Abstract
Background
Mosquito-borne diseases such as malaria and encephalitis are still the cause of several hundred thousand deaths annually. The excessive use of chemical insecticides for transmission control has led to environmental pollution and widespread resistance in mosquitoes. Botanical insecticides' efficacies improvement has thus received considerable attention recently.
Methods
The larvicidal effects of three essential oils from the Citrus family and limonene (their major ingredient) were first investigated against malaria and filariasis mosquito vectors. An attempt was then made to improve their efficacies by preparing nanoliposomes containing each of them.
Results
The larvicidal effect of nanoformulated forms was more effective than non-formulated states. Nanoliposomes containing Citrus aurantium essential oil with a particle size of 52 ± 4 nm showed the best larvicidal activity (LC50 and LC90 values) against Anopheles stephensi (6.63 and 12.29 µg/mL) and Culex quinquefasciatus (4.9 and 16.4 µg/mL).
Conclusion
Due to the green constituents and high efficacy of nanoliposomes containing C. aurantium essential oil, it could be considered for further investigation against other mosquitoes’ populations and field trials.
Similar content being viewed by others
Background
Mosquitoes are members of the order Diptera, class Insecta, and phylum Arthropoda that live in subtropical or tropical regions [1]. They transmit many diseases such as malaria, dengue fever, yellow fever, encephalitis, and filariasis to humans [2]. For example, it has been estimated that about 229 million new malaria infections and about 0.4 million deaths occurred only in 2019; 67% of death was related to children < 5 years old [3, 4]. Anopheles stephensi Liston. is the primary malaria vector in the Indian subcontinent, Arabian Peninsula, Iran, and Afghanistan [5, 6]. On the other hand, Culex quinquefasciatus Say, the southern house mosquito, is another geographically widespread and medically important mosquito vector. It transmits viruses such as West Nile and St. Louis encephalitis and the filarial worm, Wuchereria bancrofti [7, 8].
Treating such diseases is challenging while preventing their transmission is an accessible and effective way to reduce disease burden and economic, emotional, and health consequences. For example, insecticide-treated nets (ITNs) effectively control malaria in children and adults living in areas with persistent malaria transmission. Residual spraying (IRS) and space spraying of chemical insecticides are also recommended. Besides, larvicide is recommended in endemic regions and regions [9, 10]. However, excessive use of chemical insecticides has led to environmental pollution; toxin residues in agricultural materials or drinking water are health challenges worldwide nowadays [11, 12]. Moreover, in addition to direct adverse effects on human health, they also affect non-target beneficial insects such as bee venom and other pollinating insects [13, 14].
Moreover, repetitive and non-compliant chemical insecticides have also increased mosquitoes' resistance [15, 16]. Herbal insecticides, especially essential oils (EOs), are potential alternatives to chemical ones because they are environmentally friendly, biodegradable, and generally have no toxic effects on non-target organisms [17, 18]. Some scientific studies have also recently been suggested them as alternative agents against mosquito larvae [19, 20]. However, their usage in practical conditions is questioned due to their instability and lower efficacies than chemical larvicide.
The preparation of EO-based nanoformulations is considered a promising approach to prevent oxidative destruction, improve dispersion in water, and enhance the biological efficacies of EOs [21,22,23]. In the pharmaceutical industry, one commonly used carrier is nanoliposomes; they are vesicles whose structure resembles a cell membrane [24]. Furthermore, due to their hydrophobic nature, they have a higher loading capacity of EOs than chitosan nanoparticles [25, 26]; they are thus proper candidates in developing green larvicide.
This study first investigated the larvicidal effect of the three EOs, including Citrus aurantium, Citrus limon, Citrus sinensis, and limonene (as their identified major ingredients), against A. stephensi and C. quinquefasciatus. An attempt was then made on enhancing their efficacy by preparation of nanoliposome containing each of them.
Methods
Reagents
Limonene (97%), wool fat cholesterol (97%), egg yolk lecithin (> 90%), tween 20 (99.9%), and absolute ethanol (99.9%) were bought from Merck Chemicals (Germany). In addition, C. aurantium EO (99.9%) was bought from Tabib Daru pharmaceutical Co. (Iran). C. limon EO (99.9%) was purchased from Barij Essence Co. (Iran). C. sinensis EO (99.9%) supplied by Green Plants of Life Co.
