Abstract
Background
Somatic embryogenesis is one of the most popular in vitro regeneration methods for mass micropropagation. In the present study, somatic embryogenesis via zygotic embryos was studied in Asparagus racemosus Willd. Since the callus quality plays an important role in plantlet development, therefore, compact embryogenic callus was selected for further embryogenesis.
Results
Somatic embryos were induced by zygotic embryos germinating on callus induction medium (MS media with 1.54 mg L−1 2,4-D and 0.43 mg L−1 kinetin) in dark. Thereafter, the compact embryogenic callus differentiated up on MS media with 0.1 mg L−1 NAA and 0.5 mg L−1 kinetin supplemented with various concentrations of ancymidol. An addition of 0.75 mg L−1 ancymidol resulted in significant enhancement of somatic embryo formation and no malformed embryos were observed. Scanning electron micrographs and thin sections confirmed the structures as somatic embryos. Furthermore, these embryos were transferred to the same medium supplemented with glutamine, casein hydrolysate, and 3% sucrose for conversion into green shoots. These shoots could be multiplied in vitro using BAP-supplemented medium.
Conclusions
An effective method for conservation of an overexploited threatened medicinally important species Asparagus racemosus has been developed. This is the first report of the formation of somatic embryos from zygotic embryos in A. racemosus. Ancymidol in combination with kinetin and NAA was found to be most efficient for somatic embryo maturation and germination. The established protocol would certainly advance the efficient somatic embryo induction, maturation, and germination which could be utilized for large-scale propagation of Asparagus species.
Similar content being viewed by others
Introduction
Asparagus racemosus Willd. (common name Shatavar; Asparagaceae) is an important medicinal plant native to the Indian subcontinent. The tuberous roots of A. racemosus are rich in the saponins, Shatavarin I to IV having rejuvenating and phytoestrogenic properties. These saponins are now extensively used in hormone replacement therapy instead of synthetic estrogens that are neither safe nor effective (Barrett-Connor, 1998). Roots of A. racemosus are also reported to have antioxidant, anti-ADH (Wiboonpun et al. 2004), and anti-amnesic activity (Ojha et al. 2010).
In view of the increasing world population to cross nine billion by 2050, indicating that the crop supplies must be doubled to meet the requirement in changing climatic conditions (Chaudhary et al. 2015; Chaudhary et al. 2018). A. racemosus roots are extensively collected from the wild causing mass destruction of the germplasm. This overexploitation has put considerable survival pressure on this plant making it endangered in the region of its natural existence (Kala et al. 2006; Chaudhary and Dantu, 2011). The plant is normally propagated through seeds and by splitting the cluster of roots. However, due to poor seed set and germination and low availability of crown roots, propagation of elite lines is too slow. For these reasons, commercial production of this crop has remained consistently low and not able to fulfill the high market demands. This problem could be offset by the rapid multiplication of A. racemosus through tissue culture. Adventitious regeneration from callus (Kar and Sen, 1985) and clonal propagation through axillary branching (Bopana and Saxena, 2008) have been reported earlier in A. racemosus. In vitro proliferation in this genus through somatic embryos has been reported for Asparagus species such as A. officinalis (Kohmura et al. 1994; Li and Wolyn, 1995) A. breslerianus (Mousavizadeh et al. 2017) through this process has been reported for a large number of other species such as Carica papaya L. (Anandan et al. 2012), Musa (Ma et al. 2012; Divakaran and Nair, 2011), and Cymbidium bicolor Lindl. (Mahendran and Bai, 2012). Somatic embryogenesis is the preferred method for mass proliferation because of the possibility of rapid scale-up through a bioreactor for direct plantation as in coffee (Ducos et al. 2010) or encapsulation and use as artificial seeds (Utomo et al. 2008). We have been working on developing appropriate propagation methods and agronomic practices for this plant (Chaudhary and Dantu, 2011). In this paper, we report a method for induction of embryogenic callus and differentiation of somatic embryos from zygotic embryos. These somatic embryos could be germinated to form shoots on a modified medium, which could then be multiplied through shoot proliferation for increasing their numbers. This is the first report for production of the somatic embryo using zygotic embryos for this plant and conservation of this species could be a noteworthy genetic resource for future asparagus breeding programs.
