Abstract
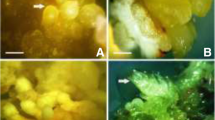
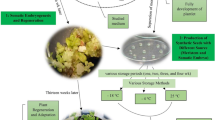
Kelussia odoratissima Mozaff. (or Kelus) is a medicinal plant native to the Zagros Mountains in Iran. This plant is widely used as a food flavoring and for its health-promoting properties. It has been considered an endangered species by the United Nations Development Programme. In this study, a somatic embryogenesis (SE) method was developed for mass propagation of Kelus. The green globular embryogenic callus was induced on cotyledonary leaves using the Murashige and Skoog (MS) medium supplemented with 1 mg/l 2,4-dichlorophenoxyaceticacid (2,4-D) and 0.25 mg/l Kinetin. Different treatments were assayed for proliferation of the embryogenic callus. The calli remained embryogenic in an MS medium containing 2,4-D (1 mg/l). The light treatments and carbon source showed significant effects (P ≤ 0.05) on the proliferation and development of somatic embryos. These treatments improved the conversion rate of the cotyledonary-stage embryos by 100%. The average numbers of embryos in the globular, heart, torpedo, and cotyledonary stages decreased by the addition of 3 g/l case in hydrolisate. The genetic stability among tissue culture-derived plants and the mother plant were assessed using the amplification fragment length polymorphism. No polymorphic band was observed among all the plants, exhibiting the genetic stability during in vitro multiplication. This research provides a promising approach for true-to-type plant multiplication of K. odoratissima through SE.




Similar content being viewed by others
References
Abdollahi Sahlabadi E, Ranjbar GA, Babaeian Jelodar NA, Bagheri NA (2012) Study of in vitro germination of the indangered medicinal plant of mountain Celery (Kelussia Odoratissima Mozaff.). Biotechcongress, Tehran, Iran. http://www.biotechcongress.ir
Ageel S, Elmeer (2011) K effects of casein hydrolysates and glutamine on callus and somatic embryogenesis of date palm (Phoenix dactylifera L.). NY Sci J 4(7):121–125
Ahmadi F, Kadivar M, Shahedi M (2007) Antioxidant activity of Kelussia odoratissima Mozaff. in model and food systems. Food Chem 105:57–64
Altamura MM, Rovere FD, Fattorini L, D’Angeli S, Falasca G (2016) Recent advances on genetic and physiological bases of in vitro somatic embryo formation. In: Germanà MA, Lambardi Maurizio (eds), In vitro embryogenesis in higher plants, methods in molecular biology, vol. 1359, Springer, New York. doi:10.1007/978-1-4939-3061-6
Askari-Khorasgani O, Mortazaeinezhad F, Otroshy M, Golparvar AR (2013) Breaking seed dormancy of endangered medicinal plant kelussia odoratissima using zygotic embryo culture technique. Tech J Eng App Sci 3(15):1712–1718
Aversano R, Di Dato F, Di Matteo A, Frusciante L, Carputo D (2011) AFLP analysis to assess genomic stability in solanum regenerants derived from wild and cultivated species. Plant Biotechnol Rep 5:265–271
Azad MAK, Yokota S, Begum F, Yoshizawa N (2009) Plant regeneration through somatic embryogenesis of a medicinal plant, Phellodendron amurense Rupr. In Vitro Cell Dev Biol-Plant 45:441–449. doi:10.1007/s11627-008-9171-9
Basavaraj S, Rangaswamy K, Rao AM, Prameela H, Bhagyashree M (2016) Morphological and molecular characterisation of somaclonal variants in tissue culture banana variety grand naine. Adv Life Sci 5:1205–1210
Beena MR, Martin KP (2003) In vitro propagation of the rare medicinal plant Ceropegia Candelabrum L. through somatic embryogenesis. In Vitro Cell Dev Biol—Plant 39:510–513
Berthouly M, Michaux-Ferrière N (1996) High frequency somatic embryogenesis in Coffea canephora. induction conditions and histological evolution. Plant Cell Tiss Org 44:169–176. doi:10.1007/BF00048196
