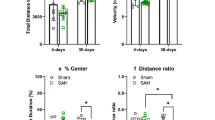
Abstract
Subarachnoid hemorrhage (SAH) is a severe form of stroke that can cause unpredictable and diffuse cerebral damage, which is difficult to detect until it becomes irreversible. Therefore, there is a need for a reliable method to identify dysfunctional regions and initiate treatment before permanent damage occurs. Neurobehavioral assessments have been suggested as a possible tool to detect and approximately localize dysfunctional cerebral regions. In this study, we hypothesized that a neurobehavioral assessment battery could be a sensitive and specific method for detecting damage in discrete cerebral regions following SAH. To test this hypothesis, a behavioral battery was employed at multiple time points after SAH induced via an endovascular perforation, and brain damage was confirmed via postmortem histopathological analysis. Our results demonstrate that impairment of sensorimotor function accurately predict damage in the cerebral cortex (AUC 0.905; sensitivity 81.8%; specificity 90.9%) and striatum (AUC 0.913; sensitivity 90.1%; specificity 100%), while impaired novel object recognition is a more accurate indicator of damage to the hippocampus (AUC 0.902; sensitivity 74.1%; specificity 83.3%) than impaired reference memory (AUC 0.746; sensitivity 72.2%; specificity 58.0%). Tests for anxiety-like and depression-like behaviors predict damage to the amygdala (AUC 0.900; sensitivity 77.0%; specificity 81.7%) and thalamus (AUC 0.963; sensitivity 86.3%; specificity 87.8%), respectively. This study suggests that recurring behavioral testing can accurately predict damage in specific brain regions, which could be developed into a clinical battery for early detection of SAH damage in humans, potentially improving early treatment and outcomes.









Similar content being viewed by others
Data Availability
The authors declare that all supporting data are available within this article.
References
Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. The Lancet. 2017;389(10069):655–66. https://doi.org/10.1016/s0140-6736(16)30668-7.
Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997;28(3):660–4. https://doi.org/10.1161/01.str.28.3.660.
Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41(8):e519–36. https://doi.org/10.1161/STROKEAHA.110.581975.
Gaastra B, Carmichael H, Galea I, Bulters D. Long-term fatigue following aneurysmal subarachnoid haemorrhage and the impact on employment. Eur J Neurol. 2022;29(12):3564–70. https://doi.org/10.1111/ene.15533.
Sanchez B, Delemos CD, Sandhu KS, et al. Aneurysmal subarachnoid hemorrhage survivors show long-term deficits in spatial reference memory in a pilot study of a virtual water maze paradigm. Clin Neurol Neurosurg. 2021;207:106788. https://doi.org/10.1016/j.clineuro.2021.106788.
Tang WK, Wang L, Kwok Chu Wong G, et al. Depression after subarachnoid hemorrhage: a systematic review. J Stroke. 2020;22(1):11–28. https://doi.org/10.5853/jos.2019.02103.
Wenneberg SB, Block L, Sorbo A, et al. Long-term outcomes after aneurysmal subarachnoid hemorrhage: a prospective observational cohort study. Acta Neurol Scand. 2022;146(5):525–36. https://doi.org/10.1111/ane.13674.
Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology. 1998;50(5):1413–8. https://doi.org/10.1212/wnl.50.5.1413.
De Marchis GM, Filippi CG, Guo X, et al. Brain injury visible on early MRI after subarachnoid hemorrhage might predict neurological impairment and functional outcome. Neurocrit Care. 2015;22(1):74–81. https://doi.org/10.1007/s12028-014-0008-6.
Stienen MN, Germans MR, Zindel-Geisseler O, et al. Longitudinal neuropsychological assessment after aneurysmal subarachnoid hemorrhage and its relationship with delayed cerebral ischemia: a prospective Swiss multicenter study. J Neurosurg. 2022;137(6):1–9. https://doi.org/10.3171/2022.2.JNS212595.