Materials and methods
Plant material and medium
Asparagus racemosus accession no. IC471921 was obtained from National Bureau of Plant Genetic Resources, New Delhi, and established in the Botanical Garden of the Institute. Embryos were dissected under aseptic conditions from berries sterilized in 70% ethanol followed by direct flaming.
MS (Murashige and Skoog, 1962) basal medium supplemented with 3% (w/v) sucrose and 0.8% (w/v) agar was used for all experiments unless otherwise stated. Medium taken in suitable culture vials was sterilized at 121 °C and 15 Psi for 15 min. For callus induction, young embryos were cultured on MS medium supplemented with various concentrations of either 2,4-D (0, 1.1, 1.54, or 2.2 mg L−1) alone or in combination with 0.43 mg L−1 kinetin. The callus from callus induction medium (CIM) was transferred to somatic embryo induction (SEI) medium. SEI medium was first standardized for the growth regulators NAA (0.05, 0.1 mg L−1) and kinetin (0.5, 1.0 mg L−1) and then for ancymadol (0.5, 0.75, or 1.0 mg L−1). Developed somatic embryos from the SEI medium were transferred for germination (somatic embryo germination, SEG medium) to MS with 0.1 mg L−1 NAA, 0.5 mg L−1 kinetin, and 0.75 mg L−1 ancymidol supplemented with either (i) 600 mg L−1 glutamine and 400 mg L−1 casein hydrolysate, or (ii) 800 mg L−1 glutamine and 500 mg L−1 casein hydrolysate. These media contained either 3 or 5% sucrose. For shoot multiplication and elongation, germinating shoots were transferred to MS supplemented with BAP (0, 0.002, 0.01, 0.02, 0.04, 0.06, 0.08, and 0.1 mg L−1). To achieve shoot proliferation and cluster multiplication, healthy cultures were further subcultured to three concentrations of BAP (0.04, 0.06, 0.08 mg L−1).
Cultures of zygotic embryos for callus induction were incubated either in dark or under 16 h photoperiod for the initial 4 weeks after which all cultures were maintained under 16 h photoperiod and 30 μE m−2 s−1 irradiance provided by cool white fluorescent tubes.
Histology and SEM
The elongated bipolar embryo-like structures were fixed in formalin:acetic acid:70% ethanol (1:1:18) and dehydrated through a xylene-tertiary butyl alcohol series before gradual infiltration with xylene-paraffin wax and embedding in molten paraffin wax. The specimens were sectioned at 8 μm (MICROM HM 340E microtome, Germany), stained with 1% hematoxylin and erythrosine and observed in a Nikon optical microscope E200 (Nikon, Japan), and photographed using Nikon Digital Sight.
For scanning electron microscopy (SEM), calli-bearing somatic embryos were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4 for 24 h at 4 °C (De Mason et al. 1989). After washing in the same buffer, the material was dehydrated in a graded ethanol series, critical point dried, and coated with a thin layer of gold. The processed material was scanned and photographed in a Leo 435 VP Scanning Electron Microscope at an acceleration voltage of 15–30 kV at All India Institute of Medical Sciences, New Delhi.
Statistical analysis
Data for each experiment was recorded by counting the number of somatic embryos per culture and presented as mean ± standard error (SE). Quantitative data were analyzed using one-way analysis of variance (ANOVA) and comparisons between the mean values of treatment were made by Duncan’s multiple range test (DMRT) at 0.05 level of probability using SPSS 18.0.
Results
The simple sterilization procedure resulted in recovering 100% sterile cultures. Zygotic embryos cultured on 2,4-D alone did form callus but the presence of kinetin was absolutely necessary for the development of embryogenic callus (Table 1). MS culture media containing 0.43 mg L−1 kinetin and 1.54 mg L−1 2,4-D resulted in 74% cultures that formed creamish, hard, nodular, compact callus (compact embryogenic callus (CEC)), and also showed some globular (Fig. 1a) and bipolar embryos. The callus was regularly maintained on this medium for the continuous formation of somatic embryos.
