Abstract
Aedes aegypti and Anopheles stephensi have challenged human health by transmitting several infectious disease agents, such as malaria, dengue fever, and yellow fever. Larvicides, especially in endemic regions, is an effective approach to the control of mosquito-borne diseases. In this study, the composition of three essential oil from the Artemisia L. family was analyzed by Gas Chromatography–Mass Spectrometry. Afterward, nanoliposomes containing essential oils of A. annua, A. dracunculus, and A. sieberi with particle sizes of 137 ± 5, 151 ± 6, and 92 ± 5 nm were prepared. Besides, their zeta potential values were obtained at 32 ± 0.5, 32 ± 0.6, and 43 ± 1.7 mV. ATR-FTIR analysis (Attenuated Total Reflection-Fourier Transform InfraRed) confirmed the successful loading of the essential oils. Moreover, The LC50 values of nanoliposomes against Ae. aegypti larvae were 34, 151, and 197 µg/mL. These values for An.stephensi were obtained as 23 and 90, and 140 µg/mL, respectively. The results revealed that nanoliposomes containing A. dracunculus exerted the highest potential larvicidal effect against Ae. aegypti and An. stephensi, which can be considered against other mosquitoes.
Similar content being viewed by others
Introduction
Mosquitoes transmit several pathogens to humans, e.g., the Aedes genus can transmit Flaviviruses (mostly Dengue and Japanese encephalitis), Togaviruses, and Bunyaviruses1,2. These vectors are distributed worldwide, especially in tropical and temperate regions. Aedes aegypti is the most efficient vector of several arboviruses, such as dengue, yellow fever, Zika, and chikungunya3,4. It is an important threat to human health, e.g., 100 million people are affected annually by symptomatic Dengue virus infection5.
According to the WHO report, nearly 90 countries are contaminated by malaria, with more than 600,000 death in 20216,7. Anopheles stephensi as the main vector of malaria needs to be controlled8,9. However, the vector has been endemic to Arabia Peninsula and Southeast Asia since its first emergence in Djibouti in 2012. Its geographical distribution has extended to African and Asian countries and five continents of the world10,11. Various inequalities such as climate change, globalization, international transport, population movements, international conflicts, and socioeconomic conditions alongside the vector resistance to WHO-recommended pesticides have affected the vector's residence and spread12,13.
Plant-based compounds (Extract and Essential oil (EO)) have been applied as pesticides (larvicidal and adulticidal) for many years14. These compounds are biodegradable, highly effective, non-toxic to the host, and target-specific15,16. Moreover, the lack of bioinsecticide resistance is another benefit of phytochemicals17. For instance, Artemisia L. is a widespread genus of the Asteraceae family; its 500 species are mainly found in Asia, Europe, and North America18,19. Artemisia-derived EOs contain high amounts of terpenes; they are thus frequently utilized as insecticides20,21. For instance, the EO of A. annua showed efficient insecticidal properties against red flour beetle (Tribolium casteneum)22. Likewise, the insecticidal effect of A. dracunculus L. EO on Aphis gossypii was reported23. Moreover, the toxic and repellent potential of A. sieberi against Dermanyssus gallinae (poultry red mite) was reported24.
Nanoliposome are bilayered vesicles containing amphiphilic molecules similar to cell membranes; stability, durability, potency, and efficacy of the entrapped compounds in nanoliposomes are improved25,26,27. Besides, some reports on applying nanoliposomes containing EOs as larvicides have been reported. For instance, nanoliposomes containing Citrus aurantium EO with LC50 value of 4.9 µg/ml against Culex quinquefasciatus28. Moreover, nanoliposomes containing carvacrol with an LC50 value of 128 µg/mL against An. stephensi29. However, to the authors’ best knowledge, no report was available on the use of nanoliposomes containing EO as larvicides against Ae. aegypti.
For the first time, nanoliposomes containing A. annua, A. dracunculus, and A. sieberi EOs were proposed in the current study. Moreover, their larvicidal properties against Ae. aegypti and An. stephensi was investigated.
Materials and methods
Materials
Tween 20, cholesterol, and egg lecithin were obtained from Merck Chemicals Co. (Germany). The Culicidae insectary at Hormozgan University Medical Sciences supplied An. stephensi and Ae. aegypti larvae; they were continually available for bioassay testing. Besides, the mosquito colonies were maintained at relative humidity (70 ± 5%), with photoperiod cycles of 12:12 (light:dark) at 27 ± 1 °C. Moreover, the polytetrafluoroethylene (PTFE)-based membrane method was used to blood-feed adult female mosquitoes30.