Preparation of nanoliposomes
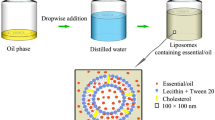
Nanoliposomes containing limonene and each EO were prepared using the ethanol injection approach with some modifications [27]. The lipid phase was first prepared by dissolving lecithin (2.5% w/v), cholesterol (0.5% w/v), tween 20 (1.0% w/v), and limonene, C. aurantium EO, C. limon EO, C. sinensis EO (2% w/v), separately in absolute ethanol. Then, one mL of each prepared solution was added dropwise to 4 mL of distilled water, and the mixtures were stirred for 40 min (2000 rpm, room temperature). The prepared samples were abbreviated as LimLipo, CALipo, CLLipo, and CSLipo, and were used for size analyses, chemical analysis, and larvicidal assays (Fig. 1). Moreover, free liposomes were also prepared similarly, without EOs or limonene.
Size analyses
The particle sizes of LimLipo, CALipo, CLLipo, and CSLipo were investigated using a dynamic light scattering (DLS) instrument (9900 series, K-One nano Ltd, Korea). The samples' particle size distribution (SPAN) was also calculated as d90-d10/d50, where d is diameter and 10, 50, and 90 are percentages of particles with lower sizes than these values. SPAN values less than 1 confirm narrow particle size distribution [28].
Confirmation of limonene and EOs in nanoliposomes
Loading of limonene and the EOs in nanoliposomes was investigated using ATR-FTIR analysis (Model Tensor II, Bruker, USA). Free liposomes, LimLipo, CALipo, CLLipo, and CSLipo, were centrifuged for 60 min at 4 ºC (12,000 g). The resulting pellets were placed at room temperature to reduce their moisture for one day. They were then subjected to the instrument, and the spectra were recorded in the range of 400–4000 cm−1.
Larvicidal bioassays
Late third or early fourth instar larvae of A. stephensi and C. quinquefasciatus (Bandar Abbas strain) were reared in the insectary of Hormozgan University Medical Sciences (Iran). The colonies were maintained at 27 ± 2 ºC with 12:12 light and dark photoperiod at 65% ± 5% relative humidity. Besides, they were not exposed to any insecticides and were susceptible to all larvicides.
Limonene, C. aurantium EO, C. limon EO, and C. sinensis EO were dissolved in ethanol at a concentration equal to as-prepared nanoliposomes (2.5 w/v). Their larvicidal effects as non-formulated samples and LimLipo, CALipo, CLLipo, and CSLipo as nanoformulated samples were investigated according to the WHO guideline [29]. Briefly, beakers containing 200 mL dechlorinated water and 25 A. stephensi and C. quinquefasciatus larvae were first ready. By adding ≤ 0.8 mL of each sample; concentrations were fixed at 100, 50, 25, 12.50, 6.25, and 3.12 µg/mL. Larval mortality was recorded after 24 h exposure; no food was given to the larvae during these tests. Larvicidal effects of free liposomes were also investigated by adding equal amounts compared with samples. Besides, control groups were exposed to only 0.8 mL ethanol.
Statistical analyses
All experiments were carried out in triplicate, and final values were reported as mean ± standard deviation. Lethal concentration values (LC50 and LC90) with 95% fiducial limits and probit equations were calculated using probit analysis, as described by Finney [30]. One-Way-ANOVA or independent sample t-test with a confidence interval of 95% was used (SPSS v.21 software, USA) to compare the samples' larvicidal activity.
Results
Size analyses of the prepared nanoliposomes
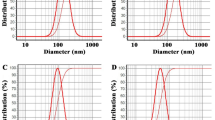
DLS analyses of the prepared nanoliposomes containing limonene, C. aurantium EO, C. limon EO, and C. sinensis EO are depicted in Fig. 2; their particle size was in the range of 42–67 nm. Their uniformity was confirmed as their particles size distributions (SAPN) were obtained as 0.97, 0.94, 0.98, and 0.99. Besides, the presence of a single sharp peak is also a sign of uniformity of nanoparticles.
Loading of limonene and EOs into nanoliposomes
ATR-FTIR spectra of free liposomes (Fig. 3A) display the bands at 2981 and 2904 cm−1; they attribute to C–C-H stretching (alkane groups). The band at 1453 cm−1 is related to CH2 bending, and the characteristic absorption at around 1385 cm−1 shows CH3 bending. The band at 1274 cm−1 is assigned to PO2 groups in lecithin. The band at 877 cm−1 is attributed to N(CH3)3, the band at 1085 cm−1 is attributed to P = O, the strong band at 1044 cm−1 is related to C-O stretching.
