Abstract
A multi-zone kinetic model coupled with a dynamic slag generation model was developed for the simulation of hot metal and slag composition during the basic oxygen furnace (BOF) operation. The three reaction zones (i) jet impact zone, (ii) slag–bulk metal zone, (iii) slag–metal–gas emulsion zone were considered for the calculation of overall refining kinetics. In the rate equations, the transient rate parameters were mathematically described as a function of process variables. A micro and macroscopic rate calculation methodology (micro-kinetics and macro-kinetics) were developed to estimate the total refining contributed by the recirculating metal droplets through the slag–metal emulsion zone. The micro-kinetics involves developing the rate equation for individual droplets in the emulsion. The mathematical models for the size distribution of initial droplets, kinetics of simultaneous refining of elements, the residence time in the emulsion, and dynamic interfacial area change were established in the micro-kinetic model. In the macro-kinetics calculation, a droplet generation model was employed and the total amount of refining by emulsion was calculated by summing the refining from the entire population of returning droplets. A dynamic FetO generation model based on oxygen mass balance was developed and coupled with the multi-zone kinetic model. The effect of post-combustion on the evolution of slag and metal composition was investigated. The model was applied to a 200-ton top blowing converter and the simulated value of metal and slag was found to be in good agreement with the measured data. The post-combustion ratio was found to be an important factor in controlling FetO content in the slag and the kinetics of Mn and P in a BOF process.













Similar content being viewed by others
Abbreviations
- A :
-
Interfacial area (m2)
- C jm :
-
Concentration of jth component in metal, j = Si, C, Mn, and P (wt pct)
- C ji :
-
Concentration of jth component on the reaction interface (wt pct)
- \( C_{\text{jd}}^{\text{return}} \) :
-
Concentration of jth component of refining droplets (wt pct)
- Cp,m :
-
Heat capacity of bulk metal (J/kg)
- C p,s :
-
Heat capacity of slag (J/kg)
- d p :
-
Diameter of the droplet (m)
- D :
-
Diffusion coefficient of slag (m2/s)
- F G,T :
-
Temperature-corrected oxygen flow rate (Nm3/min)
- h :
-
Height of the cavity (m)
- k a :
-
Apparent rate constant (mol/m2 s atm)
- k g :
-
Gas phase mass transfer coefficient (mole/m2 s atm)
- \( k_{\text{d}}^{\text{em}} \) :
-
Overall mass transfer coefficient of droplet (m/s)
- \( k_{\text{jm}}^{\text{d}} \) :
-
Mass transfer coefficient in metal side of droplet (m/s)
- k ds :
-
Mass transfer coefficient in slag side of droplet (m/s)
- \( k_{\text{m}}^{\text{sm}} \) :
-
Overall mass transfer coefficient at slag–bulk metal interface (m/s)
- \( k_{\text{gm}}^{\text{m}} \) :
-
Mass transfer coefficient in the melt in jet impact area (m/s)
- L h :
-
Lance height (between lance tip and bath surface (m)
- m d :
-
Mass of a single droplet (kg)
- m d,p :
-
Average mass of droplets belongs to pth size class (kg)
- \( m_{\text{d}}^{\text{return}} \) :
-
Weight of a single droplet returns to the bath (kg)
- M :
-
Molecular weight (g/mol)
- \( N_{p}^{{{\text{eject}},{\text{t}}}} \) :
-
Number of droplets of pth class size ejects to the bath at blowing time t (–)
- \( N_{p}^{{{\text{return}},{\text{t}}}} \) :
-
Number of droplets of pth class size returns to the bath at blowing time t (–)