Chemical compositions of the EOs
The chemical compound of the EOs was performed using an Agilent type 6890 GC–MS device equipped with a BPX5 silica capillary column (30 m × 0.25 μm, layer thickness of 0.25 μm) as described in our previous report31. To identify the EO's constituent compositions, 1 µL n-hexane was added in column chromatography. The temperature was scheduled; the oven temperature was set to 50 °C for 5 min. Then, the temperature was increased to 240 °C at a rate of 3 °C min−1, in continue, the temperature was increased to 300 °C at a rate of 15 °C min−1 for 3 min. Finally, the transfer line temperature was adjusted to 250 °C by split 1 to 35. Helium was used as the carrier gas at a flow rate of 0.5 mL min−1. The mass spectrometer (Agilent 5973 model) was scanned between 40 to 500 amu with an ionization voltage of 70 eV and ionization source temperature of 220 °C. The software used was Chemstation. Identification of the spectra was done with the help of their inhibition index and its comparison with the indices found in the source books and papers, using the mass spectra of standard compounds and the information available in the computer and virtual library32.
Preparation of nanoliposomes containing EOs
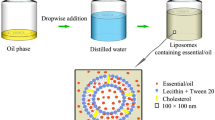
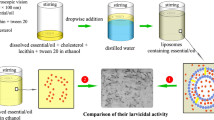
Nanoliposomes were prepared using ethanol injection method28. The mixture of lecithin (3% w/v), cholesterol (0.5% w/v), tween 20 (2% w/v), and each EO (2% w/v) was added in absolute ethanol and stirred overnight (2000 RPM) to oily phase prepared. After that, 1 mL of the oily phase was added to the 4 mL of distilled water. Finally, the mixture was mixed for 1 h at 2000 RPM and room temperature to stabilize the formed nanoliposomes. Meanwhile, free nanoliposomes were prepared using the above method but without EO.
Investigation of size and zeta potential of the nanoliposomes
Three prepared nanoliposomes and free nanoliposomes were poured into a quartz cell and transferred to the DLS machine to investigate particle size and particle size distribution (SPAN). The SPAN was calculated through the following equation: d90 − d10/d50. Also, the zeta potential of the samples was measured at room temperature33.
Investigation loading of the EOs in nanoliposomes
The loading of EOs was confirmed by ATR-FTIR qualitative method. For this purpose, each EO, free liposome, and nanoliposome containing each EO was subjected to the FTIR machine, and spectra in the 400 to 4000 wavenumber cm−1 were recorded34.
Mosquito rearing and larvicidal bioassays
The World Health Organization-recommended protocol was applied for the larvicidal bioassay tests35. In a 400 mL beaker containing 200 mL water, 25 larvae of An. stephensi or Ae. aegypti in the late third and early fourth instars were subjected to 12.5, 25, 50, 100, and 20 µg/mL of nanoliposome containing each EO. Besides, 1 mL of ethanol and free nanoliposomes were added to three bakers as control and negative control groups. Larval mortality was then noted after 24 h exposure. Three replicates for larvicidal bioassay were carried out, and larvicidal effects are presented as mean ± standard deviations. Besides, LC50 values with upper and lower confidence limits were calculated using the CalcuSyn software (free version). The non-overlap between the samples’ upper and lower confidence limits was considered a significant difference.
Ethical approval
The ethics committee has approved this research at Fasa University of Medical Sciences, Iran, IR.FUMS.REC.1401.145. Besides, this study did not include human investigation, so consent to participate is not applicable.
Results
Identified compounds in the EOs
Identified compounds in the EOs are listed in Table 1. Artemisia ketone (26.2%), camphor (19.2%), 1,8-cineole (12.3%), trans-caryophyllene (4.5%), and camphene (4.4%) are five major compounds of A. annua EO. Besides, estragole (67.6%), cis-ocimene (8.7%), ɣ-terpinene (7.6%), trans-ocimene (4.3%), and α-pinene (1.6%) are five major compounds in A. dracunculus EO. Moreover, camphor (28.3%), β-thujone (15.9%), α-thujone (8.4%), 1,8-cineole (1.8%), and borneol (4.2%) are five major compounds in A. sieberi EO.
Physical properties of the nanoliposomes
DLS diagrams of nanoliposomes containing EOs of A. annua, A. dracunculus, and A. sieberi and free nanoliposomes are depicted in Fig. 1A–D. Particle sizes were obtained as 137 ± 5, 151 ± 6, 92 ± 5, and 55 ± 4 nm, and their SPAN values were 0.96, 0.97, 0.97, and 0.96. Moreover, their potential zeta diagrams are shown in Fig. 2A–D; obtained values were 32 ± 0.5, 32 ± 0.6, 43 ± 1.7, and 23 ± 1.2 mV.