In limonene-loaded nanoliposomes (Fig. 3B; LimLipo), expanding the peak in 3345 cm−1 is due to increased hydrogen bands between tween 20, OH of cholesterol, and carbonyl groups (C = O) of fatty acid esters in lecithin structure. The bands at 2955, 2918, and 2850 cm−1 show –CH stretching in alkane groups. The peak at 1637 cm−1 relates to binding between lecithin and cholesterol, the band at 1225 cm−1 is assigned to PO2 groups in lecithin, and the band at 961 cm−1 is attributed to N(CH3)3. The most characteristic peak at about 886 cm−1 is attributed to the terminal methylene group out of plane bending in limonene. All the bands attributed to the functional groups of limonene and liposomes, including (methyl, hydroxyl, ester) were also presented in the limonene-loaded nanoliposome spectrum.
In the ATR-FTIR spectra of CALipo (Fig. 3C), the broadband at about 3369 cm−1 is related to OH stretching vibrations that confirmed increasing hydrogen bands (tween, OH cholesterol, and carbonyl groups of fatty acid esters in lecithin structure). The bands at 2922 and 2852 cm−1 correspond to the –CH stretching (alkyl chains in lecithin, cholesterol, and C. aurantium EO). The peak at 1708 cm−1 corresponds to the stretching vibration of carbonyl C = O, the absorption band at 1644 cm−1 assigns to binding between lecithin and cholesterol. The band at 1208 cm−1 is attributed to PO2 groups in lecithin, and the band at 961 cm−1 is assigned to N(CH3)3. The main characteristic peak in C. aurantium EO and limonene is related to the terminal methylene group out of plane bending at 887 cm−1.
The ATR-FTIR spectra of CLLipo (Fig. 3D) showed the broadband at about 3367 cm−1 which could be attributed to OH stretching vibrations; this broadening of infrared spectra is related to forming hydrogen bonds in tween, OH of cholesterol, and carbonyl groups of fatty acid esters in lecithin structure. The bands at 2921 and 2851 cm−1 show –CH stretching vibration related to alkane groups. The strong peak at 1708 cm−1 corresponds to the stretching vibration of carbonyl C = O, and the peak at 1645 cm−1 could be related to the binding between lecithin and cholesterol. The peak at 1206 cm−1 corresponds to PO2 groups in lecithin, the band at 962 cm−1 is related to N(CH3)3. The peak at about 886 cm−1 is attributed to the terminal methylene group out of plane bending in limonene as the main compound of C. limon EO.
The ATR-FTIR spectra of CSLipo (Fig. 3E) displayed the broadband at about 3331 cm−1. It relates to OH stretching vibrations in hydrogen-bonded (tween, OH of cholesterol, and carbonyl groups of fatty acid esters in lecithin structure). The band at 1222 cm−1 corresponds to PO2 groups in lecithin, and the absorption bands at 2924 and 2852 cm−1 relate to alkane groups stretching. The strong peak at 1626 cm−1 could be related to the binding between lecithin and cholesterol. The band at 972 cm−1 corresponds to N(CH3)3. The peak at about 877 cm−1 is assigned to the terminal methylene group out of plane bending in limonene as the main compound of C. sinensis EO. Eventually, the peak at about 886 cm−1 was evidence of limonene presence. The ATR-FTIR analyses of nanoliposomes containing EOs and limonene have made us notice a similitude of absorption bands and merely small differences between their intensities. This result also confirmed only physical interactions between EOs or limonene and free liposomes.
Larvicidal effect of samples
As shown in Fig. 4, free liposomes did not affect the larvae. Viabilities of both A. stephensi and C. quinquefasciatus were 97–100% after treatment with different amounts of free liposomes, equal to the values of the samples containing EOs or limonene were treated.
Probit regression lines parameters of A. stephensi larvae exposed to different concentrations of samples are listed in Table 1. Among the non-formulated samples (limonene, C. aurantium EO, C. limon EO, and C. sinensis EO), LC50 and LC90 values of C. aurantium EO were significantly higher than others (one-way ANOVA, P < 0.05). The best-observed LC50 values (6.63 µg/mL) and LC90 value were related to CALipo (12.29 µg/mL).