Dayyani M, Sadeghirad B, Grotta JC, et al. Prophylactic therapies for morbidity and mortality after aneurysmal subarachnoid hemorrhage: a systematic review and network meta-analysis of randomized trials. Stroke. 2022;53(6):1993–2005. https://doi.org/10.1161/STROKEAHA.121.035699.
Etminan N, Vergouwen MD, Ilodigwe D, Macdonald RL. Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab. 2011;31(6):1443–51. https://doi.org/10.1038/jcbfm.2011.7.
Oka F, Chung DY, Suzuki M, Ayata C. Delayed cerebral ischemia after subarachnoid hemorrhage: experimental-clinical disconnect and the unmet need. Neurocrit Care. 2020;32(1):238–51. https://doi.org/10.1007/s12028-018-0650-5.
Dodd WS, Laurent D, Dumont AS, et al. Pathophysiology of delayed cerebral ischemia after subarachnoid hemorrhage: a review. J Am Heart Assoc. 2021;10(15):e021845. https://doi.org/10.1161/JAHA.121.021845.
Terpolilli NA, Brem C, Buhler D, Plesnila N. Are we barking up the wrong vessels? Cerebral microcirculation after subarachnoid hemorrhage. Stroke. Oct2015;46(10):3014–9. https://doi.org/10.1161/STROKEAHA.115.006353.
Dreier JP, Woitzik J, Fabricius M, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129(Pt 12):3224–37. https://doi.org/10.1093/brain/awl297.
Helbok R, Madineni RC, Schmidt MJ, et al. Intracerebral monitoring of silent infarcts after subarachnoid hemorrhage. Neurocrit Care. 2011;14(2):162–7. https://doi.org/10.1007/s12028-010-9472-9.
Westermaier T, Pham M, Stetter C, et al. Value of transcranial Doppler, perfusion-CT and neurological evaluation to forecast secondary ischemia after aneurysmal SAH. Neurocrit Care. 2014;20(3):406–12. https://doi.org/10.1007/s12028-013-9896-0.
Shimamura N, Fumoto T, Naraoka M, et al. Irreversible neuronal damage begins just after aneurysm rupture in poor-grade subarachnoid hemorrhage patients. Transl Stroke Res. 2021;12(5):785–90. https://doi.org/10.1007/s12975-020-00875-0.
Jeon H, Ai J, Sabri M, Tariq A, Macdonald RL. Learning deficits after experimental subarachnoid hemorrhage in rats. Neuroscience. 2010;169(4):1805–14. https://doi.org/10.1016/j.neuroscience.2010.06.039.
Turan N, Heider RA, Nadeem M, et al. Neurocognitive outcomes in a cisternal blood injection murine model of subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2020;29(11):105249. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105249.
Buhler D, Schuller K, Plesnila N. Protocol for the induction of subarachnoid hemorrhage in mice by perforation of the Circle of Willis with an endovascular filament. Transl Stroke Res. 2014;5(6):653–9. https://doi.org/10.1007/s12975-014-0366-6.
Shah KA, White TG, Powell K, Woo HH, Narayan RK, Li C. Trigeminal nerve stimulation improves cerebral macrocirculation and microcirculation after subarachnoid hemorrhage: an exploratory study. Neurosurgery. 2022;90(4):485–94. https://doi.org/10.1227/NEU.0000000000001854.
Papp EA, Leergaard TB, Calabrese E, Johnson GA, Bjaalie JG. Waxholm Space atlas of the Sprague Dawley rat brain. Neuroimage. 2014;97:374–86. https://doi.org/10.1016/j.neuroimage.2014.04.001.
Garman RH. Histology of the central nervous system. Toxicol Pathol. 2011;39(1):22–35. https://doi.org/10.1177/0192623310389621.
Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36(4):557–65. https://doi.org/10.1002/ana.410360404.
Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11(5):491–8. https://doi.org/10.1002/ana.410110509.
Baron JC, Yamauchi H, Fujioka M, Endres M. Selective neuronal loss in ischemic stroke and cerebrovascular disease. J Cereb Blood Flow Metab. 2014;34(1):2–18. https://doi.org/10.1038/jcbfm.2013.188.