Furthermore, due to the high concentration, the sedimentation in all three nanoliposomes started after about 6 h. After overnight, two-phase suspensions were observed, with a clear supernatant and an agglomerate of nanoparticles below; no oily phases were observed at the top of the solutions. Agglomerate differs from aggregate; in the first one, the boundary between the nanoparticles is preserved and can be re-dispersed. In the next one, the nanoparticles become one and cannot be re-dispersed easily36,37. However, due to the presence of surfactant in the prepared nanoliposomes, these suspensions were re-dispersed with a simple shake, and their size did not change much from the initial state (data not shown). It is added that because the preparation site of nanoliposomes (Fasa University of Medical Sciences, Iran) and the larvicide testing site (Hormozgan University of Medical Sciences, Iran) are about 500 km away, the larvicide tests were conducted after 6 months of preparation. Therefore, it can be concluded that this re-dispersion did not affect nanoliposome efficacy. However, if the larvicidal test could have been performed immediately after preparation, the results would have been more accurate. However, in practical conditions, a larvicide usually is used for several months or years after manufacturing, so when the efficacy of these nanoliposomes was proper after six months, it can be concluded that they have good stability.
Successful loading of the EOs in the nanoliposomes
ATR-FTIR spectrum of A. annua EO (Fig. 3A) displayed the broadband at 3514 cm−1 attributed to OH and the characteristic peaks at 3084 and 3028 cm−1 can be related to C–H SP2 hybrid of alkyne. Besides, the spectra at 2963 and 2928 cm−1 corresponded to –CH stretching vibration due to alkanes, the spectra at 2872 and 2725 cm−1 indicating CH stretching vibration in aldehyde structure, the spectrum at 1743 cm−1, related to C=O, and the spectra at 1646, 1445 cm−1 can be related to C=C in aromatic compounds. The peak at 1445 cm−1 is attributed to the bending vibration of alcohol C–OH. The peaks at 1167, 1071, and 1004 cm−1 can be related to the stretching vibrations of C–O. The spectrum at 970 cm−1 is allocated to =C–H out-of-plane bending vibration from aromatics, and the characteristic band at 749 cm−1 is attributed to C–H vibration in benzene.
ATR-FTIR spectrum of A. dracunculus EO (Fig. 3B) displayed the peaks at 3076 and 3032 cm−1; they are attributed to SP2 hybrid of alkyne, the spectra at 2976, 2953, 2933, 2906, and 2834 cm−1 displayed –CH stretching vibration in SP3. The characteristic band at 1727 and 1638 cm−1 can be allocated to carbonyl groups. The band at 1509 cm−1 can be related to the C=C vibration in the aromatic ring and the characteristic band at 1243 cm−1 is attributed to C–O stretching vibration. Besides, the spectrum at 1035 cm−1 can be related to C–H bending absorption; also, the spectrum at 808 cm−1 is allocated to C–H vibration in benzene.
ATR-FTIR spectrum of A. sieberi EO (Fig. 3C) indicated the spectrum at 3467 cm−1 assigned to OH stretching due to phenolic compound in the EO. The characteristic peaks at 2958, 2924, and 2872 m−1 are ascribed to C–H stretching due to aliphatic compound, and the strong band at 1741 corresponded to (C=O), Carbonyl stretch representing aldehyde or ketones. The peak at 1454 cm−1 exhibited CH2 bending, and the absorption at about 1367 cm−1 is allocated to CH3 bending.
ATR-FTIR spectrum of free liposome (Fig. 3D) displayed the broad band between 3200 and 3600 cm−1 attributed to the presence of the hydroxyl group (OH), and the spectra at 2977, 2929, and 2900 cm−1 are attributed to C–C–H stretching. The absorption at 1645 cm−1 corresponded to the presence of the carbonyl group, and the spectrum at 1453 cm−1 indicates CH2 bending. Besides, the absorption at around 1383 cm−1 can be related to CH3 bending. The characteristic spectrum at 1085 cm−1 confirmed that the presence of P=O, the absorption at 1044 cm−1 could be related to C–O stretching, the characteristic absorption at 934 cm−1 corresponded to N(CH3)3, and the spectrum at 877 cm−1 represented the P–O stretching due to presence of lecithin.
It is evidenced from the blank and liposome containing EO for all absorption bands of interest that little difference was observed because the functional group of EO overlapped with the strong bands of the blank liposome.
ATR-FTIR spectrum of liposome containing A. annua EO (Fig. 3E) represented the broad and characteristic peak between 3200 and 3600 cm−1 attributed to the hydroxyl group due to hydrogen bonding between plant phenolic compound in the EO, carbonyl, and phosphate groups of lecithin. The absorptions at 2977 and 2929, 2900 cm−1 are allocated to symmetric and anti-symmetric vibration of CH2 in the alkyl chain in EO, tween 20, lecithin, and cholesterol. The spectrum at 1645 cm−1 is attributed to the carbonyl group. The phosphate stretching in 1274 and 1085 cm−1 is attributed to the interaction of compounds in the EO and fatty acid chains or polar heads in the nanoliposome.