However, LC50 values of nanoformulations were lower than their non-formulated state; this difference was only significant between C. limon EO and CLLipo as well as C. aurantium EO and CALipo (Independent sample t-test, P < 0.05). Besides, the LC90 values of all nanoformulations were significantly more deleterious to larvae than their non-formulated forms (Independent sample t-test, P < 0.05).
Probit regression lines parameters of C. quinquefasciatus exposed to different concentrations of samples are listed in Table 2. Among the non-formulated samples, the LC50 value of limonene was higher than others; however, this difference was only significant compared to C. sinensis EO (one-way ANOVA, P < 0.05). Besides, LC90 values of all non-formulated samples were not significantly different together (one-way ANOVA, P > 0.05). In all samples, the best-observed LC50 (4.9 µg/mL) and LC90 (16.4 µg/mL) values were related to CALipo.
Furthermore, the LC50 values of LimLipo, CALipo, and CLLipo were significantly more effective than their non-formulated states (Independent sample t-test, P < 0.05); no significant difference was seen between C. sinensis EO and CSLipo (Independent sample t-test, P > 0.05). LC90 values of LimLipo and CALipo were significantly more efficient than their non-formulated states (Independent sample t-test, P < 0.05).
Discussions
As detailed in our previous report, limonene was identified as the major compound in the used EOs; it composed 31.4, 61.8, and 61.8% of identified compounds in C. aurantium, C. limon, and C. sinensis EOs [31]. Sabinene (15.6%), ɣ-terpinene (6.0%), linalool (5.6%), and nerolidol (5.1%) were the other four major compounds of C. aurantium EO. Alpha-pinene, sabinene, cis-limonene oxide, and trans-limonene oxide with a portion of 3.5, 17.0, 2.3, and 3.1% were the other four C. limon EO compounds. In C. sinensis EO, trans-p-2,8-Menthadien-1-ol, cis-limonene oxide, trans-limonene oxide, and trans-carveol were identified as the other four major compounds (5.0, 2.6, 2.3, and 2.9%). Limonene is a colorless terpene with a pleasant lemon-like odor [32, 33].
The literature reported a nanoliposome containing limonene with a particle size of 100 nm; however, it was used as an antibacterial agent [34]. In another research, liposomes containing C. limon EO with a particle size of 114 nm was also reported [35]. Interestingly, we could not find any report on the preparation of liposomes containing C. sinensis and C. aurantium EOs.
The larvicidal effects of C. aurantium EO were previously reported; the LC50 value against A. stephensi was 31.20 ppm, but details on C. quinquefasciatus were not available [36, 37]. However, in the current study, LC50 of C. aurantium EO was observed at 62.49 μg/mL; the difference was related to the used strain of A. stephensi (Bandar Abbas vs. Beech). Besides, the LC50 value of C. sinensis EO against larvae of C. quinquefasciatus in two separate reports was reported at 304 and 452 ppm; however, used strains were not mentioned [38, 39]. Furthermore, no report was found on the larvicidal effect of limonene and C. limon EO, LimLipo, CLLipo, CALipo, and CSLipo against A. stephensi and C. quinquefasciatus. The present research is thus a new venture into this specific field. Interestingly, the best-obtained result in the current study was related to nanoliposomes containing C. aurantium EO with LC50 values of 6.63 and 4.9 μg/mL against larvae A. stephensi and C. quinquefasciatus. These values were more potent than available reports as EO-based nano larvicides. For instance, nanocrystal emulsion of Ficus glomerata with LC50 value of 17 μg/mL against A. stephensi [40]. Moreover, LC50 values of Azadirachta indica and Lippia alba EOs nanoemulsions were reported at 11.75 and 38.22 μg/mL against C. quinquefasciatus [41, 42].
The current data also emphasize the superior activity of nanoformulations over their non-formulated states; it is consonant with the literature. When a solute such as EO is dissolved in the appropriate solvent, droplet size depends on the solution's preparation conditions (temperature and time) is around 0.3 – 10 nm [43, 44]. On the other hand, many droplets are incorporated into a nanocarrier (e.g., nanoliposome) during the formulating process; due to the presence of packages containing EOs, the larvicidal efficacies are improved [45,46,47]. Furthermore, nanostructures' high surface energy leads to a more efficient interaction between nanoparticles and the target organism [48]. Besides, nanosize carriers improve EO's ability to pass through tiny pores in larval bodies [49]. The higher physical stability of nanoformulated EO could also improve larvicidal effects [50].
