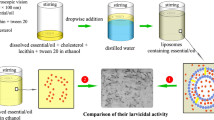
Abstract
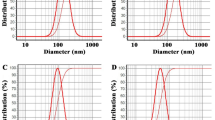
The low efficacy of essential oil-based larvicides and their components’ volatility have challenged their use as green alternatives. In this study, the larvicidal effect of essential oils of Satureja khuzestanica and Zataria multiflora with their ingredients, carvacrol, was first investigated against two medically important mosquitoes. After confirming each sample’s successful loading in the nanoliposomes with a vesicle size of 110 ± 8, 70 ± 5, and 76 ± 5 nm, their larvicidal effects were also evaluated. These effects of nanoliposomal forms were greater than that of non-formulated states. The best-observed LC50 against Anopheles stephensi and Culex quinquefasciatus were related to nanoliposomes containing essential oils of Z. multiflora (10.88 µg/mL) and S. khuzestanica (16.74 µg/mL). These samples could be used as green larvicides for investigation against other mosquito populations.



Similar content being viewed by others
Data Availability
Ok.
Abbreviations
- GC-MS:
-
Gas chromatography-mass spectrometry
- DLS:
-
Dynamic light scattering
- ATR-FTIR:
-
Attenuated total reflection-Fourier transform infrared
- EO:
-
Essential oil
- CarLipo:
-
Nanoliposomes containing carvacrol
- SKEO:
-
Satureja khuzestanica Essential oil
- SKLipo:
-
Nanoliposomes containing Satureja khuzestanica essential oil
- ZMEO:
-
Zataria multiflora Essential oil
- ZMLipo:
-
Nanoliposomes containing Zataria multiflora essential oil
References
Calzolari, M. (2016). Mosquito-borne diseases in Europe: An emerging public health threat. Reports in Parasitology, 5, 1–12. https://doi.org/10.2147/rip.s56780.
Corby-Harris, V., Drexler, A., De Jong, L. W., Antonova, Y., Pakpour, N., Ziegler, R., et al. (2010). Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes. PLoS Pathogens, 6(7), e1001003. https://doi.org/10.1371/journal.ppat.1001003.
Akbarzadeh, M., Soltani, A., Moemenbellah-Fard, M. D., Khoshnoud, M. J., & Azizi, K. (2020). Larvicidal, repellent, and histopathologic effects of Citrullus colocynthis against the malaria vector. Toxicological and Environmental Chemistry, 102(1–4), 92–104. https://doi.org/10.1080/02772248.2020.1770254.
WHO (2019) World malaria report 2019. https://www.who.int/malaria/publications/world_malaria_report/en/. Accessed Dec 2020
Ghahremani, L., Azizi, M., Moemenbellah-Fard, M. D., & Ghaem, H. (2019). Malaria preventive behaviors among housewives in suburbs of Bandar-Abbas City, south of Iran: Interventional design based on PRECEDE model. Pathog Glob Health, 113(1), 32–38. https://doi.org/10.1080/20477724.2019.1583847.
Kent, R. J., Crabtree, M. B., & Miller, B. R. (2010). Transmission of West Nile virus by Culex quinquefasciatus say infected with Culex Flavivirus Izabal. PLoS Neglected Tropical Diseases, 4(5), e671. https://doi.org/10.1371/journal.pntd.0000671.
Arensburger, P., Megy, K., Waterhouse, R. M., Abrudan, J., Amedeo, P., Antelo, B., et al. (2010). Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science, 330(6000), 86–88. https://doi.org/10.1126/science.1191864.
Hemingway, J., Beaty, B. J., Rowland, M., Scott, T. W., & Sharp, B. L. (2006). The innovative vector control consortium: Improved control of mosquito-borne diseases. Trends in parasitology, 22(7), 308–312. https://doi.org/10.1016/j.pt.2006.05.003.
Osanloo, M., Amini, S. M., Sedaghat, M. M., & Amani, A. (2019). Larvicidal activity of chemically synthesized silver nanoparticles against Anopheles stephensi. J Pharm Negat Results, 10(1), 69–72. https://doi.org/10.4103/jpnr.jpnr_18_17.