ATR-FTIR spectrum of liposome containing A. dracunculus EO (Fig. 3F) displayed the broad band between 3200 to 3600 cm−1 allocated to the OH group due to hydrogen bonding between carboxyl and phosphate groups of lecithin. The absorption at 2976 and 2928 cm−1 are allocated to C-H starching due to SP3 hybrids of alkane in EO, tween 20, lecithin, and cholesterol. The spectrum at 1644 cm−1 can be attributed to the C=O. The phosphate stretching is presented in 1274 and 1085 cm−1, and the spectrum at 1044 cm−1 is attributed to C–O.
ATR-FTIR spectrum of liposome containing A. sieberi EO (Fig. 3G) showed the broadband between 3200 to 3600 cm−1 corresponded to hydroxyl groups due to hydrogen bonding interaction between EO, carbonyl, and phosphate groups. Besides, The spectra at 2978 and 2927 cm−1 allocated to C–H starching vibration related to alkanes in EO, Lecithin, tween 20, and cholesterol. Besides, the peak at 1644 cm−1 can be attributed to the C=O. The phosphate stretching presented in 1274 and 1085 cm−1 can be allocated to the interaction of compounds in EO and fatty acid chains or polar heads in nanoliposome.
Larvicidal effects of the nanoliposomes
Larvicidal effects of free nanoliposomes and nanoliposomes containing EOs of A. annua, A. dracunculus, and A. sieberi against Ae. Aegypti is illustrated in Fig. 4. Free liposomes showed negligible effects on the viability of larvae (~ 2%). Interestingly, nanoliposomes containing A. dracunculus inferred complete larvicidal effects (100%) at 100 and 200 µg/mL. Additionally, nanoliposomes containing A. annua and A. sieberi EOs caused 70 and 56% larval mortality at 200 µg/mL.
The larvicidal effects of the samples against An. stephensi is shown in Fig. 5. Free liposomes showed negligible effects on the viability of larvae (~ 4%). Besides, nanoliposomes containing A. sieberi EOs caused 77% larval mortality at 200 µg/mL. Interestingly, perfect larvicidal effects were observed after treatment with nanoliposomes containing A. dracunculus EO (50, 100, and 200 µg/mL) and nanoliposomes containing A. annua EO (200 µg/mL).
Obtained LC50 values of samples against Ae. aegypti and An. stephensi are summarized in Table 2. Nanoliposomes containing A. dracunculus EO with LC50 value of 34 (25–46) µg/mL against Ae. aegypti was significantly more potent (P < 0.05) than nanoliposomes containing A. annua and A. sieberi with LC50 values of 151 (149–153) and 197 (152–255) µg/mL. Moreover, the efficacy of nanoliposomes containing A. dracunculus EO against An. stephensi was significantly more potent (P < 0.05) than the others. LC50 values in order of efficacy were 23 (16–31), 90 (77–103), and 140 (138–141) µg/mL; these values were related to nanoliposomes containing A. dracunculus, A. annua, and A. sieberi.
Discussions
Excessive use of synthetic pesticides has led to environmental pollution with enhancement in vectors’ resistance38,39. The development of resistance to insecticides such as pyrethroids, organophosphates, organochlorines, and carbamates has precluded the successful elimination of larval stages40,41. For instance, a high rate (78%) of pyrethroids resistance in the WHO African Region has been demonstrated42. EOs consist of various natural volatile hydrocarbons and phenylpropenes molecules43. Monoterpenes are the main component of EOs which exert neurotoxic effects on insects via AChE and GABA activities16,44. However, total EOs confers substantially higher larvicidal or insecticidal effects through multi-target effects45,46. So in this study, three EOs from Asteraceae Family was used as larvicides. A. annua L. is a polyphenols-reach plant with antimalarial effects that grows in various geographical and soil pH conditions47. Another member of the Asteraceae Family, A.dracunculus has demonstrated larvicidal, antimicrobial, anticancer, and anti-inflammatory effects48,49. In addition, Artemisia sieberi has exhibited antimicrobial, antifungal, larvicidal, and insecticidal traits50,51,52.