- N B,T :
-
Modified blowing number (–)
- \( P_{{{\text{CO}}_{2} }}^{\text{b}} \) :
-
Partial pressure of CO2 (atm)
- \( P_{{{\text{O}}_{2} }}^{\text{b}} \) :
-
Partial pressure of O2 (atm)
- PCR:
-
Post-combustion ratio (–)
- Re :
-
Reynolds number (–)
- R B,T :
-
Droplet generation rate (kg/min)
- Sh :
-
Sherwood number (–)
- Sc :
-
Schmidt number (–)
- r c :
-
Decarburization rate of the droplet (wt pct/s)
- \( r_{\text{c}}^{*} \) :
-
Critical decarburization for bloating (wt pct/s)
- r cav :
-
Cavity radius (m)
- t c :
-
Contact time between the metal droplet and slag (seconds)
- t res :
-
Residence time of droplet in emulsion (seconds)
- T s :
-
Interface temperature at slag–metal (K)
- T ∞ :
-
Temperature in the emulsion medium (K)
- T 0 :
-
Initial temperature of the metal drop at the time of ejection (K)
- u :
-
Velocity of the droplet (m/s)
- V d :
-
Volume of droplet (m3)
- W c :
-
Weight of carbon (kg)
- WC j :
-
Weight of impurity (kg)
- W D :
-
Weight of dolomite (kg)
- W d,p :
-
Weight proportion of droplet belongs to pth size class (kg)
- W L :
-
Weight of lime (kg)
- W m :
-
Weight of hot metal (kg)
- \( \Delta W_{{{\text{m}},{\text{ref}}}}^{t} \) :
-
Weight of refining hot metal in a numerical time step (kg)
- ΔW MOx :
-
Sum of oxide mass in a numerical time step (kg)
- W s :
-
Weight of slag (kg)
- \( W_{\text{jm}}^{\text{eject}} \) :
-
Weight of jth element in the hot metal ejected to the emulsion (kg)
- \( W_{\text{jm}}^{\text{return}} \) :
-
Weight of jth element in the hot metal return to the bath (kg)
- \( W_{\text{sc}}^{\text{m}} \) :
-
Weight of the melted scrap (kg)
- ρ d :
-
Density of droplet (kg/m3)
- ρ d,0 :
-
Initial density of droplet (kg/m3)
- ρ m :
-
Density of the bulk metal (kg/m3)
- ρ s :
-
Density of slag (kg/m3)
- λ m :
-
Thermal conductivity of liquid metal (W/m K)
- λ s :
-
Thermal conductivity of slag (W/m K)
- cav:
-
Cavity
- d:
-
Droplet
- m:
-
Hot metal
- P :
-
Number of classes in the droplet size spectrum
- eq:
-
Equilibrium
- hs:
-
Hot spot
- iz:
-
Impact zone
- em:
-
Emulsion
- sm:
-
Slag/metal
- gm:
-
Gas/metal
References
W. Knoop, B. Deo, A. Snoeijer, G. Unen, and R. Boom: Proc. 4th Int. Conf. Molten Slags Fluxes, ISIJ, Tokyo, 1992, pp. 302–07.
H. Jalkanen and L. Holappa: VII Int. Conf. Molten Slags Fluxes Salts, The South African Institute of Mining and Metallurgy, 2004, pp. 71–76.
A. K. Shukla, B. Deo, S. Millman, B. Snoeijer, A. Overbosch and A. Kapilashrami: Steel Research International, 2010, vol. 81, pp. 940-48.
I. H. Jung, P. Hudon, M. A. Van Ende and W.Y. Kim: AISTech-Iron and Steel Technology Conference Proceedings, 2014, vol 1, pp. 1257-68.
N. Dogan, G. A. Brooks and M. A. Rhamdhani: ISIJ Int., 2011, vol. 51(7), pp. 1086-92.
F. Pahlevani, S. Kitamura, H. Shibata and N. Maruoka: Steel Res. Int., 2010, vol. 81, pp. 617-22.
Y. Ogasawara, Y. Miki, Y. Uchida and N. Kikuchi, ISIJ Int., 2013, vol. 53, pp. 1786-93.
R. Sarkar, P. Gupta, S. Basu and N. B. Ballal: Metall. Mater. Trans. B, 2015, vol. 46, pp. 961-76.
C. Kattenbelt and B. Roffel: Metall.Mater. Trans. B, 2008, vol. 39, pp. 764-69.
Y. Lytvynyuk, J. Schenk, M. Hiebler and A. Sormann: Steel Research International 2014, vol. 85, pp. 537-43.
D. Guo, D. Swickard, and J. Bradley: Iron Steel Technol., April 2014, pp. 131–40.
12. G. Li, B. Wang, Q. Liu, X. Tian, R. Zhu, L. Hu and G. Cheng: International Journal of Minerals, Metallurgy, and Materials 2010, vol. 17, pp. 715-22.