Salim-Abadi, Y., Asadpour, M., Sharifi, I., Sanei-Dehkordi, A., Gorouhi, M. A., Paksa, A., et al. (2017). Baseline susceptibility of filarial vector Culex quinquefasciatus (Diptera: Culicidae) to five insecticides with different modes of action in southeast of Iran. Journal of Arthropod-Borne Diseases, 11(4), 453–462.
Esmaili, F., Sanei-Dehkordi, A., Amoozegar, F., & Osanloo, M. (2021). A review on the use of essential oil-based nanoformulations in control of mosquitoes. Biointerface Research Applied Chemistry, 11(5), 12516–12529. https://doi.org/10.33263/briac115.1251612529.
Osanloo, M., Arish, J., & Sereshti, H. (2019). Developed methods for the preparation of electrospun nanofibers containing plant-derived oil or essential oil: A systematic review. Polymer Bulletin, 77(11), 6085–6104. https://doi.org/10.1007/s00289-019-03042-0.
Sugumar, S., Clarke, S., Nirmala, M., Tyagi, B., Mukherjee, A., & Chandrasekaran, N. (2014). Nanoemulsion of eucalyptus oil and its larvicidal activity against Culex quinquefasciatus. Bulletin of Entomological Research, 104(3), 393–402. https://doi.org/10.1017/S0007485313000710.
Jesser, E. N., Yeguerman, C. O., Gili, V. O., Santillan, G. O., Murray, A. P., & DOMINI C, Werdin González JO. (2020). Optimization and characterization of essential oil nanoemulsions using ultrasound for new ecofriendly insecticides. ACS Sustain Chem Eng, 8(21), 7981–7992. https://doi.org/10.1021/acssuschemeng.0c02224.
Osanloo, M., Sedaghat, M. M., Sereshti, H., & Amani, A. (2019). Chitosan nanocapsules of tarragon essential oil with low cytotoxicity and long-lasting activity as a green nano-larvicide. Journal Nanostructured, 9(4), 723–735. https://doi.org/10.22052/JNS.2019.04.014.
Pavela, R., Pavoni, L., Bonacucina, G., Cespi, M., Cappellacci, L., Petrelli, R., et al. (2021). Encapsulation of Carlina acaulis essential oil and carlina oxide to develop long-lasting mosquito larvicides: Microemulsions versus nanoemulsions. Journal of Pest Science. https://doi.org/10.1007/s10340-020-01327-2.
Trucillo, P., Ferrari, P., Campardelli, R., Reverchon, E., & Perego, P. (2020). A supercritical assisted process for the production of amoxicillin loaded liposomes for anti-microbial applications. Journal of Supercritical Fluids, 163, 104842. https://doi.org/10.1016/j.supflu.2020.104842.
Mishra, D. K., Shandilya, R., & Mishra, P. K. (2018). Lipid based nanocarriers: A translational perspective. Nanomedicine, 14(7), 2023–2050. https://doi.org/10.1016/j.nano.2018.05.021.
Zahin, N., Anwar, R., Tewari, D., Kabir, M. T., Sajid, A., Mathew, B., & Uddin, M. S. (2020). Nanoparticles and its biomedical applications in health and diseases: Special focus on drug delivery. Environmental Science and Pollution Research, 27(16), 19151–19168. https://doi.org/10.1007/s11356-019-05211-0.
Sebaaly, C., Jraij, A., Fessi, H., Charcosset, C., & Greige-Gerges, H. (2015). Preparation and characterization of clove essential oil-loaded liposomes. Food Chemistry, 178, 52–62. https://doi.org/10.1016/j.foodchem.2015.01.067.
Wen, Z., Liu, B., Zheng, Z., You, X., Pu, Y., & Li, Q. (2010). Preparation of liposomes entrapping essential oil from Atractylodes macrocephala Koidz by modified RESS technique. Chemical Engineering Research and Design, 88(8), 1102–1107. https://doi.org/10.1016/j.cherd.2010.01.020.