In recent years many reports on using nanostructures containing EOs as mosquito repellents or larvicides have been published. For instance, nanogel containing Zataria multiflora EO with 600 min repellent against An. stephensi compared to 242 min efficacy of DEET53. Moreover, nanoemulsion of Cinnamomum zeylanicum EO (55 ppm) caused 92% larval mortality against An. stephensi54. Besides, The perfect larvicidal effect against Cx. quinquefasciatus was achieved at 4 h of exposure with nanoemulsion Eucalyptus globulus EO55. Nowadays, it is accepted that the efficacy of nanoformulation is more than the non-formulated state of EO. For instance, the LC50 value of Mentha piperita EO against Cx. pipiens in its nanoemulsion state compare to the non-formulated state decreased from 88.90 to 31.24 μg/mL56. By the way, the LC50 value of Siparuna guianensis EO against Ae. aegypti decreased from 86.52 to 24.75 μg/mL in its nanoemulsion state57. The smaller the nanoparticle size, the greater the mobility, and more collisions with larvae (due to Brownian motion) led to better accessibility, permeability, and toxicity against the larval body58,59. The current study demonstrated nanoliposomes containing A. dracunculus with LC50 values of 34 and 23 μg/mL against Ae. aegypti and An. stephensi is a great formulation for use as a mosquito larvicide. Its efficacy is also more than many available reports. For instance, nanoemulsion containing Pterodon emarginatus at 250 μg/mL showed 100% larvicidal effects on Ae. aegypti60. Besides, the LC50 value of Lippia alba nanoemulsion against Ae. aegypti was 31.02 μg/mL61. Moreover, the LC50 value of Myrtus communis nanoemulsion against An. stephensi was reported as 26.1 μg/mL62. Besides, Eucalyptus globulus EO nanogel against An. stephensi was reported as 32 μg/mL63. The main reason for its considerable capability might be related to the high percentage of estragole (67.6%), which can be used as a larvicidal agent for mosquito control programs. In that way, our findings provide a possible way for further studies to find out the active molecule. However, further investigations must be conducted to describe the mode of action of each constituent independently.
Conclusions
The chemical compositions of three used EOs were first investigated. Artemisia ketone (26.2%), camphor (19.2%), 1,8-cineole (12.3%), trans-caryophyllene (4.5%), and camphene (4.4%) included five major compounds of A. annua EO. Besides, estragole (67.6%), cis-ocimene (8.7%), ɣ-terpinene (7.6%), trans-ocimene (4.3%), and α-pinene (1.6%) were five major compounds in A. dracunculus EO. Moreover, camphor (28.3%), β-thujone (15.9%), α-thujone (8.4%), 1,8-cineole (1.8%), and borneol (4.2%) were five major compounds in A. sieberi EO. Larvicidal effects of nanoliposomes containing each EO against An. stephensi or Ae. aegypti were then investigated. Considering the promising results of nanoliposomes containing A. dracunculus EO against two important medical species, it could be a distinct candidate against other mosquitos’ larvae.
Data availability
The data used to support the findings of this study are included within the article.
References
Weetman, D. et al. Aedes mosquitoes and aedes-borne arboviruses in Africa: Current and future threats. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph15020220 (2018).
Robert, M. A., Stewart-Ibarra, A. M. & Estallo, E. L. Climate change and viral emergence: Evidence from Aedes-borne arboviruses. Curr. Opin. Virol. 40, 41–47. https://doi.org/10.1016/j.coviro.2020.05.001 (2020).
Kamal, M., Kenawy, M. A., Rady, M. H., Khaled, A. S. & Samy, A. M. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS One 13(12), e0210122. https://doi.org/10.1371/journal.pone.0210122 (2018).
Alonso-Palomares, L. A., Moreno-García, M., Lanz-Mendoza, H. & Salazar, M. I. Molecular basis for arbovirus transmission by Aedes aegypti mosquitoes. Intervirology 61(6), 255–264. https://doi.org/10.1159/000499128 (2018).
Bhatt, S. et al. The global distribution and burden of dengue. Nature 496(7446), 504–507. https://doi.org/10.1038/nature12060 (2013).
Organization, W. H. World malaria report 2022: World Health Organization; 2022. Available from: https://who.int/publications/i/item/9789240015791.
Agyekum, T. P. et al. A systematic review of the effects of temperature on anopheles mosquito development and survival: Implications for malaria control in a future warmer climate. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph18147255 (2021).
Sinka, M. E. et al. A new malaria vector in Africa: Predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc. Natl. Acad. Sci. 117(40), 24900–24908. https://doi.org/10.1073/pnas.2003976117 (2020).
Mnzava, A., Monroe, A. C. & Okumu, F. Anopheles stephensi in Africa requires a more integrated response. Malar. J. 21(1), 1–6. https://doi.org/10.1186/s12936-022-04197-4 (2022).
Ahmed, A., Abubakr, M., Ali, Y., Siddig, E. E. & Mohamed, N. S. Vector control strategy for Anopheles stephensi in Africa. Lancet Microbe 3(6), e403. https://doi.org/10.1016/S2666-5247(22)00039-8 (2022).
Villena, O. C., Ryan, S. J., Murdock, C. C. & Johnson, L. R. Temperature impacts the environmental suitability for malaria transmission by Anopheles gambiae and Anopheles stephensi. Ecology 103(8), e3685. https://doi.org/10.1002/ecy.3685 (2022).
Organization, W. H. Global malaria report 2020: global Malaria Programme 2020.
Alonso, P. & Noor, A. M. The global fight against malaria is at crossroads. The Lancet 390(10112), 2532–2534. https://doi.org/10.1016/S0140-6736(17)33080-5 (2017).