Blum H, Beier H, Gross HJ (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99
Bomal C, Tremblay FM (1999) Effect of desiccation to low moisture content on germination, synchronization of root emergence, and plantlet regeneration of black spruce somatic embryos. Plant Cell Tiss Org Cult 56:193–200
Bomal C, Le VQ, Tremblay FM (2002) Induction of tolerance to fast desiccation in black spruce (Picea mariana) somatic embryos: relationship between partial water loss, sugars and dehydrins. Physiol Plant 115:423–530
Braybrook SA, Stone SL, Park S et al (2006) Genes directly regulated by leafy Cotyledon2 provide insight into the control of embryo maturation and somatic embryogenesis. Proc Natl Acad Sci (USA) 103:3468–3473. doi:10.1073/pnas.0511331103
Canter PH, Thomas H, Ernst E (2005) Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol 23(4):180–185
Chao W, Lin B (2012) Bioactivities of major constituents isolated from Angelica sinensis (Danggui). Chin Med 6:29
Choi J, Sung Z (1984) Two-dimensional gel analysis of carrot somatic embryonic proteins. Plant Mol Biol Rep 2:19–25. doi:10.1007/BF02885643
Dhir R, Shekhawat GS, Alam A (2014) Improved protocol for somatic embryogenesis and calcium alginate encapsulation in Anethum graveolens L.: a medicinal herb. Appl Biochem Biotechnol 173:2267–2278
Dodeman VL, Ducreux G, Kreis M (1997) Zygotic embryogenesis versus somatic embryogenesis. J Exp Bot 48:1493–1509
Elmeer KMS, Hennerty MJ (2008) Observations on the combined effects of light, NAA and 2,4-D on somatic embryogenesis of cucumber (Cucumis sativus) hybrids. Plant Cell Tiss Organ Cult 95:381–384. doi:10.1007/s11240-008-9439-0
Facchini PJ, De Luca V (2008) Opium poppy and Madagascar periwinkle: model non-model systems to investigate alkaloid biosynthesis in plants. Plant J 54:763–784. doi:10.1111/j.1365-313X.2008.03438.x
Fuentes SRL, Calheiros MBP, Manetti J et al (2000) The effects of silver nitrate and different carbohydrate sources on somatic embryogenesis in Coffea canephora. Plant Cell Tiss Org 60:5–13. doi:10.1023/A:1006474324652
Fuentes-Cerda CFJ, Monforte-González M, Méndez-Zeel M et al (2001) Modification of the embryogenic response of Coffea arabica by nitrogen source. Biotechnol Lett 23:1341–1343. doi:10.1023/A:1010545818671
Fujimura T, Komamine A (1979) Involvement of endogenous auxin in somatic embryogenesis in a carrot cell suspension culture. Z Pflanzenphysiol 95(79):13–19. doi:10.1016/S0044-328X80023-9
George EF, Hall MA, De Klerk GJ (eds.) (2008) Plant propagation by tissue culture, 3rd Edn, pp 1–28. Springer, Dordrecht
Giri CC, Zaheer M (2016) Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: recent trends and a sky eye view appraisal. Plant Cell Tissue Organ Cult 126(1):1–18
Halperin W (1964) Morphogenetic studies with partially synchronized cultures of carrot embryos. Science 146(3642):408–410
Halperin W (1995) In vitro embryogenesis: some historical issues and unresolved problems. In: Thorpe TA (ed) In vitro embryogenesis in plants. Kluwer Academic Publishers, Dordrecht, pp 1–16
Hand ML, de Vries S, Koltunow AMG (2016) A comparison of in vitro and in vivo asexual embryogenesis. In: Germanà MA, Lambardi M (eds), In vitro embryogenesis in higher plants, methods in molecular biology, vol. 1359, Springer, New York. doi:10.1007/978-1-4939-3061-6
Haque SK, Ghossh B (2016) High-frequency somatic embryogenesis and artificial seeds for mass production of true-to-type plants in Ledebouria revoluta: an important cardioprotective plant. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-016-1030-5