Kayedi, M. H., Haghdoost, A. A., Salehnia, A., & Khamisabadi, K. (2014). Evaluation of repellency effect of essential oils of Satureja khuzestanica (Carvacrol), Myrtus communis (Myrtle), Lavendula officinalis and Salvia sclarea using standard WHO repellency tests. Journal of Arthropod-Borne Diseases, 8(1), 60–68.
Lucia, A., & Guzmán, E. (2020). Emulsions containing essential oils, their components or volatile semiochemicals as promising tools for insect pest and pathogen management. Advances in Colloid and Interface Science, 287, 102330. https://doi.org/10.1016/j.cis.2020.102330.
Farahani, S., Bandani, A. R., & Amiri, A. (2020). Toxicity and repellency effects of three essential oils on two populations of Tetranychus urticae (Acari: Tetranychidae). Persian Journal Acarol, 9(1), 67–81. https://doi.org/10.22073/pja.v9i1.55853.
Amirinezhad, M., Yousefzadi, M., Arman, M., & Rahimzadeh, M. (2018). Comparison of essential oils toxicity of Satureja khuzistanica and Satureja rechingeri on larvae of the barnacle Amphibalanus amphitrite. Modares Journal of Biotechnology, 9(3), 435–440.
Bassole, I., Guelbeogo, W., Nebie, R., Costantini, C., Sagnon, N., Kabore, Z., & Traore, S. (2003). Ovicidal and larvicidal activity against Aedes aegypti and Anopheles gambiae complex mosquitoes of essential oils extracted from three spontaneous plants of Burkina Faso. Parassitologia, 45(1), 23–26.
Kelidari, H. R., Moemenbellah-Fard, M. D., Morteza-Semnani, K., Amoozegar, F., Shahriari-Namadi, M., Saeedi, M., & Osanloo, M. (2021). Solid-lipid nanoparticles (SLN) s containing Zataria multiflora essential oil with no-cytotoxicity and potent repellent activity against Anopheles stephensi. Journal of Parasitic Diseases, 45(1), 101–108. https://doi.org/10.1007/s12639-020-01281-x.
Chegini, S. G., Abbasipour, H., Karimi, J., & Askarianzadeh, A. (2018). Toxicity of Shirazi thyme, Zataria multiflora essential oil to the tomato leaf miner, Tuta absoluta (Lepidoptera: Gelechiidae). International Journal of Tropical Insect Science, 38(4), 340–347. https://doi.org/10.1017/S1742758418000097.
Youssefi, M. R., Tabari, M. A., Esfandiari, A., Kazemi, S., Moghadamnia, A. A., Sut, S., & Dall’Acqua, S. (2019). Efficacy of two monoterpenoids, carvacrol and thymol, and their combinations against eggs and larvae of the West Nile vector Culex pipiens. Molecules, 24(10), 1867. https://doi.org/10.3390/molecules24101867.
Novato, T. P., Araújo, L. X., de Monteiro, C. M., Maturano, R., Senra Tde, O., da Silva, M. R., et al. (2015). Evaluation of the combined effect of thymol, carvacrol and (E)-cinnamaldehyde on Amblyomma sculptum (Acari: Ixodidae) and Dermacentor nitens (Acari: Ixodidae) larvae. Veterinary Parasitology, 212(3–4), 331–335. https://doi.org/10.1016/j.vetpar.2015.08.021.
Traboulsi, A. F., Taoubi, K., & el-Haj S, Bessiere JM, Rammal S. (2002). Insecticidal properties of essential plant oils against the mosquito Culex pipiens molestus (Diptera: Culicidae). Pest Management Science, 58(5), 491–495. https://doi.org/10.1002/ps.486.
Govindarajan, M., Rajeswary, M., Hoti, S. L., & Benelli, G. (2016). Larvicidal potential of carvacrol and terpinen-4-ol from the essential oil of Origanum vulgare (Lamiaceae) against Anopheles stephensi, Anopheles subpictus, Culex quinquefasciatus and Culex tritaeniorhynchus (Diptera: Culicidae). Research in Veterinary Science, 104, 77–82. https://doi.org/10.1016/j.rvsc.2015.11.011.