Sousa, R., Cunha, A. C. & Fernandes-Ferreira, M. The potential of Apiaceae species as sources of singular phytochemicals and plant-based pesticides. Phytochemistry 187, 112714. https://doi.org/10.1016/j.phytochem.2021.112714 (2021).
Moemenbellah-Fard, M. D. et al. Chemical composition and repellent activity of nine medicinal essential oils against Anopheles stephensi, the main malaria vector. Int. J. Trop. Insect. Sci. 41(2), 1325–1332. https://doi.org/10.1007/s42690-020-00325-2 (2021).
Chaudhari, A. K., Singh, V. K., Kedia, A., Das, S. & Dubey, N. K. Essential oils and their bioactive compounds as eco-friendly novel green pesticides for management of storage insect pests: Prospects and retrospects. Environ. Sci. Pollut. Res. Int. 28, 18918–18940. https://doi.org/10.1007/s11356-021-12841-w (2021).
Ngegba, P. M., Cui, G., Khalid, M. Z., Li, Y. & Zhong, G. Prospects of botanical compounds and pesticides as sustainable management strategies against Spodoptera frugiperda. J. Econ. Entomol. 115(6), 1834–1845. https://doi.org/10.1093/jee/toac157 (2022).
Bora, K. S. & Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 49(1), 101–109. https://doi.org/10.3109/13880209.2010.497815 (2011).
Abad, M. J., Bedoya, L. M., Apaza, L. & Bermejo, P. The artemisia L. Genus: A review of bioactive essential oils. Molecules 17(3), 2542–2566. https://doi.org/10.3390/molecules17032542 (2012).
Willcox, M. Artemisia species: From traditional medicines to modern antimalarials–and back again. J. Altern. Complement Med. 15(2), 101–109. https://doi.org/10.1089/acm.2008.0327 (2009).
Lopes-Lutz, D., Alviano, D. S., Alviano, C. S. & Kolodziejczyk, P. P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 69(8), 1732–1738. https://doi.org/10.1016/j.phytochem.2008.02.014 (2008).
Deb, M. & Kumar, D. Bioactivity and efficacy of essential oils extracted from Artemisia annua against Tribolium casteneum (Herbst. 1797) (Coleoptera: Tenebrionidae): An eco-friendly approach. Ecotoxicol. Environ. Saf. 189, 109988. https://doi.org/10.1016/j.ecoenv.2019.109988 (2020).
Mousavi, M. & Valizadegan, O. Insecticidal effects of Artemisia dracunculus L. (Asteraceae) essential oil on adult of Aphis gossypii Glover (Hemiptera: Aphididae) under laboratory conditions. Arch. Phytopathol. Plant. Prot. 47(14), 1737–1745. https://doi.org/10.1080/03235408.2013.856098 (2014).
Tabari, M. A., Youssefi, M. R. & Benelli, G. Eco-friendly control of the poultry red mite, Dermanyssus gallinae (Dermanyssidae), using the α-thujone-rich essential oil of Artemisia sieberi (Asteraceae): Toxic and repellent potential. Parasitol. Res. 116(5), 1545–1551. https://doi.org/10.1007/s00436-017-5431-0 (2017).
Zarrabi, A. et al. Nanoliposomes and tocosomes as multifunctional nanocarriers for the encapsulation of nutraceutical and dietary molecules. Molecules https://doi.org/10.3390/molecules25030638 (2020).
Asadi, P., Mehravaran, A., Soltanloo, N., Abastabar, M. & Akhtari, J. Nanoliposome-loaded antifungal drugs for dermal administration: A review. Curr. Med. Mycol. 7(1), 71–78. https://doi.org/10.18502/cmm.7.1.6247 (2021).
Bozzuto, G. & Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 10, 975–999. https://doi.org/10.2147/ijn.S68861 (2015).
Sanei-Dehkordi, A., Moemenbellah-Fard, M. D., Saffari, M., Zarenezhad, E. & Osanloo, M. Nanoliposomes containing limonene and limonene-rich essential oils as novel larvicides against malaria and filariasis mosquito vectors. BMC Complement Med. Ther. 22(1), 1–9. https://doi.org/10.1186/s12906-022-03624-y (2022).
Sanei-Dehkordi, A., Heiran, R., Moemenbellah-Fard, M. D., Sayah, S. & Osanloo, M. Nanoliposomes containing carvacrol and carvacrol-rich essential oils as effective mosquitoes larvicides. BioNanoScience https://doi.org/10.1007/s12668-022-00971-5 (2022).
Siria, D. J. et al. Evaluation of a simple polytetrafluoroethylene (PTFE)-based membrane for blood-feeding of malaria and dengue fever vectors in the laboratory. Parasit Vectors 11(1), 236. https://doi.org/10.1186/s13071-018-2823-7 (2018).