Scanning the literature, the effect of nanoformulations compared to non-formulated EOs could be summarized as follows. First, achieved a 100% larvicidal effect at lower exposure time, e.g., the study conducted on C. quinquefasciatus a perfect larvicidal effect was achieved at low exposure time, Eucalyptus globulus EO (24 h), and its nanoemulsion (4 h) [51]. Second, an improvement of LC50 value of EOs was acquired after preparing their nanoformulated form; for instance, the LC50 value of C. sinensis EO against Culex pipiens was reported at 86.3 μg/mL, and its nanoemulsion dosage form had its LC50 value at 27.4 μg/mL [52]. However, few reports on the preparation of slow and long-release nanoformulations and the long-lasting effects of green nanolarvicides were also reported [45, 53, 54].
Conclusions
The results show that the nanoliposomes containing limonene and limonene-rich EOs were more toxic than non-nanoliposomes (max 20 folds) against malaria and filariasis mosquito larvae. Interestingly, nanoliposomes containing C. aurantium EO with LC50 values of 6.63 and 4.9 μg/mL against larvae A. stephensi and C. quinquefasciatus could be considered an alternative to synthetic insecticides for the prevention of transmission of such dreadful diseases. However, by investigating its efficacies against other medically important mosquito species, its effectiveness could be better evaluated.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ATR-FTIR:
-
Attenuated Total Reflection-Fourier Transform InfraRed
- EO:
-
Essential Oil
- LimLipo:
-
Nanoliposomes containing limonene
- CALipo:
-
Nanoliposomes containing Citrus aurantium essential oil
- CLLipo:
-
Nanoliposomes containing Citrus limon essential oil
- CSLipo:
-
Nanoliposomes containing Citrus sinensis essential oil
References
CDC. Where mosquitoes live 2021. Available from: https://www.cdc.gov/mosquitoes/about/where-mosquitoes-live.html#:~:text=Habitats,%2C%20marshes%2C%20or%20tall%20grasses.&text=Some%20mosquitoes%20prefer%20clean%20water,in%20containers%20that%20hold%20water.
Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis. 2017;17(3):101–6. https://doi.org/10.1016/s1473-3099(16)30518-7.
Hanafi-Bojd AA, Vatandoost H, Yaghoobi-Ershadi MR. Climate change and the risk of malaria transmission in Iran. J Med Entomol. 2020;57(1):50–64. https://doi.org/10.1093/jme/tjz131.
WHO. Malaria fac sheet 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/malaria#:~:text=The%20estimated%20number%20of%20malaria,of%20all%20malaria%20deaths%20worldwide.
Azari-Hamidian S, Abai M-R, Norouzi B. Mansonia uniformis (Diptera: Culicidae), a genus and species new to southwestern Asia, with a review of its medical and veterinary importance. Zootaxa. 2020;4772(2):385–95. https://doi.org/10.11646/zootaxa.4772.2.10.
Sedaghat MM, Harbach RE. An annotated checklist of the Anopheles mosquitoes (Diptera: Culicidae) in Iran. J Vector Ecol. 2005;30(2):272.
Bartholomay LC, Waterhouse RM, Mayhew GF, Campbell CL, Michel K, Zou Z, et al. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science. 2010;330(6000):88–90. https://doi.org/10.1126/science.1193162.
Arensburger P, Megy K, Waterhouse RM, Abrudan J, Amedeo P, Antelo B, et al. Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science. 2010;330(6000):86–8. https://doi.org/10.1126/science.1191864.
Ghosh A, Chowdhury N, Chandra G. Plant extracts as potential mosquito larvicides. Indian J Med Res. 2012;135(5):581–98.
Floore TG. Mosquito larval control practices: past and present. J Am Mosq Control Assoc. 2006;22(3):527–33. https://doi.org/10.2987/8756-971x(2006)22[527:mlcppa]2.0.co;2.
Pravin Y, Saranya M, Sivakumar T, Mahendran S, Mohanraj R, Dhanakkodi B. Larvicidal, pupicidal, ovicidal activity and GC-MS analysis of Spathodea campanulata P. Beauv.(Bignoniaceae) acetone leaf extract against the dengue vector mosquito Aedes aegypti (Diptera: Culicidae). Int J Curr Res Acad Rev. 2015;3(5):92–111.
Osanloo M, Amini SM, Sedaghat MM, Amani A. Larvicidal activity of chemically synthesized silver nanoparticles against Anopheles stephensi. J Pharm Negat Results. 2019;10(1):69–72. https://doi.org/10.4103/jpnr.JPNR_18_17.