N. Sasaki, G. Brooks, and M.A. Rhamdhani: Asia Steel, Yokohama, Japan, 2015.
14. S. Kitamura: Steel Research International 2010, vol. 81, pp. 766-71.
15. S. Ohguchi, D. G. C. Robertson, B. Deo, P. Grieveson and J. H. E. Jeffes: Ironmak Steelmak., 1984, vol. 11, pp. 202-13.
16. G. A. Brooks, M. A. Rhamdhani, K. S. Coley, Subagyo and Y. Pan: Metall. Mater. Trans. B, 2009, vol. 40B, pp. 353-62.
17. A. K. Hewage, B. K. Rout, G. Brooks and J. Naser: Ironmak. Steelmak. 2016, vol. 43, pp. 358-70.
18. M. S. Millman, A. Kapilashrami, M. Bramming and D. Malmberg: Imphos: Improving Phosphorus Refining, European Union, Luxembourg, 2011.
19. B. K. Rout, G. A. Brooks, Z. Li and M. A. Rhamdhani: AISTech - Iron and Steel Technology Conference Proceedings, 2015, vol. 3, pp. 3225-37.
B.K. Rout, G. Brooks, M.A. Rhamdhani, Z. Li, F.N.H. Schrama, and A. Overbosch: Metall. Mater. Trans. B (submitted, E-TP-17-603-B), 2017.
B.K. Rout, G. Brooks, M.A. Rhamdhani, Z. Li, F. Schrama, and W.van der Knoop: Metall. Mater. Trans. B (submitted, E-TP-17-604-B), 2017
22. Q. Li, M. Li, S. Kuang, & Z. Zou: Metall. Mater. Trans. B., 2015, vol. 46, pp.1494-1509.
23. S. Kitamura, T. Kitamura, K. Shibata, Y. Mizukami, S. Mukawa, and J. Nakagawa: ISIJ Int.,1991, vol 31, no. 11, pp. 1322-1328.
24. N. Dogan, G. A. Brooks and M. A. Rhamdhani: ISIJ Int. 2011, vol. 51(7), pp. 1102-09.
25. K. Chou, U. B. Pal and R. G. Reddy: ISIJ Int., 1993, vol. 33, pp. 862-68.
26. B. Deo and R. Boom: Fundamentals of Steelmaking Metallurgy, Prentice Hall, Hertfordshire,1993, pp. 194.
27. B. K. Rout, G. A. Brooks, Z. Li and M. A. Rhamdhani: AISTech - Iron and Steel Technology Conference Proceedings, 2016, vol. 1, pp 1019-26.
28. F. R Cheslak, J. A. Nicholls and M. Sichel: Journal of Fluid Mechanics 1969, vol. 36, pp. 55-63.
29. S. C. Koria and K. W. Lange: Steel Res. 1987, vol. 58, pp. 421-26.
30. P. Kozakevitch: J Metals, 1968, vol. 22, pp. 57-67.
31. H. W. Meyer, W. F. Porter, G. C. Smith and J. Szekely: J Metals, 1968, vol. 20, pp. 35-42.
32. G. Brooks, Y. H. Pan, Subagyo and K. Coley: Metall. Mater. Trans. B, 2005, vol. 36, pp. 525-35.
33. C. L. Molloseau and R. J. Fruehan: Metall. Mater. Trans. B, 2002, vol. 33, pp. 335-44.
34. H. Gaye and P. V. Riboud: Metallurgical Transactions B, 1977, vol. 8, pp. 409-15.
P. Kozakevitch, G.H. Geiger, M. Olette, and P.V. Riboud: BOF Steelmaking, Iron and Steel Society, 1975, vol. 2, Chapter V, pp. 287–88.