Andrade-Ochoa, S., Sánchez-Aldana, D., Chacón-Vargas, K. F., Rivera-Chavira, B. E., & Sánchez-Torres, L. E. (2018). Oviposition deterrent and larvicidal and pupaecidal activity of seven essential oils and their major components against Culex quinquefasciatus Say (Diptera: Culicidae): Synergism-antagonism effects. Insects, 9(1), 25. https://doi.org/10.3390/insects9010025.
Suntres, Z. E., Coccimiglio, J., & Alipour, M. (2015). The bioactivity and toxicological actions of carvacrol. Critical Reviews in Food Science and Nutrition, 55(3), 304–318. https://doi.org/10.1080/10408398.2011.653458.
Abedinpour, N., Ghanbariasad, A., Taghinezhad, A., & Osanloo, M. (2021). Preparation of nanoemulsions of Mentha piperita essential oil and investigation of their cytotoxic effect on human breast cancer lines. BioNanoScience. https://doi.org/10.1007/s12668-021-00827-4.
Siria, D. J., Batista, E. P. A., Opiyo, M. A., Melo, E. F., Sumaye, R. D., Ngowo, H. S., et al. (2018). Evaluation of a simple polytetrafluoroethylene (PTFE)-based membrane for blood-feeding of malaria and dengue fever vectors in the laboratory. Parasites & Vectors, 11(1), 236. https://doi.org/10.1186/s13071-018-2823-7.
WHO (2005) Guidelines for laboratory and field testing of mosquito larvicides. https://whqlibdoc.who.int/hq/2005/WHO_CDS_WHOPES_GCDPP_2005.13.pdf. AccessedDec2020
Finney, D. J. (1971). Probit analysis (3rd ed.). Cambridge University Press.
Can, B. K. (2008). Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Current Pharmaceutical Design, 14(29), 3106–3119. https://doi.org/10.2174/138161208786404227.
Veldhuizen, E. J., Tjeerdsma-van Bokhoven, J. L., Zweijtzer, C., Burt, S. A., & Haagsman, H. P. (2006). Structural requirements for the antimicrobial activity of carvacrol. Journal of Agriculture and Food Chemistry, 54(5), 1874–1879. https://doi.org/10.1021/jf052564y.
Pavela, R., Morshedloo, M. R., Mumivand, H., Khorsand, G. J., Karami, A., Maggi, F., et al. (2020). Phenolic monoterpene-rich essential oils from Apiaceae and Lamiaceae species: Insecticidal activity and safety evaluation on non-target earthworms. Entomologia Generalis, 40, 421–435. https://doi.org/10.1127/entomologia/2020/1131.
Khakzad, S., Rahmani, F., Hojjati, M., & Tabandeh, M. R. (2019). Anti-carcinogenic effects of Satureja khuzistanica and Zataria multiflora essential oils on K562 cell line proliferation. Journal of Food and Bioprocess Engineering, 2(2), 127–132.
Saidi, M. (2014). Antioxidant activities and chemical composition of essential oils from Satureja khuzestanica, Oliveria decumbens and Thymus daenensis. Journal of Essential Oil-Bear Plants, 17(3), 513–521. https://doi.org/10.1080/0972060X.2014.901607.
Zandi-Sohani, N., & Ramezani, L. (2015). Evaluation of five essential oils as botanical acaricides against the strawberry spider mite Tetranychus turkestani Ugarov and Nikolskii. International Biodeterioration & Biodegradation, 98, 101–106. https://doi.org/10.1016/j.ibiod.2014.12.007.
Raeisi, M., GhorbaniBidkorpeh, F., Hashemi, M., Tepe, B., Moghaddam, Z., AmanMohammadi, M., & Noori, S. M. A. (2019). Chemical composition and antibacterial and antioxidant properties of essential oils of Zataria multiflora, Artemisia deracunculus and Mentha piperita. Medical Laboratory Journal, 13(2), 1–7. https://doi.org/10.29252/mlj.13.2.1.
Ardekani, N. T., Khorram, M., Zomorodian, K., Yazdanpanah, S., Veisi, H., & Veisi, H. (2019). Evaluation of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with Zataria multiflora essential oil as potential wound dressing. International Journal of Biological Macromolecules, 125, 743–750. https://doi.org/10.1016/j.ijbiomac.2018.12.085.