Goursaud S, Martinez de Lizarrondo S, Grolleau F, et al. Delayed cerebral ischemia after subarachnoid hemorrhage: is there a relevant experimental model? a systematic review of preclinical literature. Front Cardiovasc Med. 2021;8:752769. https://doi.org/10.3389/fcvm.2021.752769.
Neifert SN, Chapman EK, Martini ML, et al. Aneurysmal subarachnoid hemorrhage: the last decade. Transl Stroke Res. 2021;12(3):428–46. https://doi.org/10.1007/s12975-020-00867-0.
Thompson JC, Chalet FX, Manalastas EJ, Hawkins N, Sarri G, Talbot DA. Economic and humanistic burden of cerebral vasospasm and its related complications after aneurysmal subarachnoid hemorrhage: a systematic literature review. Neurol Ther. 2022;11(2):597–620. https://doi.org/10.1007/s40120-022-00348-6.
Kerz T, Boor S, Ulrich A, Beyer C, Hechtner M, Mueller-Forell W. Endovascular therapy for vasospasm after aneurysmatic subarachnoid hemorrhage. Br J Neurosurg. 2016;30(5):549–53. https://doi.org/10.3109/02688697.2016.1173193.
Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10(1):44–58. https://doi.org/10.1038/nrneurol.2013.246.
Boyko M, Ohayon S, Goldsmith T, et al. Morphological and neuro-behavioral parallels in the rat model of stroke. Behav Brain Res. 2011;223(1):17–23. https://doi.org/10.1016/j.bbr.2011.03.019.
Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov Disord. 2013;28(11):1483–91. https://doi.org/10.1002/mds.25669.
Xu G, Guo J, Sun C. Eucalyptol ameliorates early brain injury after subarachnoid haemorrhage via antioxidant and anti-inflammatory effects in a rat model. Pharm Biol. 2021;59(1):114–20. https://doi.org/10.1080/13880209.2021.1876101.
Kooijman E, Nijboer CH, van Velthoven CT, et al. Long-term functional consequences and ongoing cerebral inflammation after subarachnoid hemorrhage in the rat. PLoS One. 2014;9(6):e90584. https://doi.org/10.1371/journal.pone.0090584.
Dienel A, Ammassam Veettil R, Hong SH, et al. Microthrombi correlates with infarction and delayed neurological deficits after subarachnoid hemorrhage in mice. Stroke. 2020;51(7):2249–54. https://doi.org/10.1161/STROKEAHA.120.029753.
Nishimura N, Schaffer CB. Big effects from tiny vessels: imaging the impact of microvascular clots and hemorrhages on the brain. Stroke. 2013;44(6 Suppl 1):S90–2. https://doi.org/10.1161/STROKEAHA.112.679621.
Larimer P, Strowbridge BW. Nonrandom local circuits in the dentate gyrus. J Neurosci. 2008;28(47):12212–23. https://doi.org/10.1523/JNEUROSCI.3612-08.2008.
Ramos C, Lutzu S, Yamasaki M, et al. Activation of extrasynaptic kainate receptors drives hilar mossy cell activity. J Neurosci. 2022;42(14):2872–84. https://doi.org/10.1523/JNEUROSCI.0922-21.2022.
Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3(3):238–44. https://doi.org/10.1038/72945.
Jessberger S, Clark RE, Broadbent NJ, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16(2):147–54. https://doi.org/10.1101/lm.1172609.
Han SM, Wan H, Kudo G, et al. Molecular alterations in the hippocampus after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2014;34(1):108–17. https://doi.org/10.1038/jcbfm.2013.170.
Puglisi CH, Ander BP, Peterson C, et al. Sustained ICP Elevation is a driver of spatial memory deficits after intraventricular hemorrhage and leads to activation of distinct microglial signaling pathways. Transl Stroke Res. 2023;14(4):572–88. https://doi.org/10.1007/s12975-022-01061-0.