Serrão JE, Plata-Rueda A, Martínez LC, Zanuncio JC. Side-effects of pesticides on non-target insects in agriculture: a mini-review. The Science of Nature. 2022;109(2):17. https://doi.org/10.1007/s00114-022-01788-8.
Stara J, Ourednickova J, Kocourek F. Laboratory evaluation of the side effects of insecticides on Aphidius colemani (Hymenoptera: Aphidiidae), Aphidoletes aphidimyza (Diptera: Cecidomyiidae), and Neoseiulus cucumeris (Acari: Phytoseidae). J Pest Sci. 2011;84(1):25–31. https://doi.org/10.1007/s10340-010-0322-5.
Vatandoost H, Hanafi—Bojd A. Current Resistant Status of Anopheles stephensi Liston to Different Larvicides in Hormoz gan. Pak J Biol Sci. 2005;8(11):1568–70. DOI: https://doi.org/10.3923/pjbs.2005.1568.1570
Soltani A, Vatandoost H, Oshaghi MA, Ravasan NM, Enayati AA, Asgarian F. Resistance mechanisms of Anopheles stephensi (Diptera: Culicidae) to temephos. J Arthropod Borne Dis. 2015;9(1):71–83.
Isman MB. Plant essential oils for pest and disease management. Crop Prot. 2000;19(8–10):603–8. https://doi.org/10.1016/S0261-2194(00)00079-X.
Azizi K, Shahidi-Hakak F, Asgari Q, Hatam GR, Fakoorziba MR, Miri R, et al. In vitro efficacy of ethanolic extract of Artemisia absinthium (Asteraceae) against Leishmania major L. using cell sensitivity and flow cytometry assays. J Parasit Dis. 2016;40(3):735–40. https://doi.org/10.1007/s12639-014-0569-5.
Sanei-Dehkordi A, Gholami S, Abai MR, Sedaghat MM. Essential Oil Composition and Larvicidal Evaluation of Platycladus orientalis against Two Mosquito Vectors, Anopheles stephensi and Culex pipiens. J Arthropod Borne Dis. 2018;12(2):101–7. https://doi.org/10.18502/jad.v12i2.35.
Şengül Demirak M, Canpolat E. Plant-Based Bioinsecticides for Mosquito Control: Impact on Insecticide Resistance and Disease Transmission. Insects. 2022;13(2). DOI: https://doi.org/10.3390/insects13020162
Osanloo M, Arish J, Sereshti H. Developed methods for the preparation of electrospun nanofibers containing plant-derived oil or essential oil: a systematic review. Polym Bull. 2020;77:6085–104. https://doi.org/10.1007/s00289-019-03042-0.
Prakash B, Kujur A, Yadav A, Kumar A, Singh PP, Dubey N. Nanoencapsulation: An efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control. 2018;89:1–11. https://doi.org/10.1016/j.foodcont.2018.01.018.
Esmaili F, Sanei-Dehkordi A, Amoozegar F, Osanloo M. A review on the use of essential oil-based nanoformulations in control of mosquitoes. Biointerface Res Appl Chem. 2021;11(5):12516–29. https://doi.org/10.33263/BRIAC115.1251612529.
Trucillo P, Ferrari P, Campardelli R, Reverchon E, Perego P. A supercritical assisted process for the production of amoxicillin-loaded liposomes for antimicrobial applications. J Supercrit Fluids. 2020;163: 104842. https://doi.org/10.1016/j.supflu.2020.104842.
Daraee H, Etemadi A, Kouhi M, Alimirzalu S, Akbarzadeh A. Application of liposomes in medicine and drug delivery. Artif Cells, Nanomed, Biotechnol. 2016;44(1):381–91. https://doi.org/10.3109/21691401.2014.953633.
Rahman M, Kumar V, Beg S, Sharma G, Katare OP, Anwar F. Emergence of liposome as targeted magic bullet for inflammatory disorders: current state of the art. Artif Cells, Nanomed, Biotechnol. 2016;44(7):1597–608. https://doi.org/10.3109/21691401.2015.1129617.
Charcosset C, Juban A, Valour J-P, Urbaniak S, Fessi H. Preparation of liposomes at large scale using the ethanol injection method: Effect of scale-up and injection devices. Chem Eng Res Des. 2015;94:508–15. https://doi.org/10.1016/j.cherd.2014.09.008.