Khatibi, S., Misaghi, A., Moosavy, M., AkhondzadehBasti, A., Mohamadian, S., & Khanjari, A. (2018). Effect of nanoliposomes containing Zataria multiflora Boiss. Essential oil on gene expression of Shiga toxin 2 in Escherichia coli O157: H7. Journal Applied Microbiology, 124(2), 389–397. https://doi.org/10.1111/jam.13641.
Khatibi, S. A., Misaghi, A., Moosavy, M.-H., Amoabediny, G., & Basti, A. A. (2014). Effect of preparation methods on the properties of Zataria multiflora Boiss. Essential oil loaded: nanoliposomes Characterization of size, encapsulation efficiency and stability. Pharmaceutical Sciences, 20(4), 141–148. https://doi.org/10.5681/PS.2015.003.
Liolios, C. C., Gortzi, O., Lalas, S., Tsaknis, J., & Chinou, I. (2009). Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chemistry, 112(1), 77–83. https://doi.org/10.1016/j.foodchem.2008.05.060.
Engel, J. B., Heckler, C., Tondo, E. C., Daroit, D. J., & da Silva, M. P. (2017). Antimicrobial activity of free and liposome-encapsulated thymol and carvacrol against Salmonella and Staphylococcus aureus adhered to stainless steel. International Journal of Food Microbiology, 252, 18–23. https://doi.org/10.1016/j.ijfoodmicro.2017.04.003.
Liu, H., Xu, Q., Zhang, L., & Liu, N. (2005). Chlorpyrifos resistance in mosquito Culex quinquefasciatus. Journal of Medical Entomology, 42(5), 815–820. https://doi.org/10.1093/jmedent/42.5.815.
Lima, E. P., Paiva, M. H. S., de Araújo, A. P., da Silva, ÉV. G., da Silva, U. M., de Oliveira, L. N., et al. (2011). Insecticide resistance in Aedes aegypti populations from Ceará. Brazil. Parasites & vectors, 4(1), 1–12. https://doi.org/10.1186/1756-3305-4-5.
Casimiro, S., Coleman, M., Hemingway, J., & Sharp, B. (2014). Insecticide resistance in Anopheles arabiensis and Anopheles gambiae from Mozambique. Journal of Medical Entomology, 43(2), 276–282. https://doi.org/10.1603/0022-2585(2006)043[0276:iriaaa]2.0.co;2.
Baek, S. H., Kang, J. H., Hwang, Y. H., Ok, K. M., Kwak, K., & Chun, H. S. (2016). Detection of methomyl, a carbamate insecticide, in food matrices using terahertz time-domain spectroscopy. J Infrared Millim Terahertz Waves, 37(5), 486–497. https://doi.org/10.1007/s10762-015-0234-9.
Schulze, H., Scherbaum, E., Anastassiades, M., Vorlová, S., Schmid, R. D., & Bachmann, T. T. (2002). Development, validation, and application of an acetylcholinesterase-biosensor test for the direct detection of insecticide residues in infant food. Biosensors & Bioelectronics, 17(11–12), 1095–1105. https://doi.org/10.1016/s0956-5663(02)00104-5.
Damalas, C. A., & Eleftherohorinos, I. G. (2011). Pesticide exposure, safety issues, and risk assessment indicators. International Journal of Environmental Research and Public Health, 8(5), 1402–1419. https://doi.org/10.3390/ijerph8051402.
Isman, M. B. (2000). Plant essential oils for pest and disease management. Crop protection, 19(8–10), 603–608. https://doi.org/10.1016/s0261-2194(00)00079-x.
Osanloo, M., Sedaghat, M. M., Esmaeili, F., & Amani, A. (2018). Larvicidal activity of essential oil of Syzygium aromaticum (Clove) in comparison with its major constituent, eugenol, against Anopheles stephensi. Journal of Arthropod-Borne Diseases, 12(4), 361–369.