Tariq A, Ai J, Chen G, et al. Loss of long-term potentiation in the hippocampus after experimental subarachnoid hemorrhage in rats. Neuroscience. 2010;165(2):418–26. https://doi.org/10.1016/j.neuroscience.2009.10.040.
Leon-Rodriguez A, Fernandez-Arjona MDM, Grondona JM, Pedraza C, Lopez-Avalos MD. Anxiety-like behavior and microglial activation in the amygdala after acute neuroinflammation induced by microbial neuraminidase. Sci Rep. 2022;12(1):11581. https://doi.org/10.1038/s41598-022-15617-5.
Torres ERS, Stanojlovic M, Zelikowsky M, et al. Alpha-synuclein pathology, microgliosis, and parvalbumin neuron loss in the amygdala associated with enhanced fear in the Thy1-aSyn model of Parkinson’s disease. Neurobiol Dis. 2021;158:105478. https://doi.org/10.1016/j.nbd.2021.105478.
Song JH, Jia HY, Shao TP, Liu ZB, Zhao YP. Hydrogen gas post-conditioning alleviates cognitive dysfunction and anxiety-like behavior in a rat model of subarachnoid hemorrhage. Exp Ther Med. 2021;22(4):1121. https://doi.org/10.3892/etm.2021.10555.
Sasaki T, Hoffmann U, Kobayashi M, et al. Long-term cognitive deficits after subarachnoid hemorrhage in rats. Neurocrit Care. 2016;25(2):293–305. https://doi.org/10.1007/s12028-016-0250-1.
Ma L, Jiang Y, Dong Y, Gao J, Du B, Liu D. Anti-TNF-alpha antibody attenuates subarachnoid hemorrhage-induced apoptosis in the hypothalamus by inhibiting the activation of Erk. Neuropsychiatr Dis Treat. 2018;14:525–36. https://doi.org/10.2147/NDT.S154809.
Morris PG, Wilson JT, Dunn L. Anxiety and depression after spontaneous subarachnoid hemorrhage. Neurosurgery. 2004;54(1):47–54. https://doi.org/10.1227/01.neu.0000097198.94828.e1.
Chen F, Kang Y, Yu T, et al. Altered functional connectivity within default mode network after rupture of anterior communicating artery aneurysm. Front Aging Neurosci. 2022;14:905453. https://doi.org/10.3389/fnagi.2022.905453.
Al Yassin A, Ouyang B, Temes R. Depression and anxiety following aneurysmal subarachnoid hemorrhage are associated with higher six-month unemployment rates. J Neuropsychiatry Clin Neurosci Winter. 2017;29(1):67–9. https://doi.org/10.1176/appi.neuropsych.15070171.
Peng X, Wu X, Gong R, et al. Sub-regional anterior cingulate cortex functional connectivity revealed default network subsystem dysfunction in patients with major depressive disorder. Psychol Med. 2021;51(10):1687–95. https://doi.org/10.1017/S0033291720000434.
Inan SY, Soner BC, Sahin AS. Infralimbic cortex Rho-kinase inhibition causes antidepressant-like activity in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2015;57:36–43. https://doi.org/10.1016/j.pnpbp.2014.10.008.
Zhu Y, Qi S, Zhang B, et al. Connectome-Based biomarkers predict subclinical depression and identify abnormal brain connections with the lateral habenula and thalamus. Front Psychiatry. 2019;10:371. https://doi.org/10.3389/fpsyt.2019.00371.
Wang D, Xue SW, Tan Z, Wang Y, Lian Z, Sun Y. Altered hypothalamic functional connectivity patterns in major depressive disorder. NeuroReport. 2019;30(16):1115–20. https://doi.org/10.1097/WNR.0000000000001335.
Nanegrungsunk D, Ragozzino ME, Xu HL, Haselton KJ, Paisansathan C. Subarachnoid hemorrhage in C57BL/6J mice increases motor stereotypies and compulsive-like behaviors. Neurol Res. 2021;43(3):239–51. https://doi.org/10.1080/01616412.2020.1841481.
Weinstein S, Obuchowski NA, Lieber ML. Clinical evaluation of diagnostic tests. AJR Am J Roentgenol. 2005;184(1):14–9. https://doi.org/10.2214/ajr.184.1.01840014.