K. Gu, N. Dogan, and K.S. Coley: Metall. Mater. Trans. B, 2017, pp. 1–18.
H. Sun and G. Zhang, AISTech Iron and Steel Technology Conference Proceedings, 2005, pp 257–68.
38. K. Gu, N. Dogan and K. S. Coley: Metall. Mater. Trans. B, 2017, vol. 48, pp. 2343-2353.
39. F. D. Richardson: Physical chemistry of melts in metallurgy. Academic Press (Elsevier), 1974, vol. 2, pp. 412-416.
F. Oeters: Metallurgy of Steelmaking. Verlag Stahleisen, 1994, pp. 369.
41. C. P. Manning and R. J. Fruehan: Metall. Trans. B, 2013, vol. 44B, pp. 37-44
42. S. Ban-Ya: ISIJ Int. 1993, vol. 33, pp. 2-11.
43. K. Narita, T. Makino, H. Matsumoto, A. Hikosaka and J. Katsuda: Tetsu-to-Hagané 1983, vol. 69, pp. 1722-29.
44. H. Suito and R. Inoue: ISIJ Int., 1995, vol. 35, pp. 266-71.
C. Cicutti, M. Valdez, T. Pérez, J. Petroni, A. Gómez, R. Donayo, and L. Ferro: Sixth Int. Conf. Molten Slags, Fluxes Salts, ISS, Stockholm, 2000.
46. S. C. Koria and K. W. Lange: Metall. Trans. B, 1984, vol. 15, pp. 109-16.
47. B. K. Rout, G. A. Brooks, M. A. Rhamdhani and Z. Li: Metall.Mater. Trans. B, 2016, vol 47 (6), pp. 3350-61.
48. Subagyo, G. Brooks, K. S. Coley and G. A. Irons: ISIJ Int., 2003, vol. 43 (7), pp. 983-89.
49. Subagyo, G. Brooks, and K. Coley: Canadian Metallurgical Quarterly, 2005, vol. 44(1), pp. 119–129.
50. Y. Doh, P. Chapelle, A. Jardy, G. Djambazov, K Pericleous, G Ghazal and P Gardin: Metall. Mater. Trans. B 2013, vol. 44, pp. 653-70.
51. B. T. Chao, Journal of Heat Transfer 1969, vol. 91, pp. 273-80.
P. Sulasalmi, V. Ville-Valtteri, A. Kärnä, M. Järvinen, S. Ollila, and T. Fabritius: Metall. Mater. Trans. B, 2016, vol. 47(6), pp: 3544-3556.
T. Nishi, H. Shibata, Y. Wasedaand H. Ohta: Metall. Mater. Trans. A, 2003, vol. 34(12), pp.2801-2807.
54. K. Nishioka, T. Maeda, M. Shimizu: ISIJ Int., 2006, vol. 46(3), pp.427-33.
55. N. Dogan, G. A. Brooks, and M. A. Rhamdhani: ISIJ Int., 2009, vol. 49(10), 1474–1482.
57. E. Schürmann, K. Obst, L. Fiege and H. Kaiser: Steel Res. Int., 1985, vol. 56, pp. 425-31.
58. M. Hirai, R. Tsujino, T. Mukai, T. Harada and M. Omori: Trans.ISIJ, 1987, vol. 27(10), pp. 805-813.
59. S. Li, X. Wei and L. Yu: Fuel, 2011, vol. 90, pp. 1350-60.
60. K. Ito and R. J. Fruehan: Metall. Tans. B, 1989, vol. 20, pp. 509-14.
Slag Atlas, Verlag Stahleisen GmbH, Düsseldorf, 1995, pp. 345–46.