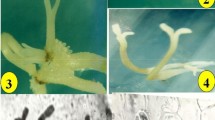
Induction of somatic embryogenesis in Asparagus racemosus from zygotic embryos. a Initiation of globular embryos (arrow marked) from embryogenic callus on CIM (bar 0.09 mm). b Globular embryo differentiation after 30 days of transfer to SEI medium. Note the smooth shining surface of the globular embryos (bar 0.05 mm). c Bipolar embryo structures developing from globular embryos 30 days after subculture to fresh SEI medium (bar 0.15 mm). d Germinating multiple somatic embryos (arrow marked) 14 days after transfer to SEG medium (bar 0.135 mm)
Somatic embryo induction could be achieved on changing the auxin from 2,4-D to NAA, increasing kinetin concentration and addition of the growth retardant ancymidol to the CIM. An optimum concentration of NAA (0.1 mg L−1) and kinetin (0.5 mg L−1) resulted in 4.83 embryos per culture (Table 2). An addition of 0.75 mg L−1 ancymidol to this medium increased the number of globular (14 per culture; Fig. 1b) embryos with 77% maturing into bipolar embryos (11 per culture; Fig. 1c) at the end of 6 weeks. The globular embryos had a shining smooth surface while the bipolar embryos appeared as elongated structures (Fig. 1b, c).
The SEI medium supplemented with glutamine (600 and 800 mg L−1), casein hydrolysate (400 and 500 mg L−1), and sucrose (3 or 5%) promoted germination of somatic embryos (Fig. 1d). Glutamine at 600 mg L−1 and casein hydrolysate at 400 mg L−1 in the presence of 3% sucrose supported germination in almost 65% of cultures within 2 weeks of transfer.
Furthermore, histological and SEM studies confirmed the structure of somatic embryos in the present work. A longitudinal section of the bipolar embryo showed a broad dome-like coleoptile enclosing the scutellum with a distinct narrow radicular end (Fig. 2a). SEM revealed that the globular embryos that were spherical and smooth surfaced (Fig. 2b) while the elongated bipolar embryos had an outer mesh-like epidermis (Fig. 2c)
Histology and SEM of somatic embryos. a L.S. of somatic embryo revealing a primary somatic embryo (pe) with shoot pole (sp) and root pole (rp). A secondary embryo (se) is arising from the body of the primary embryo. Note the coleoptile (cp) and scutellum (sc). (bar 0.42 mm). b, c SEM of somatic embryos. b globular embryos; note the distinct dome-like structures with smooth epidermal surface (c) bipolar embryo. Note the characteristic mesh-like epidermal surface (bar 30 μm, 200 μm, respectively)
Shoot multiplication was carried out on MS media supplemented with BAP. At higher BAP concentration, there was a steady increase in the multiple shoot buds but shoots were shorter. Three concentrations of BAP (0.04, 0.06, and 0.08 mg L−1) demonstrated high shoot numbers with no stunted shoots. Maximum number of shoots (15.87) per cluster was obtained on MS supplemented with 0.08 mg L−1 BAP with an average length of 2.93 cm (Table 3). Moreover, the shoots were observed thick and healthy in this medium.
Discussion
In vitro somatic embryogenesis is one of the most useful biotechnological tools used in plant breeding, propagation, and conservation approaches. There are several factors which affect somatic embryogenesis and regeneration, for example, explant type and culture media. Somatic embryo formation has been extensively studied in A. officinalis using various explants (Li and Wolyn, 1995; Levi and Sink, 1992; Dupire et al. 1999). Zygotic embryos have been utilized in several plant species such as Quercus suber (Testillano et al. 2018) and peach palm (Steinmacher et al. 2016). The responsiveness of zygotic embryos to somatic embryo formation is being reported here for the first time for any species of Asparagus.
The growth hormone plays a critical role in embryo induction, maturation, and germination. NAA is considered to be a milder auxin and has often proved to be better than 2,4-D in maturing of somatic embryos as in Centella (Martin, 2004). Therefore, 2,4-D was used in the CEI medium while NAA was studied in SEI medium. Furthermore, inclusion of ancymidol in SEI medium inhibited callus proliferation and promoted globular and bipolar embryo differentiation only in the compact and nodular callus. Ancymidol is a known inhibitor of gibberellic acid biosynthesis and by promoting the accumulation of storage protein that improves somatic embryo maturation (Li and Wolyn, 1995). During the progress of globular embryos into bipolar structures, the outer epidermal cells elongate giving a mesh-like appearance. Similar changes in epidermal cells were observed in the SEM of somatic embryos of Musa species (Pan et al. 2011). Inclusion of glutamine and casein hydrolysate in SEG medium improved both maturation and germination of the somatic embryos in the present study as have been shown in earlier studies in A. officinalis (Li and Wolyn, 1995) and chickpea (Patil et al. 2009). Varying sucrose concentrations affected somatic embryo formation, their maturation, and germination in A. officinalis (Levi and Sink, 1992). In A. racemosus, ancymidol was noticed to enhance somatic embryogenesis only in combination with a certain level of a carbon source and/or osmoticum in the medium.