Abedinpour N, Ghanbariasad A, Taghinezhad A, Osanloo M. Preparation of Nanoemulsions of Mentha piperita Essential Oil and Investigation of Their Cytotoxic Effect on Human Breast Cancer Lines. BioNanoScience. 2021;11(2):428–36. https://doi.org/10.1007/s12668-021-00827-4.
WHO. Guidelines for laboratory and field testing of mosquito larvicides 2005.
Dj F. Probit analysis. New York: Cambridge University Press; 1971.
Alipanah H, Farjam M, Zarenezhad E, Roozitalab G, Osanloo M. Chitosan nanoparticles containing limonene and limonene-rich essential oils: potential phytotherapy agents for the treatment of melanoma and breast cancers. BMC Complementary Medicine and Therapies. 2021;21(1):186. https://doi.org/10.1186/s12906-021-03362-7.
Anandakumar P, Kamaraj S, Vanitha MK. D-limonene: A multifunctional compound with potent therapeutic effects. J Food Biochem. 2021;45(1): e13566. https://doi.org/10.1111/jfbc.13566.
Vieira AJ, Beserra FP, Souza M, Totti B, Rozza A. Limonene: Aroma of innovation in health and disease. Chem Biol Interact. 2018;283:97–106. https://doi.org/10.1016/j.cbi.2018.02.007.
Umagiliyage AL, Becerra-Mora N, Kohli P, Fisher DJ, Choudhary R. Antimicrobial efficacy of liposomes containing d-limonene and its effect on the storage life of blueberries. Postharvest Biol Technol. 2017;128:130–7. https://doi.org/10.1016/j.postharvbio.2017.02.007.
Palmas L, Aroffu M, Petretto GL. Entrapment of Citrus limon var. pompia Essential Oil or Pure Citral in Liposomes Tailored as Mouthwash for the Treatment of Oral Cavity Diseases. Pharmaceuticals. 2020;13(9):216. https://doi.org/10.3390/ph13090216.
Sanei-Dehkordi A, Sedaghat MM, Vatandoost H, Abai MR. Chemical Compositions of the Peel Essential Oil of Citrus aurantium and Its Natural Larvicidal Activity against the Malaria Vector Anopheles stephensi (Diptera: Culicidae) in Comparison with Citrus paradisi. J Arthropod Borne Dis. 2016;10(4):577–85.
Mwaiko GL. Citrus peel oil extracts as mosquito larvae insecticides. East Afr Med J. 1992;69(4):223–6.
Cheah SX, Tay JW, Chan LK, Jaal Z. Larvicidal, oviposition, and ovicidal effects of Artemisia annua (Asterales: Asteraceae) against Aedes aegypti, Anopheles sinensis, and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res. 2013;112(9):3275–82. https://doi.org/10.1007/s00436-013-3506-0.
Murugan K, Mahesh Kumar P, Kovendan K, Amerasan D, Subrmaniam J, Hwang JS. Larvicidal, pupicidal, repellent and adulticidal activity of Citrus sinensis orange peel extract against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res. 2012;111(4):1757–69. https://doi.org/10.1007/s00436-012-3021-8.
Nazeer AA, Rajan HV, Vijaykumar SD, Saravanan M. Evaluation of Larvicidal and Repellent Activity of Nanocrystal Emulsion Synthesized from F. glomerata and Neem Oil Against Mosquitoes. Journal of Cluster Science. 2019;30(6):1649–61.
Anjali C, Sharma Y, Mukherjee A, Chandrasekaran N. Neem oil (Azadirachta indica) nanoemulsion—a potent larvicidal agent against Culex quinquefasciatus. Pest Manag Sci. 2012;68(2):158–63.
Ferreira RM, Duarte JL, Cruz RA, Oliveira AE, Araújo RS, Carvalho JC, et al. A herbal oil in water nano-emulsion prepared through an ecofriendly approach affects two tropical disease vectors. Rev Bras. 2019;29(6):778–84.
Ashbaugh HS, Paulaitis ME. Effect of Solute Size and Solute−Water Attractive Interactions on Hydration Water Structure around Hydrophobic Solutes. J Am Chem Soc. 2001;123(43):10721–8. https://doi.org/10.1021/ja016324k.
Carlsson J, Åqvist J. Calculations of solute and solvent entropies from molecular dynamics simulations. Phys Chem Chem Phys. 2006;8(46):5385–95. https://doi.org/10.1039/b608486a.