Silva, V. B., Travassos, D. L., Nepel, A., Barison, A., Costa, E. V., Scotti, L., et al. (2017). Synthesis and chemometrics of thymol and carvacrol derivatives as larvicides against Aedes aegypti. Journal of Arthropod-Borne Diseases, 11(2), 315–330.
Lima, T. C., Kweka, E. J., Marciale, C. M., & de Sousa, D. P. (2016). Larvicidal activity of essential oil constituents against malaria vector, Anopheles gambiae (Diptera: Culicidae). Natural Products Communications, 11(10), 1539–1540.
Vatandoost, H., & Vaziri, V. (2004). Larvicidal activity of a neem tree extract [Neemarin] against mosquito larvae in the Islamic Republic of Iran. Eastern Mediterranean Health Journal, 10(4–5), 573–581.
Hadjiakhoondi, A., Vatandoost, H., Abousaber, M., Khanavi, M., & Abdi, L. (2008). Chemical composition of the essential oil of Tagetes minuta L. and its effects on Anopheles stephensi larvae in Iran. Journal Medicinal Plants, 7(26), 33–39.
Torabi Pour, H., Shayeghi, M., Vatandoost, H., & Abai, M. R. (2016). Study on larvicidal effects of essential oils of three Iranian native plants against larvae of Anopheles stephensi (Liston). Vector Biol J, 1(2), 2–6. https://doi.org/10.4172/2473-4810.1000109.
Soonwera, M. (2015). Efficacy of essential oil from Cananga odorata (Lamk) Hook.f. & Thomson (Annonaceae) against three mosquito species Aedes aegypti (L.), Anopheles dirus (Peyton and Harrison), and Culex quinquefasciatus (Say). Parasitology Research, 114(12), 4531–4543. https://doi.org/10.1007/s00436-015-4699-1.
Sharifi-Rad, J., Sureda, A., Tenore, G. C., Daglia, M., Sharifi-Rad, M., Valussi, M., et al. (2017). Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules (Basel, Switzerland), 22(1), 70. https://doi.org/10.3390/molecules22010070.
Turek, C., & Stintzing, F. C. (2013). Stability of essential oils: A review. Comprehensive Reviews in food Science and Food Safety, 12(1), 40–53. https://doi.org/10.1111/1541-4337.12006.
Ashbaugh, H. S., & Paulaitis, M. E. (2001). Effect of solute size and solute–water attractive interactions on hydration water structure around hydrophobic solutes. Journal of the American Chemical Society, 123(43), 10721–10728. https://doi.org/10.1021/ja016324k.
Carlsson, J., & Åqvist, J. (2006). Calculations of solute and solvent entropies from molecular dynamics simulations. Physical Chemistry Chemical Physics: PCCP, 8(46), 5385–5395. https://doi.org/10.1039/b608486a.
Prakash, A., Baskaran, R., Paramasivam, N., & Vadivel, V. (2018). Essential oil based nanoemulsions to improve the microbial quality of minimally processed fruits and vegetables: A review. Food Research International, 111, 509–523. https://doi.org/10.1016/j.foodres.2018.05.066.
Das, S., Singh, V. K., Dwivedy, A. K., Chaudhari, A. K., & Dubey, N. K. (2021). Nanostructured Pimpinella anisum essential oil as novel green food preservative against fungal infestation, aflatoxin B1 contamination and deterioration of nutritional qualities. Food Chemistry, 344, 128574. https://doi.org/10.1016/j.foodchem.2020.128574.
Zou, Y., Priebe, W., & Perez-Soler, R. (1996). Lyophilized preliposomal formulation of the non-cross-resistant anthracycline annamycin: Effect of surfactant on liposome formation, stability and size. Cancer Chemotherapy and Pharmacology, 39(1–2), 103–108. https://doi.org/10.1007/s002800050544.
Park, J.-B., Noh, H.-g, Jung, J.-H., Kim, J.-M., & Kang, C.-Y. (2012). Enhanced transdermal delivery and optimization of nano-liposome preparation using hydrophilic drug. Journal of Pharmaceutical Investigation, 42(2), 57–63. https://doi.org/10.1007/s40005-012-0009-4.