In the present study, the growth hormone BAP was tested for the germination of the somatic embryo. The medium supplemented with 0.08 mg L−1 BAP was observed to be best for producing about 16 shoots per cluster. Kar and Sen (1985) has demonstrated the multiplication of shoots in A. racemosus derived from callus cultures on a medium containing BAP (1 mg L−1) and IAA (0.1 mg L−1).
Conclusion
This is the first report describing in vitro somatic embryo formation in A. racemosus. Ancymidol was found to be the key plant growth regulator for multiplication and maturation during somatic embryogenesis since along with NAA and kinetin. It also reduced the formation of malformed somatic embryos and facilitated further embryo maturation and germination. The established protocol in this study will be valuable for somatic embryogenesis induction and maintenance which could be applied for mass propagation and germplasm conservation of this species.
Availability of data and materials
Not applicable
Abbreviations
- Ancymidol:
-
α-cyclopropyl-α (4-methoxyphenyl)-5-pyrimidine methanol
- CIM:
-
Callus induction medium
- MS:
-
Murashige and Skoog
- NAA:
-
α-naphthaleneacetic acid
- SEI:
-
Somatic embryo induction
- SEG:
-
Somatic embryo germination
- SEM:
-
Scanning electron microscopy
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
References
Anandan R, Sudhakar D, Balasubramanian P, Gutierrez-Mora A (2012) In vitro somatic embryogenesis from suspension cultures of Carica papaya L. Sci Hortic 136:43–49
Barrett-Connor E (1998) Hormone replacement therapy, heart disease, and other considerations. Ann Rev Pub Health 19:55–72
Bopana N, Saxena S (2008) In vitro propagation of a high value medicinal plant: Asparagus racemosus Willd. In Vitro Cell Dev Biol 44:525–532
Chaudhary J, Dantu PK (2011) Collection, establishment, acclimatization and quantification of Shatavarin IV in the medicinally important plant—Asparagus racemosus Willd. In: Chemistry of Phytopotentials: Health, Energy and Environmental Perspectives, 1st edn. Springer, Heidelberg Dordrecht London New York, pp 83–86
Chaudhary J, Patil GB, Sonah H, Deshmukh RK, Vuong TD, Valliyodlan B, Nguyen HT (2015) Expanding omics resources for improvement of soybean seed composition traits. Front Plant Sci 6:1021
Chaudhary J, Shivaraj SM, Khatri P, Ye H, Zhou L, Klepadlo M, Dhakate P, Kumawat G, Patil G, Sonah H, Ratnaparkhe M, Deshmukh R, Nguyen HT (2018) Approaches, Applicability, and Challenges for Development of Climate-Smart Soybean. In: Kole C (ed) Genomic Designing of Climate-Smart Oilseed Crops. Springer, Cham, pp 1–74
De Mason DA, Sekhar KNC, Harris M (1989) Endosperm development in the date palm (Phoenix dactylifera) (Arecaceae). Am J Bot 6:1255–1265
Divakaran SP, Nair AS (2011) Somatic embryogenesis from bract cultures in diploid Musa acuminata cultivars from South India. Sci Hortic 131:99–102
Ducos J-P, Terrier B, Courtois D (2010) Disposable bioreactors for plant micropropagation and mass plant cell culture. Adv Biochem Eng/Biotech 115:89–115
Dupire L, De´cout E, Vasseur J, Delbriel B (1999) Histological and 2-D protein patterns comparisons between a wild type and a somatic embryogenic mutant of Asparagus officinalis L. Plant Sci 147:9–17
Kala CP, Dhyani PP, Sajwan BS (2006) Developing the medicinal plants sector in northern India: challenges and opportunities. J Ethnobiol Ethnomed 2:32
Kar DK, Sen S (1985) Propagation of Asparagus racemosus through tissue culture. Plant Cell Tissue Organ Cult 5:89–95
Kohmura H, Chokyu S, Harada T (1994) An effective micropropagation system using embryogenic calli induced from bud clusters in Asparagus offıcinalis L. J Japan Soc Hort Sci 63:51–59
