Abstract
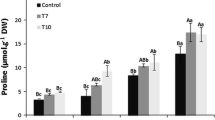
Abiotic stress is the major limiting factor of plant growth and crop yield which can be improved by osmoprotectants. Proline acts as an osmoprotectant and plays an important role in osmotic balancing, protection of sub-cellular structures, enzymes and in increasing cellular osmolarity that provide the turgor necessary for cell expansion under stress conditions. ∆1-pyrroline-5-carboxylate synthetase (P5CS), a rate-limiting enzyme in proline biosynthesis which is known for conferring enhanced salt and drought stress is subjected to feedback inhibition by proline. Therefore, in the present study, we used a mutagenized version P5CSF129A of wild P5CS which is not subjected to feedback control. Efficient in vitro transformation of embryonic structures of pigeonpea (Cajanus cajan (L.) Millsp.) was obtained using Agrobacterium tumefaciens strain LBA4404 harbouring a modified binary vector pCAMBIA 1301 carrying the hptII gene for resistance to hygromycin sulphate, GUS reporter gene, encoding β-glucuronidase, and the Vigna aconitifolia P5CSF129A genes under a constitutive 35S promoter. Embryonic structures showed blue color when tested for GUS after first cycle of antibiotic selection. Integration of T-DNA into nuclear genome of transformed plants and its sexual transmission to the progeny of the transgenic plants are confirmed by PCR amplification of 340 bp hptII, 800 bp P5CSF129A fragments and Southern blot hybridization analysis. The resultant primary transgenic plants showed more proline accumulation than their non-transformed plants. Levels of proline were also elevated in T1 transgenic plants when grown in the presence of 200 mM NaCl. In addition to their enhanced growth performance, more chlorophyll and relative water content under high salinity, these plants also had lower levels of lipid peroxidation. This suggests that overproduction of proline might play an important role against salt shock and cellular integrity.





Similar content being viewed by others
References
Anoop N, Gupta AK (2003) Transgenic indica rice cv IR-50 overexpressing Vigna aconitifolia d (1)-pyrroline-5-carboxylate synthetase cDNA shows tolerance to high salt. J Plant Biochem Biotechnol 12:109–116
Arnon DI, McSwain BD, Tsujimoto HY, Wada K (1974) Photochemical activity and components of membrane preparation from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochem Biophys Acta 357:231–245
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water studies. Plant Soil 39:205–208
Bhatnagar-Mathur P, Vincent V, Devi MJ, Lavanya M, Vani G, Sharma KK (2009) Genetic engineering of chickpea (Cicer arietinum L.) with the P5CSF129A gene for osmoregulation with implications on drought tolerance. Mol Breed 23:591–606
Dayal S, Lavanya M, Devi P, Sharma KK (2003) An efficient protocol for shoot regeneration and genetic transformation of pigeonpea (Cajanus cajan (L.) Millsp) by using leaf explants. Plant Cell Rep 21:1072–1079
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:12–14
Garcia-Mata C, Lamattina L (2001) Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol 126:1196–1204
Geetha N, Venkatachalam P, Lakshmi Sita G (1999) Agrobacterium-mediated genetic transformation of pigeonpea (Cajanus cajan L.) and development of transgenic plants via direct organogenesis. Plant Biotechnol 16(3):213–218
Gubis J, Vankova R, Cervena V, Dragunova M, Hudcovicova M, Lichtnerova H, Dokupil T, Jurekova Z (2007) Transformed tobacco plants with increased tolerance to drought. S Afr J Bot 73:505–511
Gustavo A, Joel Gonzalez C, Roberto Vazquez P, Ayra C (1998) Agrobacterium tumefaceins: a natural tool for plant transformation. Plant Biotechnol 1:3. ISSN 0717-3458
Hmida-Sayari A, Gargouri-Bouzid R, Bidani A, Jaoua L, Savoure A, Jaoua S (2005) Overexpression of D1-pyrroline-5-carboxylate synthetase increases proline production and confers salt tolerance in transgenic potato plants. Plant Sci 169:746–752
Hong Z, Lakkineni K, Zhang Z, Verma DPS (2000) Removal of feedback inhibition of pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol 122:1129–1136
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jogeswar G, Pallela R, Jakka NM, Reddy PS, Rao JV, Sreeniwasulu N, Kavi Kishor PB (2006) Antioxidative response in different sorghum species under short term salinity stress. Acta Physiol Plant 28:465–475
Karthikeyan A, Shumugiah Karutha P, Ramesh M (2011) Transgenic indica rice cv. ADT 43 expressing a Δ1-pyrroline-5-carboxylate synthetase (P5CS) gene from Vigna aconitifolia demonstrates salt tolerance. Plant Cell Tissue Organ Cult 107:383–395
Kavi Kishor PB, Sangam S, Amrutha RN, Laxmi PS, Naidu KR, Rao KRSS, Rao S, Reddy KJ, Theriappan P, Sreenivasulu N (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88:424–438