Hecht V, Vielle-Calzada JP, Hartog MV et al (2001) The Arabidopsis somatic embryogenesis receptor kINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol 127:803–816. doi:10.1104/pp.010324
Hwang SC, Ko WH (2004) Cavendish banana cultivars resistant to Fusarium wilt acquired through somaclonal variation in Taiwan. Plant dis 88:580–588
Kato M (1996) Somatic embryogenesis from immature leaves of in vitro grown tea shoots. Plant Cell Rep 15:920–923
Khan MS, Ahmad D, Adnan M, Khan MA (2014) The effect of somaclonal variation on salt tolerance and glycoalkaloid content of potato tubers. Aust J Crop Sci 8:1597
Khierallah HS, Hussein NH (2013) The role of coconut water and casein hydrolysate in somatic embryogenesis of date palm and genetic stability detection using RAPD markers. Res Biotechnol 4(3):20–28
Kintzios SE, Hiureas G, Shortsianitis E, Sereti E, Blouhos P, Manos C, Makri O, Taravira N, Drossopoulos J, Holevas CD (1998) The Effect of light on the induction, development and maturation of somatic embryos from various horticultural and ornamental species. Acta Hortic 461:427–432
Közsegui D, Johnston AJ, Rutten T et al (2011) Members of the RKD transcription factor family induce an egg cell-like gene expression program. Plant J 67:280–291. doi:10.1111/j.1365-313X.2011.04592.x
Kumar V, Sheela C (2014) High frequency somatic embryogenesis and synthetic seed production of the endangered species Swertia chirayita. Biologia 69/2:186–192
Larkin PJ, Scowcroft WR (1981) Somaclonal variation—a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214
Li D, Zhang J, Zhao J, Zhang Y, Chen F, Zhu J, Liu S, Yang Z (2006) Plant regeneration via somatic embryogenesis of Elymus sibiricus cv.‘chuancao No. 2. Plant Cell Tiss Organ Cult 84:285–292
Loyola-Vargas VM (2016) The History of somatic embryogenesis. In: Loyola-Vargas VM, Ochoa-Alejo N (eds), Somatic embryogenesis: fundamental aspects and applications, Springer, Cham. doi:10.1007/978-3-319-33705-0_25
Marques DV (1987) Study of some factors involved on in vitro callus growth and somatic embryogenesis of coffee tissues. In: Green CE, Somers DA, Hackett WP, Biesboer DD, Alan R (eds) Plant biology. vol 3. Plant tissue and cell culture, Liss Inc, New York, p 42
Martin KP (2003) Plant regeneration through somatic embryogenesis on Holostemma adakodien, a rare medicinal plant. Plant Cell Tiss Organ Cult 72:79–82
Martins M, Sarmento D, Oliveira M (2004) Genetic stability of micropropagated almond plantlets, as assessed by RAPD and ISSR markers. Plant Cell Rep 23:492–496
Mehta R, Sharma V, Sood A, Sharma M, Sharma RK (2011) Induction of somatic embryogenesis and analysis of genetic fidelity of in vitro-derived plantlets of Bambusa nutans wall., using AFLP markers. Eur J Forest Res 130:729–736
Meneses A, Flores D, Muñoz M et al (2005) Effect of 2,4-D, hydric stress and light on indica rice (Oryza sativa) somatic embryogenesis. Rev Biol Trop 53:361–368. doi:10.15517/rbt.v53i3-4.14598
Mo X, Long T, Liu Z, Lin H, Liu X, Yang Y, Zhang H (2009) AFLP analysis of somaclonal variations in Eucalyptus globulus. Biol plantarum 53:741–744
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:495–497
Naing AH, Min JS, Park KI, Chung MY, Lim SH, Lim KB, Kim CK (2013) Primary and secondary somatic embryogenesis in Chrysanthemum (Chrysanthemum morifolium) cv. ‘Baeksun’ and assessment of ploidy stability of somatic embryogenesis process by flow cytometry. Acta Physiol Plant. doi:10.1007/s11738-013-1328-4
Neuenschwander B, Baumann TW (1992) A novel type of somatic embryogenesis in Coffea arabica. Plant Cell Rep 10:608–612. doi:10.1007/BF00232380
