Abstract
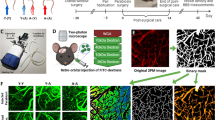
The mechanisms of cognitive decline after intraventricular hemorrhage (IVH) in some patients continue to be poorly understood. Multiple rodent models of intraventricular or subarachnoid hemorrhage have only shown mild or even no cognitive impairment on subsequent behavioral testing. In this study, we show that intraventricular hemorrhage only leads to a significant spatial memory deficit in the Morris water maze if it occurs in the setting of an elevated intracranial pressure (ICP). Histopathological analysis of these IVH + ICP animals did not show evidence of neuronal degeneration in the hippocampal formation after 2 weeks but instead showed significant microglial activation measured by lacunarity and fractal dimensions. RNA sequencing of the hippocampus showed distinct enrichment of genes in the IVH + ICP group but not in IVH alone having activated microglial signaling pathways. The most significantly activated signaling pathway was the classical complement pathway, which is used by microglia to remove synapses, followed by activation of the Fc receptor and DAP12 pathways. Thus, our study lays the groundwork for identifying signaling pathways that could be targeted to ameliorate behavioral deficits after IVH.








Similar content being viewed by others
Data Availability
The datasets generated during the RNAseq analysis are available in Online Resource 4a-e and archived in the Zenodo repository (https://doi.org/10.5281/zenodo.6395261).
References
Wiggins WS, Moody DM, Toole JF, Laster DW, Ball MR. Clinical and computerized tomographic study of hypertensive intracerebral hemorrhage. Arch Neurol. 1978;35:832–3.
Angelopoulos M, Gupta SR, Azat Kia B. Primary intraventricular hemorrhage in adults: clinical features, risk factors, and outcome. Surg Neurol. 1995;44:433–6 (discussion 437).
Steiner T, Diringer MN, Schneider D, Mayer SA, Begtrup K, Broderick J, et al. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery. 2006;59:767–73 (discussion 773-4).
Bu Y, Chen M, Gao T, Wang X, Li X, Gao F. Mechanisms of hydrocephalus after intraventricular haemorrhage in adults. Stroke Vasc Neurol. 2016;1:23–7.
Gaberel T, Magheru C, Emery E. Management of non-traumatic intraventricular hemorrhage. Neurosurg Rev. 2012;35:485–94 (discussion 494-5).
Dodel R, Winter Y, Ringel F, Spottke A, Gharevi N, Müller I, et al. Cost of illness in subarachnoid hemorrhage: a German longitudinal study. Stroke. 2010;41:2918–23.
Kreiter KT, Copeland D, Bernardini GL, Bates JE, Peery S, Claassen J, et al. Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke Am Heart Assoc. 2002;33:200–9.
Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41:e519–36.
Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 1999;27:617–21.
Garton T, Hua Y, Xiang J, Xi G, Keep RF. Challenges for intraventricular hemorrhage research and emerging therapeutic targets. Expert Opin Ther Targets. 2017;21:1111–22.
Mracsko E, Veltkamp R. Neuroinflammation after intracerebral hemorrhage. Front Cell Neurosci. 2014;8:388.
Kamal K, Keiter JA, Binyamin TR, de la Cruz Dapula JN, Vergara AR, Hawk CW, et al. Mechanical injury and blood are drivers of spatial memory deficits after rapid intraventricular hemorrhage. Neurobiol Dis. 2020;145:105084.
Peterson C, Umoye AO, Puglisi CH, Waldau B. Mechanisms of memory impairment in animal models of nontraumatic intracranial hemorrhage: a systematic review of the literature. Brain Hemorrhages [Internet]. 2021; Available from: https://www.sciencedirect.com/science/article/pii/S2589238X21000383
Milner E, Holtzman JC, Friess S, Hartman RE, Brody DL, Han BH, et al. Endovascular perforation subarachnoid hemorrhage fails to cause Morris water maze deficits in the mouse. J Cereb Blood Flow Metab [Internet]. 2014;34. Available from: https://doi.org/10.1038/jcbfm.2014.108
Regnier-Golanov AS, Gulinello M, Hernandez MS, Golanov EV, Britz GW. Subarachnoid hemorrhage induces sub-acute and early chronic impairment in learning and memory in mice. Transl Stroke Res [Internet]. 2022; Available from: https://doi.org/10.1007/s12975-022-00987-9
Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS ONE. 2009;4:e5464.
Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21.
Liao Y, Wang J, Jaehnig EJ, Shi Z, Zhang B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019;47:W199-205.
Noh J-E, Oh S-H, Park I-H, Song J. Intracerebral transplants of GMP-grade human umbilical cord-derived mesenchymal stromal cells effectively treat subacute-phase ischemic stroke in a rodent model. Front Cell Neurosci. 2020;14:546659.
Yousef H, Czupalla CJ, Lee D, Chen MB, Burke AN, Zera KA, et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat Med. 2019;25:988–1000.
Wang C, Yue H, Hu Z, Shen Y, Ma J, Li J, et al. Microglia mediate forgetting via complement-dependent synaptic elimination. Science. 2020;367:688–94.
Hwang IK, Park JH, Lee T-K, Kim DW, Yoo K-Y, Ahn JH, et al. CD74-immunoreactive activated M1 microglia are shown late in the gerbil hippocampal CA1 region following transient cerebral ischemia. Mol Med Rep. 2017;15:4148–54.
He J-H, Liu R-P, Peng Y-M, Guo Q, Zhu L-B, Lian Y-Z, et al. Differential and paradoxical roles of new-generation antidepressants in primary astrocytic inflammation. J Neuroinflammation. 2021;18:47.
Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al. SciPy 10: fundamental algorithms for scientific computing in Python. Nat Methods. 2020;17:261–72.
Karperien A, Ahammer H, Jelinek HF. Quantitating the subtleties of microglial morphology with fractal analysis. Front Cell Neurosci. 2013;7:3.
Jurga AM, Paleczna M, Kuter KZ. Overview of general and discriminating markers of differential microglia phenotypes. Front Cell Neurosci. 2020;14:198.
Sanchez B, Delemos CD, Sandhu KS, Peterson C, Cord BJ, Gurkoff GG, et al. Aneurysmal subarachnoid hemorrhage survivors show long-term deficits in spatial reference memory in a pilot study of a virtual water maze paradigm. Clin Neurol Neurosurg. 2021;207:106788.
Mangano FT, McAllister JP 2nd, Jones HC, Johnson MJ, Kriebel RM. The microglial response to progressive hydrocephalus in a model of inherited aqueductal stenosis. Neurol Res. 1998;20:697–704.
Wu K-Y, Tang F-L, Lee D, Zhao Y, Song H, Zhu X-J, et al. Ependymal Vps35 promotes ependymal cell differentiation and survival, suppresses microglial activation, and prevents neonatal hydrocephalus. J Neurosci Soc Neurosci. 2020;40:3862–79.
Fernández-Arjona MDM, León-Rodríguez A, López-Ávalos MD, Grondona JM. Microglia activated by microbial neuraminidase contributes to ependymal cell death. Fluids Barriers CNS. 2021;18:15.
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–78.
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705.
ElAli A, Rivest S. Microglia ontology and signaling. Front Cell Dev Biol. 2016;4:72.
Daëron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–34.
Lin Y, Pan Y, Wang M, Huang X, Yin Y, Wang Y, et al. Blood-brain barrier permeability is positively correlated with cerebral microvascular perfusion in the early fluid percussion-injured brain of the rat. Lab Invest. 2012;92:1623–34.
Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. 2003;3:445–53.
Fu R, Shen Q, Xu P, Luo JJ, Tang Y. Phagocytosis of microglia in the central nervous system diseases. Mol Neurobiol. 2014;49:1422–34.