Kiran Kumar Ghanti S, Sujata KG, Vijay Kumar BM, Nataraja Karba N, Janardhan Reddy K, Srinath Rao M, Kavi Kishor PB (2011) Heterologous expression of P5CS gene in chickpea enhances salt tolerance without affecting yield. Biol Plant 55(4):634–640
Krishna G, Reddy PS, Ramteke PW, Rambabu P, Tawar KB, Bhattacharya P (2011) Agrobacterium-mediated genetic transformation of pigeonpea [Cajanus cajan (L.) Millsp.] for resistance to legume pod borer Helicoverpa armigera. J Crop Sci Biotech 14((3):197–204
Kumar SG, Reddy A, Sudhakar C (2003) NaCl effects on proline metabolism in two high yielding genotypes of mulberry (Morus alba L.) with contrasting salt tolerance. Plant Sci 165:1245–1251
Kumar SM, Kumar BK, Sharma KK, Devi P (2004) Genetic transformation of pigeonpea with rice chitinase gene. Plant Breed 123:485–489
Kumar V, Shriram V, Kavi Kishor PB, Jawali N, Shitole MG (2010) Enhanced proline accumulation and salt stress tolerance of transgenic indica rice by over-expressing P5CSF129A gene. Plant Biotechnol Rep 4:37–48
Lawrence PK, Koundal KR (2001) Agrobacterium tumefaciens mediated transformation of pigeonpea (Cajanus cajan L. Mill sp.) and molecular analysis of regenerated plants. Curr Sci 80(11):1428–1432
Matysik J, Bhalu AB, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plant. Curr Sci 82:525–532
Misra N, Gupta AK (2005) Effect of salt stress on proline metabolism in two high yielding genotypes of green gram. Plant Sci 169:331–339
Molinari HBC, Marur CJ, Filho JCB, Kobayashi AK, Pileggi M, Junior RPL, Pereira LFP, Vieira LGE (2004) Osmotic adjustment in transgenic citrus rootstock Carrizo citrange (Citrus sinensis Osb. X Poncirus trifoliate L. Raf.) overproducing proline. Plant Sci 167:1375–1381
Parvanova D, Ivanov S, Konstantinova T, Karanov E, Atanassov A, Tsvetkov T, Alexieva V, Djilianov D et al (2004) Transgenic tobacco plants accumulating osmolytes show reduced oxidative damage under freezing stress. Plant Physiol Biochem 42(1):57–63
Pileggi M (2002) Genetic transformation of the lettuce cultivar Grand Rapids (Lectuca sativa L.) by Agrobacterium tumefaciens to improve osmotic stress tolerance. Gen Mol Res 1:176
Prasad V, Satyavathi VV, Sanjaya KM, Valli A, Khandelwal MS, Shaila Lakshmi Sita G (2004) Expression of biologically active hemagglutinin-neuraminidase protein 4 of Peste des petits ruminants virus in transgenic pigeon pea (Cajanus cajan (L.) Millsp.). Plant Sci 166:199–205
Sairam RK, Tyagi A (2004) Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci 86:407–421
Sairam RK, Srivastava GC, Agarwal S, Meena RC (2005) Differences in response to salinity stress in tolerant and susceptible wheat genotypes. Biol Plant 49:85–91
Sambrook J, Russell RW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbour Laboratory Press, Cold Spring Harbour
Satyavathi VV, Prasad V, Khandelwal A, Shaila MS, Lakshmi Sita G (2003) Expression of hemagglutinin protein of rinder pest virus in transgenic pigeon pea (Cajanus cajan (L.) Millsp.). Plant Cell Rep 21:651–658
Sawahel WA, Hassan AH (2002) Generation of transgenic wheat plants producing high levels of the osmoprotectant proline. Biotech Lett 24:721–725
Su J, Wu R (2004) Stress-inducible synthesis of proline in transgenic rice confers faster growth under stress conditions than that with constitutive synthesis. Plant Sci 166:941–948
Surekha Ch, Beena MR, Arundhati A, Singh PK, Tuli R, Dutta Gupta A, Kirti PB (2005) Agrobacterium-mediated genetic transformation of pigeonpea (Cajanus cajan (L.) Millsp.) using embyronal segments and development of transgenic plants for resistance against Spodoptera. Plant Sci 169:1074–1080
Vendruscolo ECG, Schuster I, Pileggi M, Scapim CA, Molinari HBC, Marur CJ, Vieira LGE (2007) Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J Plant Physiol 16:1367–1376
Yamaguchi-Shinozaki K, Shinozaki K (2005) Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci 10:88–94
Yamchi A, Jazii FR, Mousav A, Karkhane AA, Renu (2007) Proline accumulation in transgenic tobacco as a result of expression of Arabidopsis D1-pyrroline-5-carboxylate synthetase (P5CS) during osmotic stress. J Plant Biochem Biotechnol 16:9–15
Yookongkaew N, Srivatanakul M, Narangajavana J (2007) Development of genotype-independent regeneration system for transformation of rice (Oryza sativa sp. indica). J Plant Res 120:237–245
Acknowledgments
The authors are grateful to the respective institutions for providing necessary facilities for undertaking this investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Surekha, C., Kumari, K.N., Aruna, L.V. et al. Expression of the Vigna aconitifolia P5CSF129A gene in transgenic pigeonpea enhances proline accumulation and salt tolerance. Plant Cell Tiss Organ Cult 116, 27–36 (2014). https://doi.org/10.1007/s11240-013-0378-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-013-0378-z