Nic-Can GI, Avilez-Montalvo JR, Aviles-Montalvo RN, Márquez-López RE, Mellado-Mojica E, Galaz-Ávalos RM, Loyola-Vargas VM (2016) The relationship between stress and somatic embryogenesis. In: Loyola-Vargas VM, Ochoa-Alejo N (eds), Somatic embryogenesis: fundamental aspects and applications, Springer, Cham. doi: 10.1007/978-3-319-33705-0_25
Nosov AM (2012) Application of cell technologies for production of plant-derived bioactive substances of plant origin. Appl Biochem Microbiol 48(7):609–624
Oh MJ, Ahn MS, Jie EY, Liu JR, Min BW, Kim SW (2013) High-frequency plant regeneration from immature zygotic embryo cultures of Houttuynia cordata thunb via somatic embryogenesis. Plant Biotechnol Rep. doi:10.1007/s11816-013-0291-2
Ortiz BOC, Reyes MEP, Balch EPM (2000) Somatic embryogenesis and plant regeneration in Acacia farnesiana and A. schaffneri. In Vitro Cell Dev Biol-Plant 36:268–272. doi:10.1007/s11627-000-0049-8
Othmani A, Bayoudh C, Drira N, Marrakchi M, Trifi M (2009) Somatic embryo-genesis and plant regeneration in date palm Phoenix dactylifera L., cv. Boufeggousis significantly improved by fine chopping and partial desiccation of embryo-genic callus. Plant Cell Tiss Org 97:71–79
Pathak S, Mishra BK, Misra P et al (2012) High frequency somatic embryogenesis, regeneration and correlation of alkaloid biosynthesis with gene expression in Papaver somniferum. Plant Growth Regul 68:17–25. doi:10.1007/s10725-012-9689-z
Pedroso MC, Pais MS (1999) Direct somatic embryogenesis from leaves of Camellia japonica. In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants, vol 5. Kluwer, London, pp 163–178
Peraza-Echeverria S, Herrera-Valencia VA, Kay AJ (2001) Detection of DNA methylation changes in micropropagated banana plants using methylation-sensitive amplification polymorphism (MSAP). Plant Sci 161:359–367
Phillips RL, Kaeppler SM, Olhoft P (1994) Genetic instability of plant tissue cultures: breakdown of normal controls. Proc Natl Acad Sci 91:5222–5226
Powell W, Morgante M, Andre C, Hanafey M, Vogel J, Tingey S, Rafalski A (1996) The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breed 2:225–238
Prado M, Gonzalez M, Romo S, Herrera M (2007) Adventitious plant regeneration on leaf explants from adult male kiwifruit and AFLP analysis of genetic variation. Plant Cell Tiss Org 88:1–10
Raiesi S, Nadjafi F, Hadian J, Kanani MR, Ayyari M (2013) Autecological and phytochemical studies of Kelussia odoratissima Mozaff. An endangered ethnomedicinal plant of Iran. TBAP 3 (4) 285–294
Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, Borisjuk N, Brinker A, Moreno DA, Ripoll C, Yakoby N, O'Neal JM, Cornwell T, Pastor I, Fridlender B (2002) Plants and human health in the twenty-first century. Trends Biotechnol 20(12):522–531
Rathore NS, Rai MK, Phulwaria M, Rathore N, Shekhawat N (2014) Genetic stability in micropropagated Cleome gynandra revealed by SCoT analysis. Acta Physiol Plant 36:555–559
Razeghi L, Azizi M, Ziaratnia SM, Bagheri AR, Nemati SH (2015) Impact of hormonal combination on callus induction of Kelussia odoratissimia Mozaff. and evaluating its growth in broth. Iran J Med Aro Plants 30:6
Razeghi L, Azizi M, Ziaratnia SM, Bagheri AR, Nemati SH (2016) Evaluation in vitroculture of Kelussia odoratissima Mozaff and secondary metabolites production through suspension cultures. Pharm Innov J 5(1):74–80
Reinert J, Tazawa M, Semenoff S (1967) Nitrogen compounds as factors of the embryogenesis in vitro. Nature 216:1215–1216. doi:10.1038/2161215a0
Rybczynski JJ, Zdunczyk W (1986) Somatic embryogenesis and plantlet regeneration in the genus Secale. Theor Appl Genet 73:267–271
Sajjadi SE, Shokoohinia Y, Moayedi N (2012) Isolation and identification of ferulic acid from aerial parts of Kelussia odoratissima Mozaff. Jundishapur J Nat Pharm Prod 7(4):159–162
