pseudomonas aeruginosa AP02-1. Appl Microbiol Biotechnol 72(1):132–138
Persson A, Österberg E, Dostalek M (1988) Biosurfactant production by Pseudomonas Xuorescens 378: growth and product characteristics. Appl Microbiol Biotechnol 29(1):1–4
Pornsunthorntawee O, Arttaweeporna N, Paisanjit S, Somboonthanatea P, Abeb M, Rujiravanita R, Chavadeja S (2008) Isolation and comparison of biosurfactants produced by Bacillus subtilis PT2 and Pseudomonas aeruginosa SP4 for microbial surfactant-enhanced oil recovery. Biochem Eng J 42:172–179
Pruthi V, Cameotra SS (1995) Rapid method for monitoring maximum biosurfactant produced by acetone precipitation. Biotechnol Tech 9:271–276
Rahman KSM, Rahman TJ, McClean S, Marchant R, Banat IM (2002) Rhamnolipid biosurfactant production by strains of Pseudomonas aeruginosa using low-cost raw material. Biotechnol Prog 18:1277–1281
Ramana KV, Karanth NG (1989) Factors affecting biosurfactants production using Pseudomonas aeruginosa CFTR-6 under submerged conditions. J Chem Technol Biotechnol 45:249–257
Raza ZA, Khan MS, Khalid ZM (2007) Physicochemical and surface-active properties of biosurfactant produced using molasses by a Pseudomonas aeruginosa mutant. J Environ Sci Health A Tox Hazard Subst Environ Eng 42:73–80
Ron EZ, Rosenberg E (2001) Natural roles of biosurfactants. Environ Microbiol 3:229–236
Sheppard JD, Mulligan CN (1987) The production of surfactin by Bacillus subtilis grown on peat hydrolysate. Appl Microbiol Biotechnol 27:110–116
Suzuki T, Tanaka H, Itoh S (1974) Sucrose lipids of Arthrobacteria, Corynebacteria and Nocardia grown on sucrose. Agric Biol Chem 38:557–563
Wilson NG, Bradley G (1996) The eVect of immobilization on rhamnolipid production by Pseudomonas Xuorescens. J Appl Bacteriol 81(5):525–530
Varma A, Chincholkar S (eds) (2007) Microbial siderophores. Springer, New York,
Acknowledgments
We thank the Director, Prof. Joyanti Chutia, IASST, Guwahati, India for permission and support. Moreover, this work has been made possible through a project grant (BT/PR-9795/BCE/08/590/2007) funded by Department of Biotechnology, Govt of India. We thank to Dr. N. Sen Sarma, Senior Assistant Professor, IASST for facilitating the FTIR analysis at IASST, Guwahati, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saikia, R.R., Deka, S., Deka, M. et al. Isolation of biosurfactant-producing Pseudomonas aeruginosa RS29 from oil-contaminated soil and evaluation of different nitrogen sources in biosurfactant production. Ann Microbiol 62, 753–763 (2012). https://doi.org/10.1007/s13213-011-0315-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-011-0315-5