Tcholakova, S., Mitrinova, Z., Golemanov, K., Denkov, N. D., Vethamuthu, M., & Ananthapadmanabhan, K. P. (2011). Control of Ostwald ripening by using surfactants with high surface modulus. Langmuir, 27(24), 14807–14819. https://doi.org/10.1021/la203952p.
Shahzad, K., & Manzoor, F. (2021). Nanoformulations and their mode of action in insects: A review of biological interactions. Drug and Chemical Toxicology, 44(1), 1–11. https://doi.org/10.1080/01480545.2018.1525393.
Ferreira, R., D’Haveloose, N. P., Cruz, R. A. S., Araujo, R. S., Carvalho, J. C. T., Rocha, L., et al. (2020). Nano-emulsification enhances the larvicidal potential of the essential oil of Siparuna guianensis (Laurales: Siparunaceae) against Aedes (Stegomyia) aegypti (Diptera: Culicidae). Journal of Medical Entomology, 57(3), 788–796. https://doi.org/10.1093/jme/tjz221.
Osanloo, M., Sedaghat, M. M., Sereshti, H., Amani, A. (2019) Nano-encapsulated tarragon (Artemisia dracunculus) essential oil as a sustained release nano-larvicide. Journal of Contemporary Medical, 5 (2), 82–89. https://doi.org/10.22317/jcms.v5i2.570
Kavallieratos, N. G., Nika, E. P., Skourti, A., Ntalli, N., Boukouvala, M. C., Ntalaka, C. T., et al. (2021). Developing a Hazomalania voyronii essential oil nanoemulsion for the eco-friendly management of Tribolium confusum, Tribolium castaneum and Tenebrio molitor larvae and adults on stored wheat. Molecules, 26(6), 1812. https://doi.org/10.3390/molecules26061812.
Benelli, G., Pavoni, L., Zeni, V., Ricciardi, R., Cosci, F., Cacopardo, G., et al. (2020). Developing a highly stable Carlina acaulis essential oil nanoemulsion for managing Lobesia botrana. Nanomaterials, 10(9), 1867. https://doi.org/10.3390/nano10091867.
Chen, J., Wang, W., Xu, Y., & Zhang, X. (2011). Slow-release formulation of a new biological pesticide, pyoluteorin, with mesoporous silica. Journal of Agriculture and Food Chemistry, 59(1), 307–311. https://doi.org/10.1021/jf103640t.
Linxin, D., He, J., Borui, L., Nana, W., & Song, L. (2020). Study of a new 3D MOF and its adsorption, slow release and biological activity in water-soluble and oil-soluble pesticides. Polyhedron, 190, 114752. https://doi.org/10.1016/j.poly.2020.114752.
Liu, B., Wang, Y., Yang, F., Wang, X., Shen, H., Cui, H., & Wu, D. (2016). Construction of a controlled-release delivery system for pesticides using biodegradable PLA-based microcapsules. Colloids and Surfaces B: Biointerfaces, 144, 38–45. https://doi.org/10.1016/j.colsurfb.2016.03.084.
Funding
Fasa University of Medical Sciences supported this study, grant No. 97502.
Author information
Authors and Affiliations
Contributions
ASD performed larvicidal tests and performed probit analysis. RH interpreted ATR-FTIR spectra. MDMF wrote related discussion about the larvicidal assays. SS reviewed the literature and wrote the introduction. MO designed the study, prepared nanoformulations, and drafted the manuscript. All authors contributed to the drafting of the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics Approval
This study has been approved by the ethics committee at Fasa University of Medical Sciences, IR.FUMS.REC.1400.114. Besides, this research did not involve in vivo or human study.
Informed Consent
This research did not involve human study; thus, no consent form was used.
Consent for Publication
Ok.
Competing Interests
None
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sanei-Dehkordi, A., Heiran, R., Moemenbellah-Fard, M.D. et al. Nanoliposomes Containing Carvacrol and Carvacrol-Rich Essential Oils as Effective Mosquitoes Larvicides. BioNanoSci. 12, 359–369 (2022). https://doi.org/10.1007/s12668-022-00971-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-022-00971-5