Sebastiani MS, Ficcadenti N (2016) In vitro plant regeneration from cotyledonary explants of Cucumis melo L. var. cantalupensis and genetic stability evaluation using RAPD analysis. Plant Cell Tiss Organ Cult 124:69–79
Shareef HJ, Khaun AM, Abdulrahman DA (2016) Improving the germination of somatic embryos in date palm Berhi cultivar in vitro. IJAAR 8 (1):17–23
Shojaei ZA, Ebrahimi A, Salimi M (2011) Chemical composition of three ecotypes of wild celery (Kelussia odoratissima). J Herbs Spices Med Plants 17(1):62–68
Slazak B, Sliwinska E, Saługa M, Ronikier M, Bujak J, Słomka A, Göransson U, Kuta E (2015) Micropropagation of Viola uliginosa (Violaceae) for endangered species conservation and for somaclonal variation-enhanced cyclotide biosynthesis. Plant Cell Tiss Org 120:179–190
Terryn N, Van Montagu M, Inzé D, Goosens A (2006) Functional genomic approaches to study and engineer secondary metabolites in plant cell cultures. In: Bogers LJ, Craker LE, Jange D (eds) Medicinal and aromatic plants. Springer, The Netherlands, pp 291–300
Vázquez-Flota FA, Monforte-González M, de Lourdes Miranda-Ham M (2016) Application of somatic embryogenesis to secondary metabolite-producing plants. In: Loyola-Vargas VM, Ochoa-Alejo N (eds), Somatic embryogenesis: fundamental aspects and applications, Springer, Cham. doi:10.1007/978-3-319-33705-0$425
Von Arlond S In: De Klerk GJ (eds) (2008) Somatic embryogenesis. In: George EF, Hall MA Plant propagation by tissue culture, 3rd edn. Springer, Dordrecht, pp 335–354
Vos P, Hogers R, Bleeker M, Reijans M, Van de Lee T, Hornes M, Friters A, Pot J, Paleman J, Kuiper M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic acids res 23:4407–4414
Vroh-Bi I, Anagbogu C, Nnadi S, Tenkouano A (2011) Genomic characterization of natural and somaclonal variations in bananas (Musa spp.). Plant Mol Biol Rep 29:440–448
Wickramasuriya AM, Dunwell JM (2015) Global scale transcriptome analysis of Arabidopsis embryogenesis in vitro. BMC Genomics 16:301. doi:10.1186/s12864-015-1504-6 2015_BMCB_301_29152.
Wu YC, Hsieh C (2011) Pharmacological effects of Radix Angelica sinensis (Danggui) on cerebral in fraction. Chin Med 6:32. doi:10.1186/1749-8546-6-32
Yadav K, Aggarwal A, Singh N (2013) Evaluation of genetic fidelity among micropropagated plants of Gloriosa superba L. using DNA-based markers—a potential medicinal plant. Fitoterapia 89:265–270
Yantcheva A, Vlahova M, Antanassov A (1998) Direct somatic embryogenesis and plant regeneration of carnation (D ianthus caryophyllus L.). Plant Cell Rep 18:148–153
Zheng Q, Perry S (2014) Alterations in the transcriptome of soybean in response to enhanced somatic embryogenesis promoted by orthologs of AGAMOUS-like 15 and AGAMOUS-like 18. Plant Physiol 164:1365–1377. doi:10.1104/pp.113.234062
Acknowledgements
The project was supported a grant from the Agricultural Biotechnology Research Institute of Iran.
Author information
Authors and Affiliations
Contributions
ME is the project leader and this manuscript is a part of the results of a research project on Kelussiaodoratissima carried out at the ABRII-Isfahan Branch. AM and RA cooperated in the research.
Corresponding author
Additional information
Communicated by Sergio J Ochatt.
Rights and permissions
About this article
Cite this article
Ebrahimi, M., Mokhtari, A. & Amirian, R. A highly efficient method for somatic embryogenesis of Kelussia odorotissima Mozaff., an endangered medicinal plant. Plant Cell Tiss Organ Cult 132, 99–110 (2018). https://doi.org/10.1007/s11240-017-1314-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1314-4