Jairaman A, McQuade A, Granzotto A, Kang YJ, Chadarevian JP, Gandhi S, et al. TREM2 regulates purinergic receptor-mediated calcium signaling and motility in human iPSC-derived microglia. Elife [Internet]. 2022;11. Available from: https://doi.org/10.7554/eLife.73021
Buerke M, Prufer D, Dahm M, Oelert H, Meyer J, Darius H. Blocking of classical complement pathway inhibits endothelial adhesion molecule expression and preserves ischemic myocardium from reperfusion injury1. JPET. 1998;286:429–38.
D’Ambrosio AL, Pinsky DJ, Connolly ES. The role of the complement cascade in ischemia/reperfusion injury: implications for neuroprotection. Mol Med. 2001;7:367–82.
Ducruet AF, Zacharia BE, Sosunov SA, Gigante PR, Yeh ML, Gorski JW, et al. Complement inhibition promotes endogenous neurogenesis and sustained anti-inflammatory neuroprotection following reperfused stroke. PLoS ONE. 2012;7:e38664.
Bayry J, Misra N, Latry V, Prost F, Delignat S, Lacroix-Desmazes S, et al. Mechanisms of action of intravenous immunoglobulin in autoimmune and inflammatory diseases. Transfus Clin Biol. 2003;10:165–9.
Remlinger J, Madarasz A, Guse K, Hoepner R, Bagnoud M, Meli I, et al. Antineonatal Fc receptor antibody treatment ameliorates MOG-IgG-associated experimental autoimmune encephalomyelitis. neurol neuroimmunol neuroinflamm [Internet]. 2022;9. Available from: https://doi.org/10.1212/NXI.0000000000001134
Deczkowska A, Weiner A, Amit I. The physiology, pathology, and potential therapeutic applications of the TREM2 signaling pathway. Cell. 2020;181:1207–17.
Acknowledgements
We would like to thank Mr. James Graham from the UC Davis Nutrition Department for conducting corticosterone analyses. We would like to thank Dr. Douglas Rowland for his support for the MR imaging. RNA libraries were prepared and sequenced by the skilled team at the UC Davis Genomics Shared Resource. Finally, we would like to thank Ms. Angelica Michelle Bachman and Dr. Melissa Bauman from the Rodent Behavior Core at UC Davis.
Funding
This work was supported by the National Institutes of Neurological Disorder and Stroke grants K08NS105914 (BW) and R01NS106950 (BPA, FRS). Dr. Rowland is supported by the Chan Zuckerberg Initiative Donor-Advised Fund (2019–198156) of the Silicon Valley Community Foundation. The skilled team at the UC Davis Genomics Shared Resource is funded by a UC Davis Comprehensive Cancer Center Support Grant awarded by the National Cancer Institute (NCI P30CA093373). Dr. Melissa Bauman from the Rodent Behavior Core at UC Davis is supported by a MIND Institute Intellectual and Developmental Disabilities Research Center grant (P50HD103526).
Author information
Authors and Affiliations
Contributions
Conceptualization: Ben Waldau, Frank Sharp; methodology: Catherine Peterson, Chloe Puglisi, Heather Hull, Cameron Hawk, Venina Kalistratova; formal analysis and investigation: Chloe Puglisi, Bradley Ander, Janet Keiter, Catherine Peterson, Ali Izadi, Gene Gurkoff, Ben Waldau; writing—original draft preparation: Chloe Puglisi, Bradley Ander, Janet Keiter; writing—review and editing: Ben Waldau, Frank Sharp, Chloe Puglisi, Bradley Ander; funding acquisition: Ben Waldau, Frank Sharp, Bradley Ander; supervision: Ben Waldau, Frank Sharp.
Corresponding author
Ethics declarations
Ethical Approval
This study was approved by the University of California at Davis Institutional Animal Care and Use Committee. The welfare of animals was monitored by a veterinarian.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Puglisi, C.H., Ander, B.P., Peterson, C. et al. Sustained ICP Elevation Is a Driver of Spatial Memory Deficits After Intraventricular Hemorrhage and Leads to Activation of Distinct Microglial Signaling Pathways. Transl. Stroke Res. 14, 572–588 (2023). https://doi.org/10.1007/s12975-022-01061-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-01061-0