Armario A. The forced swim test: Historical, conceptual and methodological considerations and its relationship with individual behavioral traits. Neurosci Biobehav Rev. 2021;128:74–86. https://doi.org/10.1016/j.neubiorev.2021.06.014.
Kremer M, Becker LJ, Barrot M, Yalcin I. How to study anxiety and depression in rodent models of chronic pain? Eur J Neurosci. 2021;53(1):236–70. https://doi.org/10.1111/ejn.14686.
Rowland MJ, Garry P, Westbrook J, Corkill R, Antoniades CA, Pattinson KTS. Acute impairment of saccadic eye movements is associated with delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2017;127(4):754–60. https://doi.org/10.3171/2016.8.JNS16408.
Gonzalez NR, Boscardin WJ, Glenn T, Vinuela F, Martin NA. Vasospasm probability index: a combination of transcranial doppler velocities, cerebral blood flow, and clinical risk factors to predict cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007;107(6):1101–12. https://doi.org/10.3171/JNS-07/12/1101.
Rehman S, Sahle BW, Chandra RV, et al. Sex differences in risk factors for aneurysmal subarachnoid haemorrhage: Systematic review and meta-analysis. J Neurol Sci. 2019;406:116446. https://doi.org/10.1016/j.jns.2019.116446.
Cai Y, Liu Z, Jia C, et al. Comparison of sex differences in outcomes of patients with aneurysmal subarachnoid hemorrhage: a single-center retrospective study. Front Neurol. 2022;13:853513. https://doi.org/10.3389/fneur.2022.853513.
Turan N, Heider RA, Zaharieva D, Ahmad FU, Barrow DL, Pradilla G. Sex differences in the formation of intracranial aneurysms and incidence and outcome of subarachnoid hemorrhage: review of experimental and human studies. Transl Stroke Res. 2016;7(1):12–9. https://doi.org/10.1007/s12975-015-0434-6.
Felbor U, Kessler B, Mothes W, et al. Neuronal loss and brain atrophy in mice lacking cathepsins B and L. Proc Natl Acad Sci U S A. 2002;99(12):7883–8. https://doi.org/10.1073/pnas.112632299.
Zhang C, Brandon NR, Koper K, Tang P, Xu Y, Dou H. Invasion of peripheral immune cells into brain parenchyma after cardiac arrest and resuscitation. Aging Dis. 2018;9(3):412–25. https://doi.org/10.14336/AD.2017.0926.
Yin L, Yu T, Cheng L, et al. Laser speckle contrast imaging for blood flow monitoring in predicting outcomes after cerebral ischemia-reperfusion injury in mice. BMC Neurosci. 2022;23(1):80. https://doi.org/10.1186/s12868-022-00769-x.
Markov DD. Sucrose preference test as a measure of anhedonic behavior in a chronic unpredictable mild stress model of depression: outstanding issues. Brain Sci. 2022;12(10):1287. https://doi.org/10.3390/brainsci12101287.
Funding
This work is supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R21NS114763 and the US Army Medical Research and Materiel Command (USAMRMC) under award number W81XWH-18–1-0773.
Author information
Authors and Affiliations
Contributions
Daniel G. Lynch, Kevin A. Shah, and Keren Powell interpreted data and wrote the manuscript. Steven Wadolowski, Willians Tambo Ayol, and Prashin Unadkat acquired and analysed data. Joshua J. Strohl and Patricio T. Huerta performed the statistical analysis. David Eidelberg and Patricio T. Huerta critically revised the manuscript. Chunyan Li conceived and designed experiments, acquired and analyzed data, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Daniel G. Lynch and Kevin A. Shah are co first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lynch, D.G., Shah, K.A., Powell, K. et al. Neurobehavioral Impairments Predict Specific Cerebral Damage in Rat Model of Subarachnoid Hemorrhage. Transl. Stroke Res. (2023). https://doi.org/10.1007/s12975-023-01180-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12975-023-01180-2