Rahmani, H. et al. Chitosan nanoparticles containing α-pinene and Rosmarinus officinalis L. essential oil: Effects on human melanoma cells’ viability and expression of apoptosis-involved genes. Polym. Bull. https://doi.org/10.1007/s00289-023-04839-w (2023).
Adams, R. P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry (Texensis Publishing, 2017).
Sanei-Dehkordi, A., Hatami, S., Zarenezhad, E., Montaseri, Z. & Osanloo, M. Efficacy of nanogels containing carvacrol, cinnamaldehyde, thymol, and a mix compared to a standard repellent against Anopheles stephensi. Ind. Crops Prod. 189, 115883. https://doi.org/10.1016/j.indcrop.2022.115883 (2022).
Sanei-Dehkordi, A. et al. Larvicidal effects of nanoliposomes containing clove and cinnamon essential oils, eugenol, and cinnamaldehyde against the main malaria vector, anopheles stephensi liston. Psyche A J. Entomol. 2022, 1–8. https://doi.org/10.1155/2022/9991238 (2022).
Organization, W. H. Guidelines for laboratory and field testing of mosquito larvicides. World Health Organization, 2005.
Nichols, G. et al. A review of the terms agglomerate and aggregate with a recommendation for nomenclature used in powder and particle characterization. J. Pharm. Sci. 91(10), 2103–2109. https://doi.org/10.1002/jps.10191 (2002).
Sokolov, S. V., Tschulik, K., Batchelor-McAuley, C., Jurkschat, K. & Compton, R. G. Reversible or not? Distinguishing agglomeration and aggregation at the nanoscale. Anal. Chem. 87(19), 10033–10039. https://doi.org/10.1021/acs.analchem.5b02639 (2015).
Woodrow, J. E., Gibson, K. A. & Seiber, J. N. Pesticides and Related toxicants in the atmosphere. Rev. Environ. Contam. Toxicol. 247, 147–196. https://doi.org/10.1007/398_2018_19 (2019).
Rani, L. et al. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 283, 124657. https://doi.org/10.1016/j.jclepro.2020.124657 (2021).
Bellinato, D. F. et al. Resistance status to the insecticides temephos, deltamethrin, and diflubenzuron in Brazilian Aedes aegypti populations. Biomed. Res. Int. 2016, 8603263. https://doi.org/10.1155/2016/8603263 (2016).
Tong, H., Su, Q., Zhou, X. & Bai, L. Field resistance of Spodoptera litura (Lepidoptera: Noctuidae) to organophosphates, pyrethroids, carbamates and four newer chemistry insecticides in Hunan, China. J. Pest. Sci. 86(3), 599–609. https://doi.org/10.1007/s10340-013-0505-y (2013).
Ranson, H. et al. Pyrethroid resistance in African anopheline mosquitoes: What are the implications for malaria control?. Trends Parasitol. 27(2), 91–98. https://doi.org/10.1016/j.pt.2010.08.004 (2011).
Noorpisheh Ghadimi, S., Sharifi, N. & Osanloo, M. The leishmanicidal activity of essential oils: A systematic review. J. Herb. Med. Pharmacol. 9(4), 300–308. https://doi.org/10.34172/jhp.2020.38 (2020).
Chaudhari, A. K. et al. Essential oils and their bioactive compounds as green preservatives against fungal and mycotoxin contamination of food commodities with special reference to their nanoencapsulation. Environ. Sci. Pollut. Res. Int. 26(25), 25414–25431. https://doi.org/10.1007/s11356-019-05932-2 (2019).
Jankowska, M., Rogalska, J., Wyszkowska, J. & Stankiewicz, M. Molecular targets for components of essential oils in the insect nervous system-A review. Molecules https://doi.org/10.3390/molecules23010034 (2017).
Jankowska, M., Lapied, B., Jankowski, W. & Stankiewicz, M. The unusual action of essential oil component, menthol, in potentiating the effect of the carbamate insecticide, bendiocarb. Pestic. Biochem. Physiol. 158, 101–111. https://doi.org/10.1016/j.pestbp.2019.04.013 (2019).
de Monbrison, F. et al. In vitro antimalarial activity of flavonoid derivatives dehydrosilybin and 8-(1;1)-DMA-kaempferide. Acta Trop. 97(1), 102–107. https://doi.org/10.1016/j.actatropica.2005.09.004 (2006).
Raeisi, M. et al. Essential oil of tarragon (Artemisia dracunculus) antibacterial activity on Staphylococcus aureus and Escherichia coli in culture media and Iranian white cheese. Iran. J. Microbiol. 4(1), 30–34 (2012).
Osanloo, M. et al. Nanoemulsion and nanogel containing Artemisia dracunculus essential oil; Larvicidal effect and antibacterial activity. BMC Res. Notes 15(1), 276. https://doi.org/10.1186/s13104-022-06135-8 (2022).