Levi A, Sink KC (1992) Asparagus somatic embryos: production in suspension culture and conversion to plantlets on solidified medium as influenced by carbohydrate regime. Plant Cell Tissue Organ Cult 31:115–122
Li B, Wolyn DJ (1995) The effects of ancymidol, abscisic acid, uniconazole and paclobutrazol on somatic embryogenesis of Asparagus. Plant Cell Rep 14:529–534
Ma L, Xie L, Lin G, Jiang S, Chen H, Li H, Takáč T, Šamaj J, Xu C (2012) Histological changes and differences in activities of some antioxidant enzymes and hydrogen peroxide content during somatic embryogenesis of Musa AAA cv. Yueyoukang 1. Sci Hortic 144:87–92
Mahendran G, Bai VN (2012) Direct somatic embryogenesis and plant regeneration from seed derived protocorms of Cymbidium bicolor Lindl. Sci Hortic 135:40–44
Martin KP (2004) Plant regeneration through somatic embryogenesis in medicinally important Centella asiatica L. In Vitro Cell Dev Biol 40:586–591
Mousavizadeh SJ, Mashayekhi K, Hassandokht MR (2017) Indirect somatic embryogenesis on rare octoploid Asparagus breslerianus plants. Sci Hortic 226:184–190
Murashige T, Skoog F (1962) A revised medium for the rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ojha R, Sahu AN, Muruganandam AV, Singh GK, Krishnamurthy S (2010) Asparagus racemosus enhances memory and protects against amnesia in rodent models. Brain and Cognition 74:1–9
Pan X, Yang X, Lin G, Zou R, Chena H, Šamaj J, Chunxiang X (2011) Ultrastructural changes and the distribution of arabinogalactan proteins during somatic embryogenesis of banana (Musa spp. AAA cv. ‘Yueyoukang 1’). Physiol Plant 142:372–389
Patil G, Patel R, Jaat R, Pattanayak A, Jain P, Srinivasan R (2009) Glutamine improves shoot morphogenesis in chickpea (Cicer arietinum L.). Acta Physiol Plant 31:1077–1084
Steinmacher DA, Heringer AS, Jimenez VM, Quoirin MGG, Guerra MP (2016) Somatic embryogenesis in Peach-Palm (Bactris gasipaes) using different explant sources. In Vitro Embryogenesis in Higher Plants:279–288
Testillano PS, Pintos B, Gomez-Garay A, Risueno MC (2018) Stress-induced microspore embryogenesis by anther culture of Quercus suber L. Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants:93–105
Utomo HS, Wenefrida I, Meche MM, Nash JL (2008) Synthetic seed as a potential direct delivery system of mass-produced somatic embryos in the coastal marsh plant smooth cordgrass (Spartina alterniflora). Plant Cell Tissue Organ Cult 92:28–291
Wiboonpun NP, Phuwapraisirisan S, Tip-Pyang S (2004) Identification of antioxidant compound from Asparagus racemosus. Phytother Res 18:771–773
Acknowledgements
The authors gratefully acknowledge the Director of the Institute for providing the facilities to carry out the work. Director, NBPGR, New Delhi, is duly acknowledged for providing the plant material. All India Institute of Medical Sciences, New Delhi, is acknowledged for providing SEM facility.
Funding
Funding by University Grants Commission, New Delhi, through a major project vide letter no. F.32-382/2006 (SR) is acknowledged by PKD and JC.
Author information
Authors and Affiliations
Contributions
PKD and JC conceived and planned the study. JC and PKD contributed to drafting the manuscript. JC performed the data analysis and interpretation. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chaudhary, J., Dantu, P.K. Induction of somatic embryos in cultures of Asparagus racemosus Willd: an endangered medicinally important plant. Bull Natl Res Cent 43, 113 (2019). https://doi.org/10.1186/s42269-019-0157-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-019-0157-z