Osanloo M, Sedaghat MM, Sereshti H, Amani A. Chitosan nanocapsules of tarragon essential oil with low cytotoxicity and long-lasting activity as a green nano-larvicide. J Nanostruct. 2019;9(4):723–35. https://doi.org/10.22052/JNS.2019.04.014.
Kavallieratos NG, Nika EP, Skourti A, Ntalli N, Boukouvala MC, Ntalaka CT, et al. Developing a Hazomalania voyronii Essential Oil Nanoemulsion for the Eco-Friendly Management of Tribolium confusum, Tribolium castaneum and Tenebrio molitor Larvae and Adults on Stored Wheat. Molecules. 2021;26(6):1812.
Benelli G, Pavoni L, Zeni V, Ricciardi R, Cosci F, Cacopardo G, et al. Developing a Highly Stable Carlina acaulis Essential Oil Nanoemulsion for Managing Lobesia botrana. Nanomaterials. 2020;10(9):1867.
Shahzad K, Manzoor F. Nanoformulations and their mode of action in insects: a review of biological interactions. Drug Chem Toxicol. 2021;44(1):1–11. https://doi.org/10.1080/01480545.2018.1525393.
Jesser EN, Yeguerman CO, Gili VO, Santillan GO, Murray AP, Domini C, et al. Optimization and characterization of essential oil nanoemulsions using ultrasound for new ecofriendly insecticides. ACS Sustainable Chem Eng. 2020;8(21):7981–92. https://doi.org/10.1021/acssuschemeng.0c02224.
Ferreira R, D’Haveloose NP, Cruz RAS, Araujo RS, Carvalho JCT, Rocha L, et al. Nano-emulsification Enhances the Larvicidal Potential of the Essential Oil of Siparuna guianensis (Laurales: Siparunaceae) Against Aedes (Stegomyia) aegypti (Diptera: Culicidae). J Med Entomol. 2020;57(3):788–96. https://doi.org/10.1093/jme/tjz221.
Sugumar S, Clarke S, Nirmala M, Tyagi B, Mukherjee A, Chandrasekaran N. Nanoemulsion of eucalyptus oil and its larvicidal activity against Culex quinquefasciatus. Bull Entomol Res. 2014;104(3):393–402. https://doi.org/10.1017/S0007485313000710.
M Azmy R, E El Gohary EG, M Mahmoud D, AM Salem D, A Abdou M, S Salama M. Assessment of larvicidal activity of nanoemulsion from Citrus sinensis essential oil on Culex pipiens L.(Diptera: Culicidae). Egypt J Aquat Biol Fish. 2019;23(3):61–7. DOI: https://doi.org/10.21608/EJABF.2019.35100
Amado JRR, Prada AL, Diaz JG, Souto RNP, Arranz JCE, de Souza TP. Development, larvicide activity, and toxicity in nontarget species of the Croton linearis Jacq essential oil nanoemulsion. Environ Sci Pollut Res. 2020;27:9410–23. https://doi.org/10.1007/s11356-020-07608-8.
Essa E, Mo’men SA, Rady MH, Ma’moun SA, Barakat EM, Salama MS. Eucalyptus oil nano-emulsion encapsulated in chitosan beads as a new approach in control of Culex pipiens larvae. Int J Mosq Res. 2019;6(5):63–9.
Acknowledgements
Not applicable
Funding
This study was supported by Fasa University of Medical Sciences, Fasa, Iran: grant No. 400154.
Author information
Authors and Affiliations
Contributions
ASD performed larvicidal tests and probit analysis and drew regression lines of probit. MDMF wrote the introduction and a part of the discussion about the larvicidal tests. MS designed nanoliposomes. EZ interpreted ATR-FTIR spectra. MO designed the study, prepared the nanoformulation, and analyzed the data. All authors contributed to the drafting of the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been approved by the ethics committee at Fasa University of Medical Sciences, IR.FUMS.REC.1400.114. Besides, as this research did not involve in vivo or human study, consent to participate was not used.
Consent for publication
Not applicable.
Competing interests
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sanei-Dehkordi, A., Moemenbellah-Fard, M.D., Saffari, M. et al. Nanoliposomes containing limonene and limonene-rich essential oils as novel larvicides against malaria and filariasis mosquito vectors. BMC Complement Med Ther 22, 140 (2022). https://doi.org/10.1186/s12906-022-03624-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-022-03624-y