Irshaid, F., Mansi, K., Bani-Khaled, A. & Aburjia, T. Hepatoprotetive, cardioprotective and nephroprotective actions of essential oil extract of Artemisia sieberi in alloxan induced diabetic rats. Iran. J. Pharm. Res. 11(4), 1227–1234 (2012).
Farzaneh, M., Ahmadzadeh, M., Hadian, J. & Tehrani, A. S. Chemical composition and antifungal activity of the essential oils of three species of Artemisia on some soil-borne phytopathogens. Commun. Agric. Appl. Biol. Sci. 71(3 Pt B), 1327–1333 (2006).
Boroomand, N., Sadat-Hosseini, M., Moghbeli, M. & Farajpour, M. Phytochemical components, total phenol and mineral contents and antioxidant activity of six major medicinal plants from Rayen, Iran. Nat. Prod. Res. 32(5), 564–567. https://doi.org/10.1080/14786419.2017.1315579 (2018).
Moemenbellah-Fard, M. D. et al. A natural nanogel with higher efficacy than a standard repellent against the primary malaria mosquito vector, Anopheles stephensi liston. Chem. Pap. 76(3), 1767–1776. https://doi.org/10.1007/s11696-021-02006-x (2022).
Firooziyan, S. et al. Preparation of nanoemulsion of Cinnamomum zeylanicum oil and evaluation of its larvicidal activity against a main malaria vector Anopheles stephensi. J. Environ. Health Sci. Eng. 19(1), 1025–1034. https://doi.org/10.1007/s40201-021-00667-0 (2021).
Sugumar, S. et al. Nanoemulsion of eucalyptus oil and its larvicidal activity against Culex quinquefasciatus. Bull. Entomol. Res. 104(3), 393–402. https://doi.org/10.1017/S0007485313000710 (2014).
Jesser, E. N. et al. Optimization and characterization of essential oil nanoemulsions using ultrasound for new ecofriendly insecticides. ACS Sustain. Chem. Eng. 8(21), 7981–7992. https://doi.org/10.1021/acssuschemeng.0c02224 (2020).
Ferreira, R. et al. Nano-emulsification enhances the larvicidal potential of the essential oil of Siparuna guianensis (Laurales: Siparunaceae) against Aedes (Stegomyia) aegypti (Diptera: Culicidae). J. Med. Entomol. 57(3), 788–796. https://doi.org/10.1093/jme/tjz221 (2020).
Shahzad, K. & Manzoor, F. Nanoformulations and their mode of action in insects: A review of biological interactions. Drug Chem. Toxicol. 44(1), 1–11. https://doi.org/10.1080/01480545.2018.1525393 (2021).
Sanei-Dehkordi, A. et al. Chitosan nanoparticles containing Elettaria cardamomum and Cinnamomum zeylanicum essential oils; Repellent and larvicidal effects against a malaria mosquito vector, and cytotoxic effects on a human skin normal cell line. Chem. Pap. 76, 6545–6556. https://doi.org/10.1007/s11696-021-01829-y (2021).
Oliveira, A. E. et al. Development of a larvicidal nanoemulsion with Pterodon emarginatus vogel oil. PLoS One 11(1), e0145835. https://doi.org/10.1371/journal.pone.0145835 (2016).
Ferreira, R. M. et al. A herbal oil in water nano-emulsion prepared through an ecofriendly approach affects two tropical disease vectors. Rev. Bras. Farmacogn. 29(6), 778–784. https://doi.org/10.1016/j.bjp.2019.05.003 (2019).
Firooziyan, S. et al. Nanoemulsion of Myrtus communis essential oil and evaluation of its larvicidal activity against Anopheles stephensi. Arab. J. Chem. 15(9), 104064. https://doi.org/10.1016/j.arabjc.2022.104064 (2022).
Alipanah, H. et al. Nanoemulsion and nanogel containing Eucalyptus globulus essential oil; Larvicidal activity and antibacterial properties. Interdiscip. Perspect. Infect. Dis. 2022, 1616149. https://doi.org/10.1155/2022/1616149 (2022).
Funding
Fasa University of Medical Sciences supported this study (grant No. 401243).
Author information
Authors and Affiliations
Contributions
A.S.D. reared mosquitoes and carried out larvicidal bioassays. A.G. wroth introduction and discussion. E.Z. interpreted ATR-FTIR. H.Q. and M.N. reviewed the literature. M.O. designed the study, prepared nanoliposomes, analyzed data, and drafted the M.S. All authors contributed to drafting and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sanei-Dehkordi, A., Ghasemian, A., Zarenezhad, E. et al. Nanoliposomes containing three essential oils from the Artemisia genus as effective larvicides against Aedes aegypti and Anopheles stephensi. Sci Rep 13, 11002 (2023). https://doi.org/10.1038/s41598-023-38284-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38284-6
- Springer Nature Limited