62. A. Kondratiev and E. Jak: Metall. Mater. Trans. B, 2001, vol. 32, pp. 1015-25.
63. K Chiba, A Ono, M Saeki, M Yamauchi, M Kanamoto and T Ohno: Ironmak. Steelmak., 1993, vol. 20, pp. 215-20.
64. F. E. Rote and R. A. Flinn: Metall. Trans., 1972, vol. 3, pp. 1373-84.
65. Y. E. Lee and L. Kolbeinsen: ISIJ Int. 2007, vol. 47, pp. 764-65.
66. M. Ishiguro: Tetsu-to-Hagane, 1971, vol. 57, pp. 267-70.
67. S. C. Koria and K. W. Lange: Steel Res. 1987, vol. 58, pp. 421-26
68. S. Ban-Ya: ISIJ Int., 1993, vol. 33, pp. 2-11.
Acknowledgments
The authors wish to acknowledge the financial support provided by Tata Steel to carry out this research work. BKR would like to thank Mariana Adderley for the constructive and helpful discussion regarding this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted June 23, 2017.
Appendix
Appendix
A.1 Mass Transfer Coefficient in Hot Metal and Slag
The mass transfer coefficient in the hot metal has been calculated by the following relationship,[23]
where km is the mass transfer coefficient in metal phase (cm/s); ε is the stirring energy (W/t); H and L are the bath depth (cm) and diameter of the furnace, respectively; and T is the temperature in the impact zone (K). The total stirring energy was calculated by using the combined effect of the top and bottom gas injection in the BOF.[67]
The slag phase mass transfer coefficient was given by[7]:
where ks is the mass transfer coefficient in slag phase (cm/s); R gas constant (J mol−1 K−1); and a and b are the empirical parameters, assumed to be 1.7 and 0.25, respectively.[7]
A.2 Calculation of Cavity Height and Radius
The height and radius of the individual cavity formed by the top jet can be expressed as follows:
where Lh is the lance height (m) and the dimensionless momentum flow rate and is defined as
where \( \dot{m}_{\text{n}} \) is the momentum flow rate of the each nozzle, which is related to the total momentum flow rate, \( \dot{m}_{t} \) by the following equations:
Total momentum flow rate:
where nn is the number of nozzles in the lance tip; nangle is the nozzle angle (rad); dth is the throat diameter of the lance (m); P0 is the top supply pressure (Pa); and Pa is the ambient pressure (Pa).
A.3 Calculation of Equilibrium Distribution Ratios
Silicon distribution ratio[42]
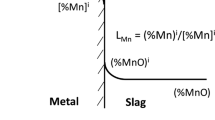
Manganese distribution ratio[43]:
where the apparent equilibrium constant k ’Mn is defined as follows
T. Fe denotes total Fe and MMn and MMnO are the molar mass (g/mol) of Mn and MnO, respectively.
Phosphorus distribution ratio
The phosphorus equilibrium distribution ratio at the slag–metal interface can be written as[68] follows:
where Kp is the equilibrium constant; f p is the activity coefficient of P; ho is the Henrian activity of oxygen; C is the conversion factor which related (pct P) with the mole fraction of PO2.5; and γP2O5 is the activity coefficient of PO2.5.
The equilibrium constant for the phosphorus oxidation reaction can be expressed as[63]
Here ho is the Henrian activity of oxygen, determined by assuming FeO-O equilibrium.
KF is the equilibrium constant for reaction [Fe] + [O] = (FeO), ΔGo = -128,090+57.99 T.[63] γFeo and γP2O5 are the activity coefficients of FeO and PO2.5, determined by Regular solution model proposed by Ban-Ya.[40] Henrian activity coefficient f p was determined by employing the first-order interaction parameter.\( \log \left( {f_{p} } \right) = e_{p}^{\text{p}} \left[ {{\text{pct}}\;{\text{P}}} \right] + e_{p}^{\text{c}} \left[ {{\text{pct}}\;{\text{C}}} \right], \)where e p p = 0.063 and e c p = 0.19.[7]
Rights and permissions
About this article
Cite this article
Rout, B.K., Brooks, G., Rhamdhani, M.A. et al. Dynamic Model of Basic Oxygen Steelmaking Process Based on Multi-zone Reaction Kinetics: Model Derivation and Validation. Metall Mater Trans B 49, 537–557 (2018). https://doi.org/10.1007/s11663-017-1166-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-017-1166-7